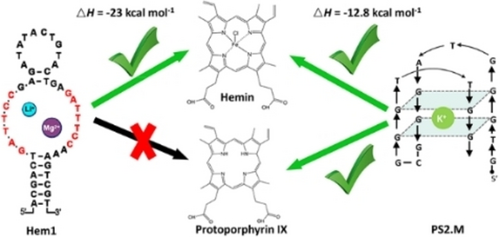
Selective Hemin Binding by a Non-G-quadruplex Aptamer with Higher Affinity and Better Peroxidase-like Activity
Graphical Abstract
Abstract
Previous aptamers for porphyrins and metalloporphyrins were all guanine-rich sequences that can fold in G-quadruplex structures. Due to stacking-based binding, these aptamers can hardly tell different porphyrins apart, and they can also bind other planar molecules, hindering their practical applications. In this work, we used the capture selection method to obtain aptamers for hemin and protoporphyrin IX (PPIX). The hemin aptamer (Hem1) features two highly conserved repeating binding loops, and it cannot form a G-quadruplex, which was supported by its Mg2+-dependent but K+-independent hemin binding and CD spectroscopy. Isothermal titration calorimetry revealed much higher enthalpy change for the new aptamer, and the best aptamer showed a Kd of 43 nM hemin. Hem1 can also enhance the peroxidase-like activity of hemin. This work demonstrates that aptamers have alternative ways to bind porphyrins allowing selective recognition of different porphyrins.
Porphyrin binding aptamers are of great interest for biosensors, catalysis, and understanding the origin of life.1-3 In 1996, Li and Sen immobilized N-methyl mesoporphyrin IX (NMM) to select DNA sequences that can catalyze porphyrin metalation, and they obtained G-quadruplex (G4) DNA.4 These DNA strands were later found to bind hemin yielding peroxidase-like activity.5, 6 This discovery led to the use of G4/hemin complexes for the design of biosensors, degradation of organic pollutants, and therapy.1, 2 The Pei group used the library immobilization method7-9 to select aptamers for NMM.10 Another NMM selection resulted in a modified RNA aptamer, which could also bind hemin.11 Niles and Marletta immobilized mesoprotoporphyrin IX (MPIX) to retain RNA aptamers and eluted aptamers with heme.12 The resulting sequences were G-rich as well. Later, a hemin-agarose column was used to select RNA aptamers by the Ito group.13 The best aptamer named 6c5 contained stretches of guanines that can also support G4. Two other groups selected DNA aptamers using hemin-agarose,14, 15 and both resulted in G4 sequences.
So far, it is generally perceived that porphyrin-binding aptamers are G4s, and stacking with a G-quartet is the main binding interaction.16-19 As a result, these aptamers are general porphyrin binders that can hardly distinguish different porphyrins. In addition, G4 DNAs can nonspecifically bind other planar molecules, such as malachite green, sulforhodamine B and thioflavin T, leading to significant interference and hindering practical applications.20-22 Besides the aromatic rings that can stack with a G-quartet, porphyrins also have peripheral and metal coordinated ligands that may interact with non-G4 aptamers. Non-G4 aptamers allow discrimination of different porphyrins, making more robust hemin/aptamer complexes and providing versatility in the design of biosensors, better catalysts and new smart DNA nanostructures.23-26
In biology, hemin is derived from protoporphyrin IX (PPIX) by chelating an iron ion.27 So far, no aptamer selection was carried out using PPIX. In all the above hemin selections, hemin was immobilized via one of the carboxyl groups. Immobilization of hemin may mask some of the potential aptamer binding sites. The capture-SELEX method immobilizes a DNA library and allows the use of free target molecules,28-30 which may produce new and better aptamers.31, 32 In this work, we used capture-SELEX to isolate aptamers for hemin and PPIX.
