Observing the Entry Events of a Titanium-Based Photoredox Catalytic Cycle in Real Time
Graphical Abstract
Abstract
Titanium-based catalysis in single electron transfer (SET) steps has evolved into a versatile approach for the synthesis of fine chemicals and first attempts have recently been made to enhance its sustainability by merging it with photo-redox (PR) catalysis. Here, we explore the photochemical principles of all-Ti-based SET-PR-catalysis, i.e. in the absence of a precious metal PR-co-catalyst. By combining time-resolved emission with ultraviolet-pump/mid-infrared-probe (UV/MIR) spectroscopy on femtosecond-to-microsecond time scales we quantify the dynamics of the critical events of entry into the catalytic cycle; namely, the singlet-triplet interconversion of the do-it-all titanocene(IV) PR-catalyst and its one-electron reduction by a sacrificial amine electron donor. The results highlight the importance of the PR-catalyst's singlet-triplet gap as a design guide for future improvements.
Titanium-based catalytic transformations1 are highly intriguing for a variety of reasons. Firstly, Ti belongs to the ten most abundant elements of the earth's crust, thus, rendering these processes more sustainable and more cost-effective as compared to those relying on precious metals. In addition, Ti itself is non-toxic and hence, catalyst and by-products can be expected to be environmentally benign.2 Finally, Ti-catalysis has matured into a versatile tool for synthesizing fine chemicals by making use of two complementary reactivity patterns; namely, hydro-functionalization, requiring the metal only at its highest oxidation state, +IV, and redox transformations, which toggle the metal between TiIV and its low-valent forms, TiII or TiIII.1a-1d
Ti-catalysis in SETs via the TiIV/TiIII redox couple is particularly compelling because it offers facile chemoselective access to radical intermediates via inner-sphere electron transfer (ET) to a metal-bound substrate.3 A superb TiIII catalyst is the coordinatively unsaturated species TiIII(Cp)2Cl, originally introduced by Nugent and RajanBabu.4 Owing to its remarkable functional group tolerance,2 it has emerged as an ideal green catalyst for engaging a wide range of substrates such as epoxides, enones, nitriles, alkyl halides, and others.1a-1d, 2, 3, 5 It is prepared in situ from the higher valent dichlorido precursor, TiIV(Cp)2(Cl)2, using a metal reductant6 and coexists in solution with its homodimer.7 Enantiomerically pure titanocene precursors even opened up the avenue to enantioselective transition-metal catalyzed radical reactions, which can then be utilized to reductively de-symmetrize meso-epoxides8 and to facilitate regiodivergent ring-openings of racemic or enantiomerically pure epoxides.9 Finally, the outstanding utility of TiIII(Cp)2Cl as both, a stoichiometric reagent and a catalyst, is showcased by numerous applications in natural product synthesis.10
The sustainability of Ti-based SET-catalysis can be improved further by avoiding the stoichiometric metal reductant. To this end, Ti-catalyzed reactions were recently coupled to PR-catalysis.11 Apart from replacing the metal with photons, this strategy allowed also for overcoming the limited oxidizing power of the TiIV radical intermediate, which may impede a desired reductive elimination.12 However, as is common in the field, bipyridine complexes of Ru and Ir were used as PR-co-catalysts and thus, sustainability was gained only at the expense of utilizing a precious metal in the photocycle.
Here, we elucidate the photochemical principles of PR-catalysis based exclusively on the TiIV/TiIII redox couple, i.e. without any PR-co-catalyst, as we proposed earlier.13 Specifically, we reveal here the elementary dynamics following optical excitation of a titanocene PR-catalyst and in particular, the critical events of entry into the catalytic cycle that transforms a targeted substrate. Apart from time-resolved fluorescence spectroscopy, we deploy UV/MIR-spectroscopy on femtosecond-to-microsecond time scales and take full advantage of this technique's unique sensitivity to changes of the molecular and electronic structures of dynamically evolving systems featuring transition metals14 and/or systems implicated in organocatalytic cycles.15
UV-MIR-spectroscopy requires the PR-catalyst to feature distinctive vibrations that are IR-active in a spectral region accessible with state-of-the-art ultrafast laser sources. Unfortunately, this is not the case for TiIV(Cp)2(Cl)2, whose Cl−Ti−Cl stretching and Cp-ring modes are located well below 1000 cm−1. We therefore investigated instead the IR-amenable model, TiIV(Cp)2(NCS)2 (1), in which the chlorido ligands are substituted by isothiocyanato ligands. Their stretching motions give rise to two characteristic IR-bands in the Fourier-transform infrared (FTIR) spectrum (cf. Figure 1a).16 The resonance at 2022 cm−1 results from the out-of-phase combination (νop) of the two N=C=S-oscillators whereas the band at 2059 cm−1 is due to their in-phase combination (νip).
