Traffic Lights for Catalysis: Stimuli-Responsive Molecular and Extended Catalytic Systems
Graphical Abstract
Just as flowers blooming in response to the sun attract bees to continue their natural life cycle, molecules which respond to external stimuli can be used to control catalytic cycles. Development of stimuli-responsive molecular and extended catalytic platforms is a pathway for precise tuning of reaction yield, rate, or enantioselectivity that could pave the way for the next era of sustainable chemistry.
Abstract
The advances made in the field of stimuli-responsive catalysis during the last five years with a focus on the novel recently-emerged directions and applications have been surveyed. Metal-free catalysts and organometallic complexes, as well as biomimetic systems and extended structures, which display switchable catalytic activity for a variety of organic transformations, are discussed. Light-activated systems comprised of photochromic molecules capable of modulating reaction rate, yield, or enantioselectivity based on geometric and electronic changes associated with photoisomerization are the focus of the detailed discussion. Alternative stimuli, including pH and temperature, which could be applied either alone or in combination with light, are also addressed. Recent advances clearly demonstrate that the capability to finely tune catalyst behavior via an external stimulus is a powerful tool that could alter the landscape of sustainable chemistry.
1 Introduction
Stimuli-responsive or “switchable” catalysis represents a rapidly expanding field due to its potential to alter the state-of-the-art of organic synthesis on both the laboratory and industrial scales.1-10 For example, the ability to alter reaction outcomes, including yield, selectivity, or enantiomeric excess, as well as the ability to switch between two distinct classes of organic transformations with a single catalyst will undoubtedly play a role in the next era of sustainable chemistry.1-38 Indeed, precise control of reaction progress, intermediates, and product distribution through an accessible external stimulus offers an opportunity to minimize the energy and resources spent on unproductive reaction pathways resulting in undesirable by-products. In this review, we survey switchable catalysts as ones whose behavior is altered by an applied external stimulus, resulting in alternation of the observable outcomes of a given reaction. In this direction, switchable catalysis offers the ability to alter catalyst behavior after it has been introduced to the reaction conditions, which could be an avenue to instantaneous control of ongoing reactions (e.g., reaction intermediates). At the same time, synthesis of novel, often time-, labor-, and cost-intensive catalysts may not be necessary if the catalyst properties could be reversibly and externally modulated in situ.
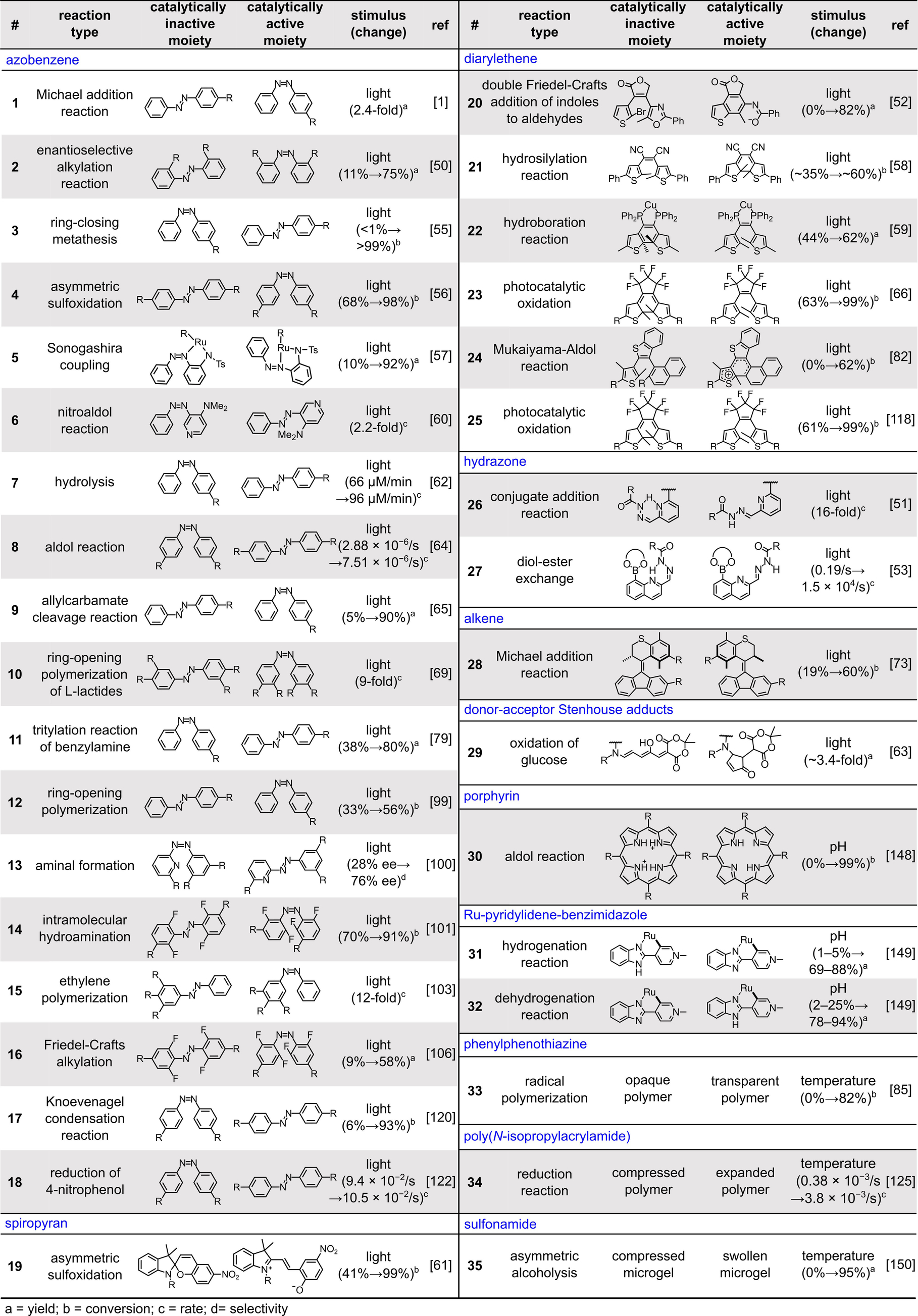
Early studies mostly focused on development of catalysts which could be selectively activated or deactivated to start or stop organic reactions.39-42 Recent progress in this area has not been limited to the development of catalysts which can be turned on or off by an external stimulus, but rather has expanded to allow for tunable formation of reaction intermediates. Whereas “on/off” switchable catalysis is a pathway for immediate control of reaction yield, possible modulation of reaction intermediates and mechanistic pathways allows for tuning of product selectivity. A combination of approaches using various stimuli has resulted in switchable catalysts capable of controlling many critical organic transformations, including cross-coupling, asymmetric sulfoxidation, transesterification, conjugate addition, and aldol addition reactions, among many others.8, 39, 43-66 Given the broad range of reactions studied in the field of switchable catalysis, enormous growth of this field is expected in the near future engaging multidisciplinary research teams from the organic, inorganic, and polymer chemistry communities, as well as in materials science and engineering.