The structure of hemin is shown in Figure 1A. It has two carboxyl groups and the central iron is coordinated to a Cl− ligand. After replacing the Fe−Cl by two protons, the molecule becomes PPIX. The hemin selection was performed by immobilization of a DNA library containing an N30 region (30 random nucleotides, Table S1) via hybridization to a biotinylated DNA attached to a streptavidin column.28, 33 The initial library contained 500 pmol DNA (3×1014 random sequences). The immobilized library was eluted with free hemin and the eluted strands were amplified by PCR (Figure S1). The initial 11 rounds of selection used 100 μM hemin, and the next 9 rounds used 10 μM hemin. The same method was also used for the PPIX selection, for which 50 μM PPIX was used throughout the 16 rounds. Finally, the PCR products from the last rounds were sequenced.
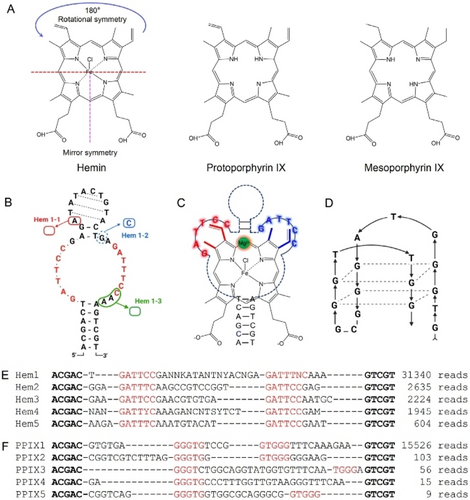
(A) The structures of hemin, PPIX and MPIX. (B) The predicted secondary structure of the Hem1 aptamer. Three mutants were tested. (C) A model of hemin binding by the Hem3 aptamer. (D) A proposed PS2.M aptamer structure.34 The top 5 sequences of the (E) hemin and (F) PPIX selections. The conserved sequences are shown in red. The boldface region can form base pairs. Some degenerate bases existed in Hem1.
The top 5 sequences of the hemin selection analyzed by the Geneious software represented 96.4 % of the final library, and they can be assigned to the same family (Figure 1E). We named the most abundant sequence Hem1 (Figure S2). To our surprise, these sequences were not rich in guanine and most sequences did not contain even two consecutive guanines. We analyzed all the top 50 sequences (99.85 % of the final library), and none of them can fold into a typical G4 structure. Instead, they contained two loops connected by two base paired stems. The conserved sequences in the loops are a repeat of GATT(T/C)C (Figure 1B). We further analyzed the top 50 sequences in an earlier round (round 11), and only one sequence can fold into a G4 structure (Table S2). So, it appeared that G4 sequences were at disadvantage using this capture-SELEX method.
As shown in Figure 1A, the peripheral ligands in the bottom half of hemin has a mirror symmetry, while those in the top half has a 180° rotational symmetry. The two binding loops in the Hem1 family aptamers (especially Hem3) also have a rotation symmetry if we consider that some T and C bases are interchangeable based on the sequence alignment. Thus, if Hem1 family aptamers have a simple stem-loop structure, the aptamer loops might interact with hemin via its top part as shown in Figure 1C.
We then analyzed the final library for the PPIX selection, and the first sequence (named PPIX1) was dominating (98.7 %, Figure 1F). By aligning with other sequences, the conserved region is GGGTG…GTGGG. While some previous aptamers also have such a motif, none of them have the exact sequence between the two GGGTG motifs (Table S3). So, we believe this is also a new aptamer. The PPIX1 aptamer has a high guanine content in the random region (36 %), although it did not appear that PPIX1 has four stretches of guanines either.
Porphyrins have rich electronic absorption features useful for binding assays. We first used UV/Vis spectroscopy to evaluate aptamer binding.17, 35 The spectrum of free hemin depends on the way of sample preparation. For example, using a Triton X-100 solution to dissolve hemin resulted in a sharp peak at 388 nm (Figure 2A), whereas directly dissolving hemin in water resulted in a broad absorption peak (Figure 3C, 3D). Adding the Hem1 aptamer resulted in a sharp Soret peak at 411 nm (Figure 2A), which was consistent with the literature of aptamer binding to hemin. We used the absorbance ratio at 411 nm and 388 nm to quantify the binding (Figure 2D), although Kd cannot be fitted due to the use of too high concentration of hemin (10 μM, in the titration region).36 Free PPIX showed a broad peak at 388 nm, and adding Hem1 did not shift this peak (Figure 2B, 2D), suggesting that Hem1 cannot bind PPIX. Given the structural similarity between hemin and PPIX, the Fe−Cl part of hemin likely involved in binding. Similarly, no binding was detected between MPIX and Hem1 (Figure 2C). We then added the PPIX1 aptamer to hemin and to PPIX (Figure S3), and a large shift was obtained in both cases. Therefore, the Hem1 aptamer is selective for hemin, which was not achieved previously.