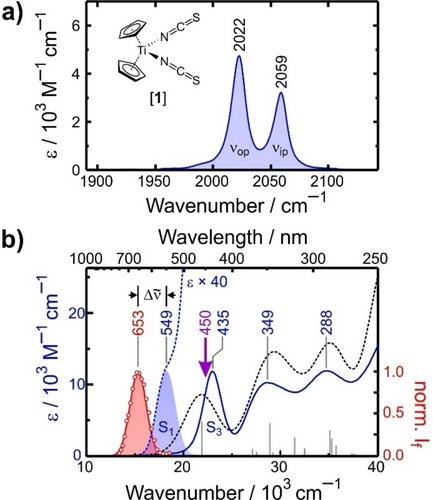
a) Stationary FTIR-spectrum of 1 in liquid MeCN solution at room temperature. b) Experimental UV/Vis (solid and dashed blue curves, left ordinate) and luminescence (red symbols, right ordinate) spectra of 1 in liquid MeCN at 295 K. Blue and red-shaded spectra are Gaussian fits. The dashed black curve is the TD-DFT-spectrum and results from convoluting the sticks (fosc, gray sticks, cf. SI for details) with a Gaussian profile. The Stokes-shift is indicated by  .
.
The electronic absorption spectrum of 1 in acetonitrile (MeCN) solution at 295 K is displayed in Figure 1b (blue curve). It consists of three bands peaking at 435 nm, 349 nm, and 288 nm, and upon expanding the spectrum vertically (dashed blue), a distinct shoulder at longer wavelengths is noted, which can be fitted very well with a Gaussian profile (pale blue) centered at 549 nm. Compound 1 has a d0-configured Ti-center and thus, transitions in the UV-to-visible (UV/Vis) region have nearly pure ligand-to-metal charge-transfer (LMCT) character.
According to time-dependent (TD) density functional theory (DFT, cf. Supporting Information, SI), the shoulder is due to the S0→S1 transition whereas the 435 nm-band originates from the S0 → S3 transition. Importantly, TD-DFT also confirms that the electronic structure of 1 is fully analogous to that of TiIV(Cp)2(Cl)2 (see SI). Finally, we also tested the catalytic activities of (−)-menthyl-substituted17 TiIV(Cp)2(L)2 with L=Cl and NCS (cf. Figure 2) in the atom-economical arylation of epoxide-derived radicals and found for irradiation with 453 nm-light that switching from chloride to pseudohalide even enhances the conversion efficiency slightly. Thus, complex 1 is a very sensible IR-model system.
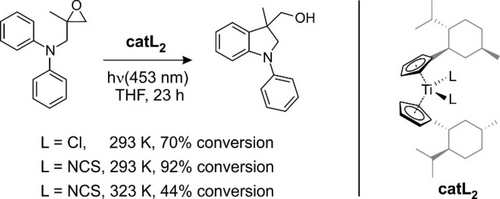
Efficiency of the radical arylation of epoxides coupled to photoredox catalysis based on TiIV(Cp)2(L)2 with L=Cl and NCS. The substrate, 1,2-epoxy-2-methyl-3-(diphenylamino)propane, also serves as the reductive quencher and the reaction mixture was irradiated with 453 nm light from a light emitting diode.
Our model was reported to be non-emissive in room temperature solutions but luminescence attributed to the triplet ground state, T1, could be collected from cryomatrices at 77 K.18 To understand this behavior, we initially examined the luminescence of 1 in MeCN at 287 K with time-correlated single-photon counting (TCSPC, see SI) and excitation at 450 nm. We were able to record emission traces at selected detection wavelengths,  , between 540 nm and 750 nm, from which the stationary emission spectrum was then reconstructed as shown in Figure 1b (open circles, see SI for data processing). Again, the data can be fitted very well with a Gaussian profile (shaded pale red) peaking at 653 nm, in accordance with the cryomatrix data.18 The Stokes-shift amounts to ≈2900 cm−1 and is thus much smaller than previously thought.18 A TCSPC-trace recorded at 650 nm is shown in Figure 3a (red dots only, label 0) together with a least squares fit to a biexponential decay. Time constants of (157±20) ps and (10.1±0.1) ns with relative amplitudes of 0.956±0.005 and 0.044±0.005 were found for the fast and slow components, respectively. We attribute the biexponential kinetics to thermally activated delayed fluorescence (TADF) as sketched in the Jablonski diagram in Figure 3b.