In the present review, we consider the directions and applications that have emerged in the field of switchable catalysis during the last five years. Within this timeframe, there are a great number of studies which have been conducted in this area, and among those, approximately 80 % utilize light as the external stimulus. For this reason, we will begin our discussion with a focus on photoswitchable catalysts, considering examples of metal-free and organometallic systems, as well as extended structures and biomimetics. Herein, we have specifically focused on photoswitchable catalysis as a conceptually different field compared to photocatalysis. In the realm of photoswitchable catalysis, light is employed to modulate the properties of catalytically active species, e.g., their geometric or electronic parameters while in photocatalysis, light is utilized to initiate a catalytic cycle. Overall, light as an external stimulus offers high spatiotemporal resolution, which allows for immediate and localized control of reaction progress. In addition, an excitation wavelength can be tuned as a function of a considered metal or ligand while maintaining relative ease of use of the corresponding experimental setup. Moreover, choice of light as a stimulus is advantageous since it does not require exogenous reagents to be introduced to the reaction conditions, but at the same time, light alone can have critical limitations (e.g., limited penetration depth). Thus, following the discussion of light-activated catalysts, we consider alternative stimuli, pH or temperature. In each of the mentioned cases, we discuss possible next steps based on the authors’ views when suitable.
To demonstrate the utility of stimuli-responsive catalysis, we bring to the discussion several distinct platforms such as molecular metal-free and organometallic catalysts, as well as extended catalytic systems. Focusing on advances occurring during the last five years showcases the exceptional speed at which the field of switchable catalysis is evolving. We highlight successful research avenues but also outline the challenges that require attention from multidisciplinary research teams. For instance, the systems activated by multiple orthogonal stimuli for synthesis of a variety of finely-tuned products by a single catalyst have not been achieved yet. Despite challenges and obstacles, the current progress in the field of switchable catalysis demonstrates enormous potential for the development of desirable sustainable pathways for chemical synthesis.
2 Photoswitchable Catalysis
2.1 Organocatalysis
2.1.1 Changes of catalyst geometric parameters as a function of light
Some of the first approaches for the regulation of catalytic performance were realized through functionalization of molecular organocatalysts with photoresponsive moieties (Schemes 1 and 2) which allowed for tailoring variables such as reaction selectivity, yield, or rate. One of the mechanisms for such modulation of catalytic performance relies on alteration of either geometric parameters or physicochemical properties of the photoswitch-catalyst pair. For instance, photoswitches for which isomerization is accompanied with large structural transformations (e.g., azobenzene, hydrazone, or rotaxane derivatives), have been utilized to selectively block or expose catalytically active sites using an excitation wavelength as a “knob” for catalytic activity regulation.1, 50, 51, 67-69 This particular approach relies on rational design of a photoresponsive unit which undergoes, for instance, E-to-Z isomerization (e.g., azobenzene or hydrazone derivatives).1, 50, 51, 69 In this case, one photoisomer blocks the catalytically active site (Scheme 1) while the other provides open access for the substrate to reach this site. Thus, the photoswitchable moiety behaves similar to a gate, allowing or preventing catalyst-substrate interactions. Many of these studies employ azobenzene-based catalysts since the cis-to-trans isomerization could modulate catalytic processes by regulating the spatial separation of substrates and catalysts. For example, incorporation of azobenzene within crown ethers has been shown to yield photoswitchable phase transfer catalysts (PTCs).50
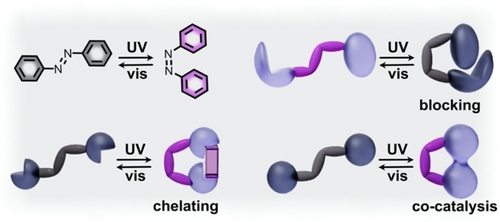
Possible mechanisms for control of catalytic pathways shown on the example of azobenzene photoisomerization which include ion binding, active site blocking, or modulation of the spatial arrangement of two active sites to promote co-catalysis. Gray and purple colors represent two distinct photostates.
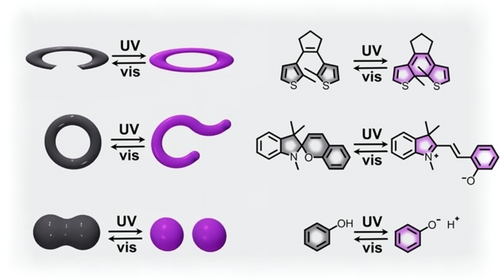
Schematic representation of diarylethene derivatives (top), spiropyran-based compounds (middle), and photoacids (bottom) used for modulation of catalytic activity. Gray and purple colors represent two distinct photostates.