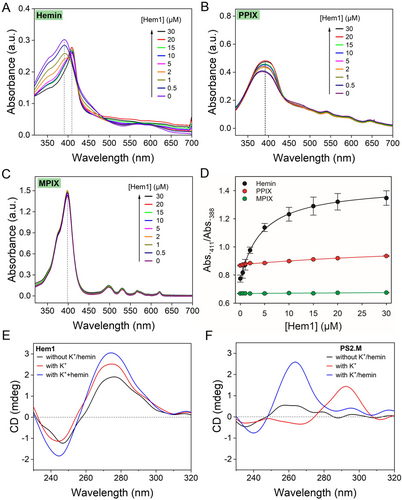
UV/Vis spectra of the Hem1 aptamer mixed with (A) hemin, (B) PPIX, and (C) MPIX. Hemin was directly dissolved in a Triton X-100 containing buffer. (D) Quantification of change in absorbance ratios of the samples. The samples contained 10 μM hemin in buffer (100 mM NaCl, 5 mM MgCl2, pH 7.2, 0.01 % Triton X-100). CD spectra of (E) Hem1, and (F) PS2.M aptamer (5 μM) and after adding 20 mM K+ and 20 μM hemin.
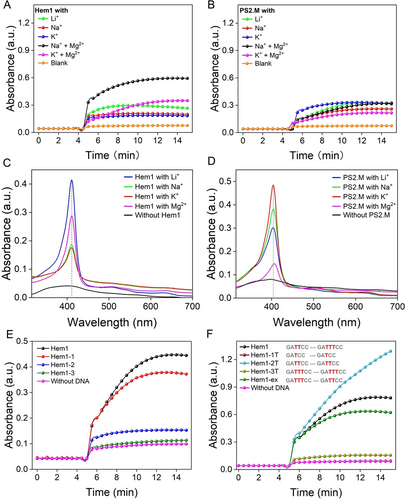
The catalytic activity of the (A) Hem1 and (B) PS2.M aptamers with different cations by monitoring TMB oxidation at 658 nm. The reactions were performed using 1 μM hemin and 2 μM DNA in buffer, pH 7.2 with 0.01 % TritonX-100, 2 mM H2O2 and 1 mM TMB. UV/Vis spectra of 5 μM (C) Hem1 and (D) PS2.M aptamers binding with 5 μM hemin in buffers with different cations (100 mM Li+, Na+ or K+, or 5 mM Mg2+). Hemin was first dissolved in a Triton X-100 free buffer and then diluted in buffer containing Triton X-100. (E, F) Activity comparison of Hem1 with a few mutants.
In addition, we employed a competitive binding assay using the UV/Vis method to assess the binding three aptamers: PS2.M, Hem1, and PPIX1 (Figure S4). Adding PPIX displaced hemin from the PS2.M and PPIX1 aptamers but not from the Hem1 aptamer. This experiment also indicated Hem1 is selective for hemin, and hemin/Hem1 is a more stable complex resisting nonspecific displacement.
G4 structures have characteristic CD signatures. Hem1 had a positive CD peak near 278 nm (characteristic of a typical B-type DNA), which was not changed by the addition of K+ and hemin (Figure 2E), confirming that Hem1 is not a G4. In contrast, PS2.M (a classical hemin binding G4 DNA, Figure 1D)5 had a large positive peak at 295 nm after adding K+, and the peak shifted to 264 nm after further addition of hemin, suggesting a change from an antiparallel G4 to a parallel G4 (Figure 2F).15 Therefore, CD spectroscopy also verified Hem1 being a non-G4 aptamer. In addition, CD also showed that PPIX1 is not a G4 structure either (Figure S5).