, between 540 nm and 750 nm, from which the stationary emission spectrum was then reconstructed as shown in Figure 1b (open circles, see SI for data processing). Again, the data can be fitted very well with a Gaussian profile (shaded pale red) peaking at 653 nm, in accordance with the cryomatrix data.18 The Stokes-shift amounts to ≈2900 cm−1 and is thus much smaller than previously thought.18 A TCSPC-trace recorded at 650 nm is shown in Figure 3a (red dots only, label 0) together with a least squares fit to a biexponential decay. Time constants of (157±20) ps and (10.1±0.1) ns with relative amplitudes of 0.956±0.005 and 0.044±0.005 were found for the fast and slow components, respectively. We attribute the biexponential kinetics to thermally activated delayed fluorescence (TADF) as sketched in the Jablonski diagram in Figure 3b.
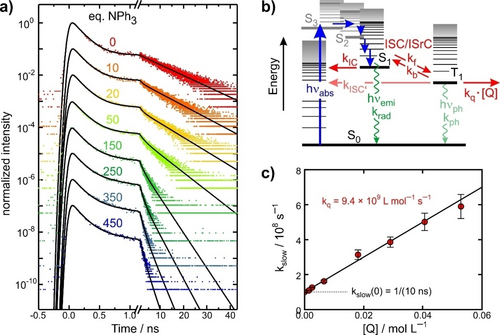
a) TCSPC fluorescence decay traces (dots) of 1 in liquid MeCN solution at 287 K and in the presence of various equivalents of NPh3 (excitation wavelength 450 nm, data are shifted vertically for clarity). Solid curves represent fits to biexponential kinetics. The topmost data were obtained without quencher. b) Jablonski-diagram for data interpretation. Without quencher, the S1 and T1-states equilibrate by ISC and ISrC, characterized kinetically by rate constants, kf and kb. Both states can radiatively decay to the ground state via fluorescence (with rate krad) and phosphorescence (kph), respectively, or non-radiatively through IC (kIC) and T1→S0 ISC (kISC), respectively. With quencher, Q=NPh3, at concentration, [Q], T1 can also decay into a TiIII-product via a bimolecular SET described by rate, kq. The associated integrated kinetic equations for the three states, S0, S1, and T1, as well as for the ET-product are given in the SI. c) Kinetic Stern–Volmer plot using the inverse time constant of the slowly decaying component of the data from panel a).
The 450 nm-excitation accesses S3, which relaxes within the time resolution to the S1-state. Emitted photons are then collected from S1 and the prompt sub-nanosecond decay of the TCSPC trace results primarily from its depopulation due to intersystem crossing (ISC) to T1 and internal conversion (IC) to the ground state, S0. The slow decay on the 10 ns-time scale is caused mostly by the thermally-induced (uphill) intersystem re-crossing (ISrC) from T1 to S1. As shown in the SI, the time constants of the fast and slow decays together with their relative amplitudes can be used to determine the rate constants for ISC and ISrC, kf and kb, respectively, as well the IC-rate, kIC. We find 1/kf=(223±40) ps, 1/kb=(2.64±0.26) ns, and 1/kIC=(593±20) ps, which allows us to extract a value for the triplet-singlet gap according to ΔES1→T1=E(T1)−E(S1)=−RT⋅ln(kf/3kb)=−(3.3±0.2) kJ/mol.
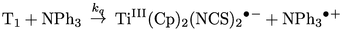 ()
()where the rate constant, kq, quantifies the bimolecular kinetics. A full kinetic analysis of all processes sketched in Figure 3b including the quenching reaction is given in the SI. A Stern–Volmer plot of the slow luminescence decay rate (cf. Figure 3b) yields a value for kq of (9.4±0.2) ⋅ 109 M−1 s−1, which is only about a factor of two smaller than an estimate of the diffusion-limited on-contact ET-rate (cf. SI).