Overall, crown ether derivatives have been used as PTCs in many organic reactions because of their ability to solubilize ionic species in organic solvents through coordination of the ions within the crown ether structure.70-72 For example, chelation of the cationic species of a salt by crown ethers stabilizes the cation and enhances the solubility of the salt. Consequently, the anionic species can exhibit increased basicity or nucleophilicity since it is no longer stabilized by the counterion. As a result, reaction yield can be altered by addition of PTCs.69 For instance, azobenzene-functionalized binaphthyl crown ether (ABCE) was utilized to promote alkylation of glycine Schiff bases by the reaction with potassium hydroxide in toluene (Figure 1 and Table 1, #2).50 Irradiation of the ABCE solution by 365 nm caused photoisomerization of trans-azobenzene to cis-azobenzene, which effectively opened the aperture of the crown ether as shown in Figure 1. In the “open aperture” state, the crown-ether-based PTC was able to facilitate the base-catalyzed alkylation of glycine, resulting in high yield because potassium cations were efficiently solubilized through their coordination within the aperture of the crown ether. In contrast, switching from cis- to trans-azobenzene reduced the aperture size of the crown ether, resulting in a drastic decrease of reaction yield.50 In addition, cis-to-trans photoisomerization resulted in a significant decrease in enantioselectivity, indicating that the reversible change in crown ether geometry could be used to control enantiomeric ratios of the products. Although more detailed studies are necessary to uncover mechanistic details, especially those pertaining to tunable enantioselectivity, this work clearly demonstrates that integration of the azobenzene-based photoswitches offers noninvasive control of both reaction yield and the enantiomeric ratio of the product mixture.50
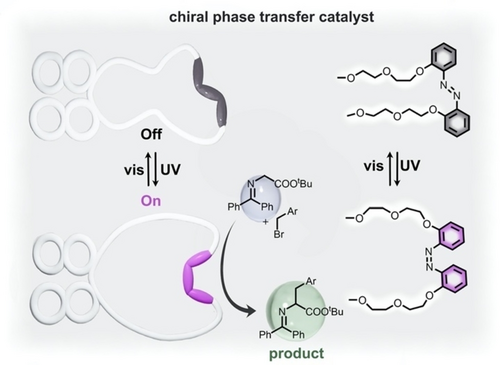
(left) Schematic representation of an azobenzene-based crown ether chiral PTC in the inactive (gray) and active (purple) forms. Alkylation reactions are carried out by the cis-azobenzene-containing catalyst. (right) Structures of azobenzene-based crown ether before (gray) and after (purple) UV irradiation. Adapted with permission.50 Copyright 2021 American Chemical Society.
A conceptually different approach to switchable catalysis based on coupling of azobenzene photoswitches with crown ethers has been demonstrated using two 15-crown-5 ether moieties connected by a central azobenzene unit (Table 1, #10).69 In contrast to the previously described study, which utilized azobenzene photoisomerization to change the size of the crown ether aperture, an alternative approach relied on azobenzene trans-to-cis transformations to modulate the distance between the two crown ether groups.69 In this case, the cis-azobenzene isomer brought the two crown ether groups in proximity to promote strong binding of cations by both crown ethers simultaneously. This approach was employed to probe base-catalyzed ring-opening polymerization of l-lactides in which the acetate anions were activated by binding of the potassium cations within the cis-azobenzene-containing crown ether catalysts. The authors suggest that electrostatic interactions between the potassium and acetate ions when the potassium cation was not chelated by the crown ether (i.e., in the presence of cis-azobenzene) could cause poor chain end activation. As a result, irradiation with 320-nm light, leading to photoisomerization from trans- to cis-azobenzene, coincided with a nine-fold increase of l-lactide polymerization rate.69
Azobenzene-based photoswitches have also been employed for the design of biphasic organocatalysts which promote solution-phase reactions and can be precipitated from solution upon photoisomerization to facilitate separation of catalysts from the product mixture.1 For instance, Han and co-workers developed a series of azobenzene-based organocatalysts bearing catalytically active tertiary amine groups along with amide groups to facilitate hydrogen bonding interactions. Notably, the E-isomers of these catalysts exhibited planar structures with significant π-π stacking interactions, while photoisomerization to the Z-isomers caused a loss of crystalline packing resulting in a phase transition. That is, E-to-Z photoisomerization coincided with a phase transition from crystalline solids to liquids. As a result, this concept was applied for facile recycling of homogeneous catalysts. For example, a reaction mixture consisting of the soluble Z-enriched catalyst (formed upon irradiation with a 340-nm wavelength) led to higher reaction rates than those of the insoluble E-enriched catalyst by an order of magnitude for Michael addition reactions. Moreover, a 2.4-fold increase in product yield was detected for the Z-isomer. After reaction completion, 87 % of the catalyst could be recovered by irradiation at 430 nm to induce Z-to-E photoisomerization. Upon photoisomerization, the catalyst precipitated from the product mixture and could be recovered using filtration and re-used in at least three subsequent cycles.1 Thus, integration of azobenzene photoswitches in organocatalysts is an avenue not only for controlling reaction rates and yields but also achieving homogeneous catalyst recycling.