A key feature of previous G4-based aptamers is that their hemin complexes possessed peroxidase-like activity.1, 5 We then measured the activity using 3,3′,5,5′-tetramethylbenzidine (TMB) as a substrate and monitored the rise of the 658 nm peak corresponding to the one-electron oxidation charge-transfer product. The formation of G4 structures is often promoted by K+ ions,37 even for some exotic G4s.38 We then examined the effect of metal ions on the catalytic activity.5 As shown in Figure 3A, the activity of Hem1 was the highest in the presence of Mg2+ followed by Li+, whereas little time-dependent reaction was observed with Na+ or K+. For the PS2.M DNAzyme, the highest activity was achieved with K+ (Figure 3B). A careful measurement of the initial rate further confirmed the difference of Hem1 and PS2.M in metal preference (Figure S6). The requirement of Mg2+ but not K+ also suggested that Hem1 is not a G4. We further measured the influence of Mg2+ concentration, and the activity was higher with more Mg2+ (Figure S7).
To further confirm our observations, we measured the UV/Vis spectra in different metal ions. Interestingly, the highest Soret peak was seen with Li+ followed by Mg2+, while Na+ or K+ ions showed the lowest peak (Figure 3C). The similar behavior of Li+ and Mg2+ is reminiscent of the diagonal relationship, and thus charge density seems to be critical for the binding. Again, PS2.M showed the highest peak with K+ and Na+ (Figure 3D).
The Mfold-predicted secondary structure of Hem1 is shown in Figure 1B, showing two stem regions connecting the two conserved loops. We then used the activity assay to understand the structure of Hem1. We first designed Hem1-1, which removed an adenine allowing the formation of a more stable hairpin on the top (Figure 1B, red). The activity of Hem1-1 was comparable to Hem1 (Figure 3E), confirming the top stem-loop structure. We then replaced a guanine by a cytosine on the basis of Hem1-1 (Figure 1B), and this mutation was expected to form an extra base pair to shrink the loop region (named Hem1-2). However, Hem1-2 showed almost no activity enhancement. Then, three more bases (CAA) were removed from the loop (Figure 1B, green), which also inhibited the activity (Figure 3E). It appears that the loop region needs to have some flexibility outside the conserved nucleotides.
Based on the sequence alignment, it seems that the two loop sequences can be identical, although the loop sequences in Hem1 were not exactly the same. Here, we systematically varied the number of thymine bases in the loops, and in each case, the two loops were kept the same to test the symmetry idea mentioned above (Figure 3F). When only one thymine was present (Hem1-1T), the activity was fully lost. The highest activity was seen with two thymines (Hem1-2T), and this is the same as Hem3 in Figure 1E. When we added another thymine (Hem1-3T), the activity dropped significantly again. Since Hem1 is not fully symmetrical, we also exchanged its two loop (Hem1-ex) and a similar activity was observed. Overall, the aptamer can have two identical loop sequences, and Hem3 (Hem1-2T) appeared to have even better activity. UV/Vis titration also indicated that Hem1-2T has stronger binding to hemin compared to Hem1 (Figure S8).
It is interesting to note that Hem1-2T showed a more sustained activity, whereas the activity of Hem1 plateaued quickly at a lower absorbance value suggesting deactivation of the catalyst (Figure 3F). Thus, it might be that a role of the aptamers is to protect hemin from degradation during the peroxidation reaction,39 and a tighter binder can better protect hemin.