However, the quenching reaction (1) still needs to be verified by directly monitoring the T1- population and the putative radical anion product, TiIII(Cp)2(NCS)2⋅− (2), must be identified. To this end, we carried out next UV/MIR-spectroscopy with 450 nm-pump pulses. Representative results are shown in Figure 4a and the full spectro-temporal evolution from 200 fs all the way up the 300 μs is presented in the SI as an animation.
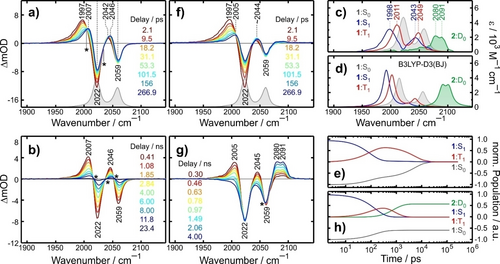
a) and b) 450 nm-pump/MIR-probe spectra 1 in liquid MeCN at 295 K for various representative time delays. Asterisks mark Isosbestic points. The gray spectrum at the bottom of panel a) represents the stationary FTIR-spectrum of 1. c) Constituent MIR-spectra of the S1 (blue curve), T1 (red curve), and S0-states (gray) from the global target analysis. The green-shaded spectrum is the MIR-spectrum of 2 in its doublet ground state. d) DFT-predicted IR-spectra for the same four states (cf. SI for details). e) Population time-traces in the absence of a quencher. f) and g) same as for panels a) and b) except that data were collected in the presence of 10 eq. of NPh3. h) same as for panel e) but in the presence of NPh3. Note that the lifetime of 2 is ≈1 μs due to back ET (not included here, cf. SI).
At early delays (Figure 4a), two negative bands are detected that spectrally coincide with the FTIR-resonances (gray). These features (aka ground-state bleach, GSB) result from the pump-induced depletion of S0. Two positive bands (termed induced absorption, IA) initially peaking at 1997 cm−1 and 2042 cm−1 are also observed, which are fingerprints of an electronically excited state. As the time delay is increased to about 300 ps, the 1997 cm−1-band gradually decays while a new IA-band at 2007 cm−1 appears. This evolution partially suppresses the GSB at 2022 cm−1 but leaves the GSB at 2059 cm−1 unaffected. Furthermore, two isosbestic points (cf. asterisks) at 2004 cm−1 and 2034 cm−1 are formed, which indicates that a simple kinetic two-state interconversion occurs within the first 300 ps after excitation.
From ca. 0.5 ns onwards (cf. Figure 4b and SI), the UV/MIR-spectrum vanishes completely while retaining its overall shape. During this period, three new isosbestic wavenumbers emerge; namely, 2015 cm−1, 2036 cm−1, and 2050 cm−1. These findings are indicative of yet another two-state interconversion, however, this time involving the return to S0 as evidenced by a simultaneous recovery of the two GSB-bands.
To test for compliance of the UV/MIR-data with the coupled rate equations derived from the Jablonski diagram (Figure 3b) a global target analysis was carried out.19 As shown in the SI, the global fit involving only the S0, S1, and T1-states is virtually indistinguishable from the data thereby lending ultimate credence to our kinetic model. The target fit yields the MIR-spectra of 1 in the S1 and T1-states (cf. Figure 4c). Qualitatively, these are very similar to the stationary FTIR-spectrum except for a distinct frequency-downshift, which can be rationalized by a diminished electron density on the ligands upon LMCT and a concomitant bond softening in the NCS-moieties. Importantly, the successive downshift in the order S0-T1-S1 is reproduced by TD-DFT ( cf. Figure 4d).
The global target analysis yields also the rate constants, kf=1/[(138±10) ps], kb=1/[(1.67±0.05) ns], and kIC=1/[(284±20) ps] and hence, the time-dependent occupations of the three states (cf. Figure 4e). Just like the TCSPC-traces, the S1-population decays bi-exponentially thus, fully corroborating our assignment of the luminescence to TADF. The forward and backward rates yield ΔES1→T1=−(3.6±0.2) kJ/mol, fully consistent with the gap obtained from TCSPC. However, the rates are larger than those obtained from time-resolved fluorescence, which can be attributed to the higher temperature (295 K vs 287 K) used to collect the UV/MIR-data.