Similar to azobenzene, hydrazone derivatives, which undergo E-to-Z isomerization around a central imine bond upon exposure to UV irradiation, also exhibit significant structural changes upon isomerization, and therefore, can be utilized to block or expose catalytically active sites.51 For example, bifunctional cinchona alkaloid-squaramide catalysts functionalized with hydrazone groups were used to control the enantiomeric excess of several conjugate addition reactions through irradiation with light (Figure 2). As shown in Figure 2, irradiation with a 395-nm excitation wavelength resulted in formation of the Z-catalyst, which was deactivated by intramolecular hydrogen bonding. In this case, the intramolecular hydrogen bonding caused the hydrazone group to block the active site and prevent binding of the substrate to the catalyst. In contrast, the formation of the E-catalyst exposed the active site to promote substrate binding and activation toward nucleophilic attack. Remarkably, the reaction rates for these catalysts in the “on” state (E-hydrazone) were 16 times higher than those of the “off” state (Z-hydrazone).51 This example illustrates that coupling of hydrazone derivatives with organocatalysts could be used for tuning the reaction rate through selective blocking of the active sites, i.e., preventing hydrogen bonding between the substrate and catalyst.51
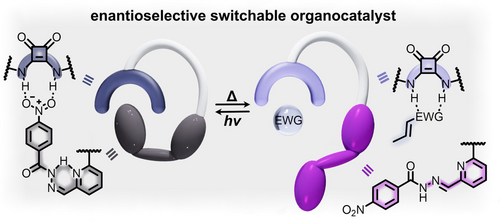
Schematic representation of a bifunctional squaramide catalyst containing a hydrazone-based photoswitchable moiety. Photoisomerization from the Z- to E-isomers (gray and purple, respectively) prevents hydrogen bonding interactions between the hydrazone functionality and the squaramide active site, allowing for activation of alkenes substituted with EWG toward Michael addition reactions. Adapted with permission under the Creative Commons Attribution 3.0 Unported License.51
Although the majority of studies in the area of stimuli-responsive photocatalysts utilize azobenzene or hydrazone derivatives as described above,1, 50, 51, 69 other classes of relatively less explored photoswitches offer other avenues for switchable catalysis.52, 73-75 For example, some overcrowded alkenes (i.e., tetrasubstituted alkenes with bulky substituents such as 9-(6-bromo-3,5,8-trimethylthiochroman-4-ylidene)-N,N-dimethyl-9H-fluoren-2-amine) exhibit photoswitchable behavior similar to E-to-Z isomerism.73 A photoresponsive tetrasubstituted alkene core has been used to build molecular motors for controlling the catalytic activity in the Michael reaction between (E)-3-bromo-β-nitrostyrene and 2,4-pentanedione.73 In the described system, formation of the E-photoisomer resulted in 60 % conversion after 18 h, whereas the Z-isomer resulted in only 19 % conversion under the same conditions, showing the potential for crowded alkenes to behave as “on/off” switchable catalysts. The other less explored stimuli-responsive molecules which could be utilized to block and expose active sites, include rotaxane and helicene derivatives.67, 76 They provide unique opportunities to expand the library of switchable catalysts because their conformational and configurational changes induced by irradiation can provide the control of chiral space.73 For instance, the position of rotaxane macrocycles around a catalytically active core could be used to regulate the facial binding of substrates as a function of an excitation wavelength.67 In a similar direction, helicene derivatives functionalized with catalytically active 4-N-methylaminopyridine groups have been shown to facilitate asymmetric Steglich rearrangements of o-carboxylazlactones.76
2.1.2 Changes in electronic properties of catalysts as a function of light
In contrast to the above discussion focusing on catalyst geometrical changes, a conceptually different approach to photoswitchable organocatalysis could be achieved through modulation of the catalyst electronic structure.52, 54, 74, 75, 77 In this direction, diarylethene derivatives (Scheme 2) have played a significant role.52, 74, 78 Their photoisomerization is based on reversible bond formation leading to alternation between conjugated and non-conjugated forms.52, 74, 78 For instance, photocyclization of diarylethene derivatives is accompanied by changes in π-conjugation of the photoswitch backbone and, in some cases, release of substituent groups.52 Indeed, Shirinian and co-workers have shown the ability of a series of halogenated diarylethene derivatives to release bromine cations upon photoisomerization (Figure 3 and Table 1, #20). Such photolysis could be used to carry out bromination reactions in good yields as well as to catalyze the double Friedel–Crafts addition of indoles to aldehydes.52 In the latter case, catalysis of the Friedel–Crafts reaction occurred through the Lewis acidic bromine cation which could only be formed through photoisomerization of the halogenated diarylethene-based photoswitch. As a result, the catalytic reaction could be initiated using UV irradiation.52
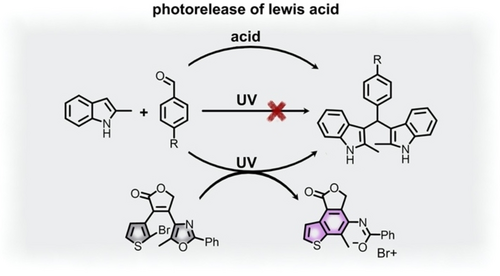
The double Friedel-Crafts addition of indoles to aldehydes (R=H or −OCH3) catalyzed by the Lewis acidic bromine cation released by diarylethene photoisomerization. Upon UV irradiation, the open form of the brominated diarylethene (gray photoisomer) undergoes skeletal rearrangement to release the bromine cation (purple photoisomer). Adapted with permission.52 Copyright 2021 American Chemical Society.