To quantitatively measure binding affinities, we used isothermal titration calorimetry (ITC). This method was rarely used for porphyrin binding aptamers likely due to the tendency of aggregation of porphyrins in water. We optimized the assay conditions by directly dissolving hemin in a Triton X-100 containing buffer (Figure S9), and we were able to achieve clean titration data. We titrated 200 μM hemin into 10 μM various aptamers and observed exothermic reactions for all of them (Figure 4). Interestingly, the Hem1-2T aptamer had the tightest binding with a Kd of 43 nM (Figure 4B). Thus, this small change in the loop has improved the Kd by 10-fold compared to Hem1. Hemin binding to PS2.M has a Kd of 0.28 μM (Figure 4C), which was five-fold weaker compared to Hem1-2T. Importantly, the released heat from PS2.M was only half of that from Hem1 or Hem1-2T (Table 1), regardless of their Kd. The ΔH and ΔS of our new aptamers were both more negative, suggesting more contacting points with hemin. Since PS2.M binds hemin by stacking,40, 41 it is understandable that the heat release was smaller. Finally, hemin binding to the PPIX1 aptamer also showed a Kd of 0.32 μM (Figure 4D).
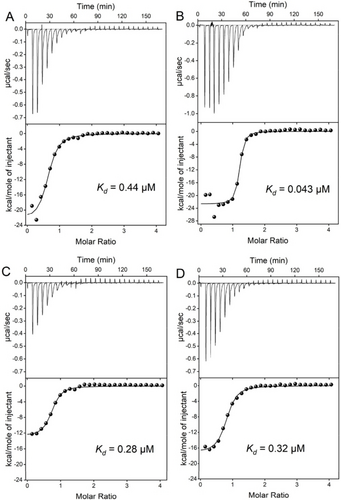
ITC traces and integrated heat of titrating 200 μM hemin into 10 μM (A) the Hem1 aptamer in Li−Mg buffer, (B) the Hem1-2T aptamer Na−Mg buffer, (C) PS2.M aptamer in Na−K buffer, and (D) PPIX1 aptamer in Na−Mg buffer.
Aptamer |
N |
Kd (μM) |
ΔH (kcal mol−1) |
ΔS (cal mol−1 K−1) |
|---|---|---|---|---|
Hem1 |
0.62±0.02 |
0.44 |
−23±1 |
−47.1 |
Hem1-2T |
1.15±0.02 |
0.043 |
−22.7±0.6 |
−42.4 |
PS2.M |
0.70±0.01 |
0.28 |
−12.8±0.4 |
−13.0 |
PPIX1 |
0.78±0.01 |
0.32 |
−17.2 ±0.4 |
−28.0 |
In a DNA library, a minimal of four sets of consecutive guanines can support a G4 structure. The probability of having it is greater than ( )8. This probably is much higher than that of a typical aptamer (around one in 1010 sequences).42 Thus, G4 sequences are statistically favored in aptamer selection. Nevertheless, our capture-SELEX still yielded non-G4 sequences. First, based on the thermodynamic differences, our aptamers are likely to have more binding points compared to simple G4 stacking based binding, which may favor non-G4 sequences during the selection. Such an advantage during selection, if existed, should be kinetic since the Kd of the G4 and non-G4 aptamers were comparable.43 Second, our buffer did not contain K+, which might be another reason for the suppression of G4 sequences. Finally, some previous hemin selections used biased library already rich in guanine (Table S4),15, 44 and this also contributed to the repeated isolation of G4.
Using capture-SELEX, we isolated aptamers for hemin and PPIX. For the hemin selection, non-G4 aptamers were obtained as confirmed by sequence analysis, UV/Vis and CD spectroscopy, metal ion requirements and mutation studies. This Hem1 aptamer also can support the peroxidase-like activity. One aptamer reached a Kd of 43 nM hemin, five-fold tighter compared to the classical PS2.M. A fundamental difference is the much higher ΔH of the non-G4 aptamers. This is the first example of an aptamer with selectivity for binding to a specific porphyrin, and it has the following implications. First, it shows the feasibility to use aptamers to recognize different porphyrins. Second, it shows that porphyrin aptamers can be non-G4, and the new aptamers already demonstrated resistance to nonspecific displacement, a practical problem facing G4 aptamers. Third, due to its closed stem structure, Hem1 can be readily used for strand displacement reactions, and this aptamer can complement the current G4-based aptamers for biosensor and nanotechnology applications.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the PhD Fellowship of the State Key Laboratory of Marine Environmental Science at Xiamen University. L. Gu received a scholarship from Chinese Scholarship Council (CSC) to visit the University of Waterloo.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.