The target analysis renders the pump-probe study directly sensitive to the occupation of T1; quite in contrast to TCSPC. In MeCN at 295 K, the T1-population builds up with a time constant of ≈90 ps and subsequently decays with a lifetime of 5.3 ns (note that the T1-lifetime is a complex function of kf, kb, and kIC; cf. SI). This is the time window, which can be exploited for reductive quenching with an amine. Having confirmed full consistency of the UV/MIR-data with the TCSPC-results and the Jablonski diagram of Figure 3b, we move on to identify the TiIII-product of the SET quenching reaction. To this end, the UV/MIR-experiment was repeated in the presence of 10 equivalents of NPh3 and representative results are depicted in Figure 4f, g (cf. SI for an animation).
During the first 300 ps, the MIR-response is very similar to that of pure 1, which demonstrates that the quencher does not perturb the initial S1-T1 equilibration significantly. However, on longer time scales, the amine profoundly affects the UV/MIR-response. Whereas in the absence of the quencher, all GSB and IA bands decay concertedly to zero within 10 ns as a result of the non-radiative return to S0, this is no longer the case when NPh3 is present. Although the IA-bands at 2005 cm−1 and 2045 cm−1 still disappear, a long-lasting GSB is now noticed. This proves that the T1-state decays without replenishing S0 via S1. Instead, a new distinctly split IA-band peaking at 2080 cm−1 and 2091 cm−1 gradually appears within a few nanoseconds. Clearly, this feature must be attributed to the product of the quenching reaction, presumably the radical anion, TiIII(Cp)2(NCS)2⋅− (2). A target analysis was carried out again, but now taking into account the bimolecular quenching reaction with the rate, kq⋅[Q]. The global fit is once again superb (cf. SI) and provides us with the MIR-spectrum of the product (cf. Figure 4c, green) as well as the populations of all species under quenching conditions (cf. Figure 4h). On much longer time scales (≈100 μs, data shown in the SI), the split product absorption finally vanishes while simultaneously, the two GSB-bands fully recover, thus, demonstrating that in the absence of an organic substrate (e.g. an epoxide), the putative radical anion decays by back-ET to replenish the parent's ground state.
To test our tentative assignment we computed the IR-spectrum of 2 as shown in Figure 4d (green). DFT predicts that the radical anion's absorption is indeed frequency-upshifted relative to that of the neutral and that it is characteristically split into two bands of equal intensity, again originating from the in-phase and out-of-phase combinations of the two local NCS-modes. The superb agreement between experiment and theory constitutes the final and unambiguous evidence that the product of the quenching reaction with NPh3 is indeed TiIII(Cp)2(NCS)2⋅−.
Unfortunately, TD-DFT falls short in predicting the triplet-singlet gap. Depending upon the exchange-correlation functional, values for ΔES1→T1 between −14 kJ/mol and −22 kJ/mol were obtained; i.e. roughly a factor of four larger than the experimental value, and sadly, even using coupled-cluster theory including spin-orbit coupling does not lead to a significant improvement (see SI).
At any rate, our results clearly emphasize the importance of a long T1-lifetime for an efficient entry into the redox catalytic cycle utilizing titanocene di(pseudo)halides. The lifetime in turn depends critically on the magnitude of ΔES1→T1 in relation to the temperature (see SI). Indeed, for a given ΔES1→T1, a decrease of temperature should lower the ISrC rate, kb, by virtue of detailed balance. According to the TCSPC-data, the T1-lifetime in MeCN is 3.2 ns at room temperature whereas it shortens to 2.8 ns by raising T by only 30 K. Yet, this small perturbation impedes the catalytic efficiency measurably as evidenced by a smaller overall chemical conversion for a given reaction time (see Figure 2). Working as close as possible to the solvent's freezing point might appear favorable; however, the consecutive steps within the redox cycle (e.g. substrate binding and H-atom transfer) may then become too slow. Electronic structure theory is required here to develop general ligand design principles for (i) optimizing the T1-S1 gap and (ii) enhancing the extinction coefficient for Vis-absorption without (iii) sacrificing the near-diffusion-limited character of the reductive ET quenching reaction ultimately leading to the TiIII intermediate. Unfortunately, sufficiently accurate predictions of the spin-state energetics of transition-metal complexes remains still a formidable challenge for DFT and TD-DFT.
Acknowledgments
We gratefully acknowledge financial support by the German Research Foundation through grant numbers VO 593/8-1, GA 619/13-1 and the Priority Program 2102 “Light-induced reactivity of metal complex”, grant number VO 593/7-1. M.L. thanks the Jürgen Manchot Stiftung for a fellowship. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.