A different approach, based on selective changes in charge delocalization involving diarylethene derivatives, takes advantage of light-induced changes in π-conjugation to alter the activity of a catalytic site.74, 77 According to computational analysis, diarylethene derivatives have the potential to modulate the behavior of frustrated Lewis pair (FLP) catalysts through charge delocalization.74 It was predicted, for instance, that functionalization of a diarylethene core with amine and boronic ester groups could result in generation of an FLP capable of catalyzing hydrogen recombination reactions.74 Without exposure to an appropriate excitation wavelength, the diarylethene core is “open,” so there is minimal electronic communication between the acid and base moieties of the FLP catalyst. In contrast, exposure to UV irradiation causes photoisomerization to the colored (i.e., fully conjugated) isomer, which allows for electronic delocalization throughout the catalyst. This delocalization promotes a resonance form in which a negative charge is formally generated at the Lewis base, and a positive charge is formally located at the Lewis acid. As a result, the theoretically predicted proton affinity of the amine group of the closed diarylethene-based catalyst is significantly higher than that of the open (colorless) form.74 Likewise, the theoretical hydride affinity of the Lewis acidic boron atom is increased for the closed isomer.74
Rather than changing the electronic structure of FLPs through the photocyclization reaction of diarylethene derivatives, another avenue is to use the photoswitchable molecules which can undergo charge separation upon photoisomerization, leading to modulation of electron density, as well as proton or hydride affinity of an active site.75 For example, functionalization of borane-phosphine or borane-amine FLPs with the dihydroazulene-vinylheptafulvene photoswitch has allowed for light-induced changes in H2 activation abilities.75
Tailoring organocatalyst performance could also be accomplished via light-induced changes in its anion binding affinity.79 For instance, Venkataramani and co-workers probed tunable ion binding using tripodal 1,3,5-tris-functionalized benzenes bearing triazole-containing azobenzene photoswitches, which could undergo trans-to-cis photoisomerization under UV excitation.79 In particular, the binding affinity of triazole functionalities to chloride anions could be reduced due to formation of the cis-azobenzene form upon irradiation with a 365-nm excitation wavelength.79 In contrast, the chloride anion binding affinity was increased by photoisomerization of the catalyst to the trans-azobenzene isomer under 405-nm irradiation.79 In fact, based on isothermal titration calorimetry experiments performed on the trans- and cis-azobenzene forms of the catalyst, the dissociation constant associated with binding of chloride ions by the triazole functionalities was two times higher for the cis-azobenzene-based catalyst than the trans-azobenzene-based catalyst, which is in line with a reduced binding affinity for the cis-catalyst.79 These findings were applied toward probing tritylation reactions (using trityl chloride) in which the photoswitchable catalyst promoted reaction rates by strong binding of the chloride counterion upon exposure to a 405-nm excitation wavelength. As expected based on the determined dissociation constants, the isolated yields of tritylation reactions, carried out in the presence of the trans-catalyst, were approximately twice of those carried out in the presence of the cis-catalyst. As a result, the reaction yields could be reversibly controlled through photoswitch isomerization.79
As previously described, hydrazone derivatives could be used to modulate geometric parameters in a catalytic system, but they have also been used to tune the barrier for proton transfer steps through intramolecular hydrogen bonding in either the E- or Z-photoisomer.53 In some cases, intramolecular hydrogen bonding between the amine proton of the hydrazone core and a substituent acting as a hydrogen bond acceptor can be induced by photoisomerization, resulting in changes in pKa of the amine proton.80 Thus, hydrazone derivatives present an opportunity to modulate pH of a reaction medium without introducing additional acids or bases.81 Even slight changes in acidity or basicity can be used to promote product formation in pH-sensitive reactions (e.g., transesterification).53 On the example of the transesterification of boronate esters with diols, Kalow and co-workers utilized a hydrazone photoswitch as an internal base catalyst to facilitate the rate-determining proton transfer mechanistic step (Figure 4a).53 In these studies, E-to-Z isomerization of the hydrazone functionality resulted in breaking of intramolecular hydrogen bonding interactions between the hydrazone amide proton and proximal nitrogen atom. As a result, hydrogen bonding between the boronate ester proton and nitrogen was possible, which promoted deprotonation of the boronate ester and subsequent transesterification (Figure 4).
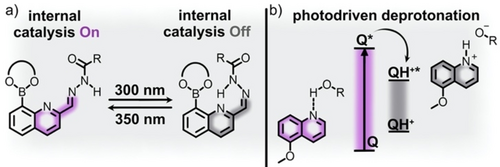
a) A hydrazone-containing boronate ester capable of internal base catalysis of transesterification reactions. The proximal nitrogen atom serves as the basic site to promote transesterification in the E-isomer (purple). Photoisomerization to the Z-isomer (gray) deactivates internal catalysis by formation of hydrogen bonding interactions at the proximal nitrogen atom. Adapted with permission.53 Copyright 2022 American Chemical Society. b) An example of a quinoline photobase (Q) promoting photolysis to form the catalytically active conjugate base from protic solvents. Adapted with permission.54 Copyright 2018 American Chemical Society.
2.1.3 Photoacids and photobases
While several well-studied classes of photochromic molecules have been successfully applied in photoswitchable catalysis as described above, a different direction involves the use of photoacids and photobases as means to achieve light-controlled organic transformations.54, 82 For example, these organic photoacids and bases exhibit significant changes in pKa depending on irradiation conditions. Unlike the previously described systems in which the photoswitch undergoes either a geometric or electronic change that perturbs the active catalyst, photoacids and photobases produce a catalytic amount of an acid or base after exposure to a particular excitation wavelength (Figure 4bb).54 For example, several studies concerning quinoline photobasicity have shown an increase in approximately 10 pKa units for quinoline derivatives exposed to UV light.54, 83 In particular, the excited states of the studied quinoline photobases are of sufficient energy to facilitate proton transfer from the reaction media (e.g., water or alcohols), which yields the conjugate base upon irradiation with an appropriate excitation wavelength (Figure 4b). The mechanism for generation of conjugate bases proceeds through abstraction of a proton from a hydrogen-bonded donor.54
While the majority of reported photoacids and bases are of the Brønsted type as described above, development of light-activated Lewis acids and bases is of particular interest due to their ability to initiate polymerizations of relatively unreactive monomers, as well as promote Diels-Alder and Friedel-Crafts reactions, among other synthetically useful transformations.82 For example, functionalization of a diarylethene-containing photoswitch with a triflate naphthalene moiety allowed for ejection of the triflate anion upon exposure to UV irradiation. As a result, a carbocation was formed at the photoactive core which was stabilized by the extended π-conjugation of the colored (i.e., UV-activated) diarylethene photoisomer. The stabilized carbocation was then evaluated in its ability to promote the bulk polymerization of epoxy monomers and the Mukaiyama-Aldol reaction between benzaldehyde and silyl enolate derivatives.82 Presence of an insoluble polymer containing end-terminal groups corresponding to the photoacid after UV excitation showed that the prepared catalyst was active toward the selected cationic polymerization reactions. As a control experiment, the described Mukaiyama-Aldol reactions were unsuccessful without UV irradiation, and addition of previously studied Brønsted photoacids could not initiate product formation.82 Thus, this approach demonstrates the successful preparation of a Lewis photoacid with switchable catalytic activity.82
Based on the mentioned studies above, it is clear that the light-induced alteration of geometric parameters of photoswitch-catalyst pairs has the potential to modulate reaction rates,1, 51, 69, 79 yield,1, 50, 52, 79 and enantiomeric ratios for chiral products.73 Rational design of photoswitchable catalysts in this area has not only resulted in “on/off” catalysts, but also in systems for which the possible outcomes are no longer binary.63, 84-86 That is, switchable catalysts could serve to promote different reaction mechanisms with regio- or enantioselectivity depending on the state of the photoswitch.73, 87, 88
Despite significant progress toward such systems, development of fully reversible switchable catalysts capable of promoting variable product formation remains a challenging task. For instance, instantaneous temporal control of organic transformations would allow for precise tuning of product formation; however, many of the reported photoswitchable organocatalysts with large geometric changes possess relatively slow photoisomerization kinetics or utilize a secondary stimulus for the reverse isomerization process (e.g., heat or acid).89-93 At the same time, photoswitchable organocatalysts relying on changes in the electronic properties of the photoswitch-catalyst pair have received well-deserved attention in recent years,94-96 but the development of a true “on/off” system, which fully suppresses reaction progress in the “off” state, has yet to be achieved. The latter issue is associated with the fact that most catalysts in the “off” state frequently possess some residual catalytic activity. This residual activity coupled with low photostationary states (PSS) for some systems (i.e., a percentage of the catalyst remains as one photoisomer despite treatment with an appropriate excitation wavelength) minimizes the change in reaction outcome upon photoisomerization.51 Addressing the mentioned challenges associated with switchable organocatalysis relies on performing deep fundamental photophysical and mechanistic studies. Thus, the combined efforts of the synthetic organic and photophysical chemistry communities are necessary to advance the development of new photoswitch-catalyst pairs with high PSS ratios and near-complete reversal of catalytic activity upon isomerization of the stimuli-responsive molecules.
2.2 Organometallic Catalysis
A fundamentally different approach toward achieving switchable catalysis is coupling of organic photoswitches with catalytically active organometallic complexes.55-60, 97-106 Similar to the purely-organic systems described above, photochromic organometallic complexes can take advantage of significant changes in geometric parameters or physicochemical properties of the complex upon isomerization of a photochromic ligand. Moreover, access to d-orbitals from transition metals expands the scope of geometries available for photoswitch integration in comparison with many organic systems. The presence of metals also provides an additional pathway that allows for the investigation of oxidation state-dependent reaction mechanisms.107, 108 For instance, ligand-to-metal or metal-to-ligand charger transfer (LMCT or MLCT, respectively), as well as photoinduced electron transfer (PET) could be used to alternate the metal center between catalytically active and inactive states.78, 109-111 Integration of photoswitchable ligands in well-known transition metal-based catalysts could be an avenue toward stimuli-controlled catalysis in several synthetically vital transformations (e.g., Suzuki or Sonogashira coupling reactions).57 In this section, photoswitch induced changes in catalytic activity through either geometric rearrangement (i.e., blocking active sites or modulation of the distance between substrates) or through alteration of the electronic structure around the metal center will be the main target of the detailed discussion.
2.2.1 Changes in geometric parameters of catalysts as a function of light
One area in which modulation of an organometallic complex's coordination geometry has been successfully used to alter catalytic activity is the noninvasive control of the products of metathesis and polymerization reactions.99 Changes in the catalyst's coordination geometry are reported to affect not only the reaction rate, but also the stereo- and regioselectivity, as well as polymer tacticity, directly affecting bulk material properties.99 For instance, a 1st generation Grubbs catalyst modified with an azobenzene-containing cyclic(alkyl)(amino)carbene ligand for olefin metathesis exhibited switchable catalytic activity based on azobenzene blocking or exposing the active site (Figure 5 and Table 1, #3).55 In particular, the formation of trans-azobenzene exposed the active site and promoted the ring-closing metathesis reaction with >99 % conversion of diethyl diallyl malonate when exposed to visible light (Figure 5).55 In contrast, irradiation with UV light caused photoisomerization to the cis-azobenzene ligand which blocked the active site, and as a result, less than 1 % conversion was detected when the reaction was performed under UV excitation. Remarkably, this system represents near complete control of catalytic activity because the “off” form of the catalyst (e.g., cis-azobenzene) possesses almost no activity toward product formation.55 Control experiments involving the ruthenium-based catalyst without the azobenzene functionality confirmed that the catalytic activity was not altered by the irradiation conditions alone, but rather could be attributed to photoisomerization of the ligand. In order to determine whether the observed changes in catalytic activity originated from the steric hindrance imposed by the cis-azobenzene photoswitch or changes in the electronic properties of the carbene moiety, a model rhodium carbonyl complex bearing the same azobenzene-carbene ligand was probed by Fourier transform infrared (FTIR) spectroscopy. Based on the FTIR stretching frequencies for the carbonyl groups, the authors calculated the Tolman electronic parameter (TEP) for the complex before and after UV irradiation. Since the TEP value was unaffected by azobenzene photoisomerization, it was concluded that changes in steric bulk at the metal center must alter the catalytic activity.55 This example provokes further investigation in the area of photochromic organometallic compounds since it clearly demonstrates the precise temporal control over reaction progress that could be achieved through rationally designed photochromic organometallic complexes.
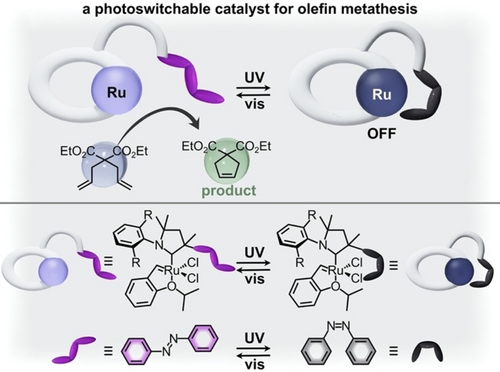
(top) Schematic representation of an azobenzene-containing ruthenium complex with the trans-isomer (purple) being the catalytically active form and the cis-isomer (gray) being catalytically inactive in the olefin metathesis reaction (substrate shown in light blue and product shown in light green). (bottom) Structures of the ruthenium complex and azobenzene photoswitch in the active (purple) and inactive (gray) forms. Adapted with permission.55 Copyright 2021 American Chemical Society.
Not only could photoswitchable ligands be utilized to block or expose active sites, but they could also be used to control the distance between two metal centers involved in cooperative catalysis.56 In Figure 6, two titanium(salen) complexes bridged by an azobenzene group could facilitate asymmetric sulfoxidation reactions only when the TiIV centers were in close proximity to one another.56 In particular, the true catalytically active species was a μ-oxo-bridged [{(salen)Ti(μ-O)}2], which could only be achieved after the trans-to-cis photoisomerization of the azobenzene ligand took place. Thus, irradiation with UV light to form cis-azobenzene promoted asymmetric sulfoxidation of a variety of prochiral sulfide substrates through the control of the spatial arrangement of two TiIV catalysts. In contrast, photoisomerization to trans-azobenzene, leading to a larger distance between the catalytic centers, decreased catalytic activity. For instance, up to 98 % conversion with 93 % chemoselectivity and >99 % enantiomeric excess was detected under UV irradiation, while only 68 % conversion, 86 % chemoselectivity, and 78 % enantiomeric excess were obtained for the same reaction conducted in the dark.56
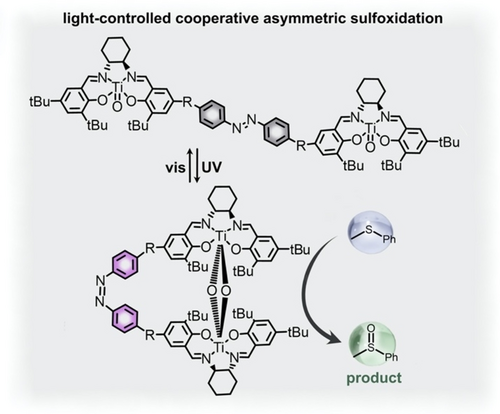
(top) A binuclear titanium(IV) salen complex possessing an azobenzene bridge (highlighted in gray). (bottom) Trans-to-cis isomerization of the azobenzene photoswitch transforms the inactive complex (gray) into the active μ-oxo-bridged [{(salen)Ti(μ-O)}2] complex (purple) catalyzing asymmetric sulfoxidation reactions (substrate shown in light blue and product shown in green). Adapted with permission.56 Copyright 2020 Royal Society of Chemistry.
Using a similar strategy, Marinetti and co-workers have synthesized a binuclear gold(I) catalyst, Au2Cl2L (L=1,2-bis(3-(diphenylphosphino)-2,6-difluorophenyl)diazene), possessing a photochromic azobenzene functionality.101 After structural confirmation of both the cis- and trans-azobenzene-containing complexes by single-crystal X-ray diffraction, their catalytic activity was evaluated on the example of intramolecular hydroamination of N-alkenyl urea.101 The spectroscopic analysis revealed an increased reaction rate for the cis-azobenzene-containing complex as compared to the trans-azobenzene-containing catalyst. For instance, 91 % conversion was detected after 44 minutes for the cis-complex, while only 70 % conversion was detected for the trans-complex under the same reaction conditions. Further, the kinetic studies for the trans-azobenzene catalyst were in line with control experiments carried out with an analogous mononuclear complex. Thus, these two examples illustrate that irradiation with UV light, resulting in formation of cis-azobenzene, enhanced catalytic activity through cooperation between the two metal centers, whereas the presence of trans-azobenzene minimized cooperativity by increasing the distance between the active sites.
Implementation of azobenzene for controlling the spatial arrangement of two metal centers has also been successfully applied in anion abstraction catalysis.106 An azobenzene core functionalized with tellurium-containing arms was used as a basis for the catalyst development.106 While the electrophilic tellurium centers exhibited strong binding of halide counterions, binding strength was modulated by photoisomerization of the azobenzene core.106 In particular, formation of cis-azobenzene through irradiation at 590 nm brought the tellurium centers in close proximity and increased the binding strength for halides. In contrast, the binding affinity was reduced for the trans-azobenzene-based catalyst. This strategy has been applied for tailoring the catalytic activity of Friedel-Crafts alkylation reactions occurring between benzhydryl chloride and 1,3,5-trimethoxybenzene.106 Indeed, reaction yield was improved from 9 % to 58 % under continuous irradiation with a 590-nm excitation wavelength, which promotes formation of the cis-azobenzene-based catalyst and led to halide abstraction from the substrate. In general, this work represents a relatively novel direction in switchable catalysis built upon synergy of photochromic moieties with chalcogen bonding motifs.
In addition to selective blocking of active sites or alternation of the spatial separation of catalytic metal centers, switchable catalysis can also be achieved through changes in the coordination environment accompanied with ligand photoisomerization. For example, an oxazolinyl-2-azopyridine ligand coordinated to rare earth metal centers (M=La or Eu) could switch between bi- and tridentate coordination arrangements.100 This system overcomes a unique challenge in that it relies on coordination of the azobenzene moiety through the nitrogen atom while maintaining efficient photoisomerization around the N=N bond. Coordination of the photoswitchable ligand to the metal center occurred through three nitrogen atoms, one from the azobenzene, one from a pyridine, and one from an oxazoline functionality. In particular, the trans-azobenzene allows for coordination of all three nitrogen atoms to a lanthanum or europium center, while irradiation with 365-nm light promoted formation of cis-azobenzene and limited the coordination of the ligand to only the pyridine and oxazoline nitrogen atoms (i.e., switching from a tridentate to bidentate ligand). Such changes in coordination modes have been associated with changes in the ratio of enantiomers present in the product mixture for intermolecular cyclization of sulfonamide and aldehyde derivatives.100 Under optimized conditions, the enantiomeric excess of the reaction could be switched from 28 % to 76 % excess of the R-enantiomer based on excitation wavelength (UV or dark, respectively).100 Thus, the trans-azobenzene ligand facilitated a tridentate binding motif which resulted in higher enantioinduction. Control reactions involving non-photochromic bidentate or tridentate oxazoline ligands revealed similar trends to that of the tridentate ligands, which resulted in higher enantioselectivity than in the case of the bidentate ligands. Further mechanistic studies are necessary to shed light on the basis for the enantioselectivity of each photoisomer, and therefore, they are critical to reveal principles for the design of new switchable catalysts.
2.2.2 Changes in electronic properties of catalysts as a function of light
Rather than geometric control of reaction rate or yield, a different approach to switchable catalysis can be achieved through modulation of the electronic properties of the metal center for catalytically active organometallic complexes.57-60, 103 For example, change in a metal center's oxidation state could significantly alter the catalytic activity. Subtle shifts in ligand field strength, as well as LMCT, MLCT, or PET could be utilized to affect the metal oxidation states.78, 109-111 Thus, in this section, we will outline the ways in which photoswitchable ligands can be used to incite changes in the electronic properties of corresponding organometallic complexes and, as a result, catalytic activity through the mentioned processes.
One approach taken by Bogliotti and co-workers relies on “photo-uncaging,” or the light-induced dissociation of ligands from metal complexes.57 In the mentioned studies, an azobenzene-based ligand caused dissociation of a phosphine group from a ruthenium complex based on changes in steric hindrance due to azobenzene photoisomerization (Figure 7). In particular, exposure to visible light promoted the trans-form of the azobenzene ligand and resulted in ejection of the phosphine ligand. While the ruthenium complex by itself was not catalytically active, the presence of free phosphine released by the ruthenium complex promoted reduction of a palladium(II) pre-catalyst to an active palladium(0) metal center. Consequently, the palladium(0) complex exhibited catalytic activity toward cross-coupling (Sonogashira) reactions between aryl iodides and trimethylsilyl acetylene. For instance, the reaction yield could be improved from 10 % to 92 % by exposure of the reaction mixture to visible light irradiation, leading to release the phosphine group. Thus, reaction rate could be tuned as a function of irradiation time since formation of the active catalyst was directly associated with the light-induced dissociation of the phosphine group.57 Although it is a significant step toward noninvasive control of critical synthetic transformations, this approach toward switchable catalysis is irreversible. For instance, the reduction of palladium(II) by the free phosphine cannot be reversed even upon photoisomerization to reform the cis-azobenzene ligand. Despite its irreversibility, “photo-uncaging” offers the advantage of being applicable to a wide variety of catalysts without requiring individualized photoswitches (i.e., novel photoswitchable ligands for each organometallic catalyst).
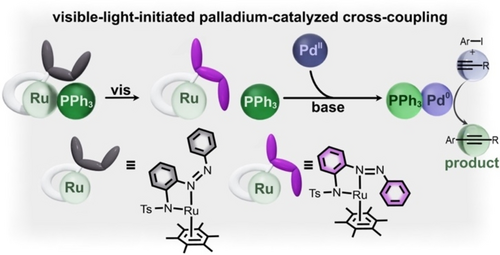
Cis-to-trans photoisomerization of an azobenzene ligand (gray to purple) initiates the dissociation of triphenylphosphine (dark green spheres) from a ruthenium complex. Triphenylphosphine then reduces a palladium(II) pre-catalyst (dark blue sphere) to the active palladium(0) catalyst (dark purple sphere) for Sonogashira type cross-coupling reactions. (substrate shown in light blue and product shown in light green). Adapted with permission under the Creative Common CC BY License.57
Tunable metal oxidation states are an attractive direction for switchable catalysis, but, in many cases, it is sufficient to alter the electron density of the metal center without utilizing complete oxidation or reduction to modulate catalytic activity. For example, the rate of industrially-relevant hydrosilylation reactions involving a platinum-based Karstedt's catalyst are sensitive to the electron density at the platinum metal center.58 In particular, coordination of electron-deficient ligands can render the platinum metal center inactive toward hydrosilylation of alkenes. While high catalytic activity is generally desirable, the high activity of platinum catalysts (even in small amounts) toward hydrosilylation can cause premature curing of polymers on the industrial scale.58, 112 Thus, selective inhibition of these catalysts through binding of electron-deficient alkenes is one attractive approach to modulate catalytic behavior.58 In this direction, reversible binding of electron-deficient ligands could alter the electron density of the catalyst and allow for tunable activity.58 Branda and co-workers demonstrated this possibility through careful design of a diarylethene-based ligand functionalized with electron-withdrawing cyano groups (Figure 8).58 In the open form of the diarylethene photoswitch, the cyano groups are located at an electron-deficient double bond which exhibited strong binding to the platinum center of Karstedt's catalyst and minimized catalytic activity. In contrast, irradiation with UV light caused photoisomerization to the closed form of diarylethene, which possesses a conjugated π-system that eliminates the electron-deficient alkene. As a result, the closed diarylethene photoswitch did not bind to the catalyst, preserving catalytic activity toward hydrosilylation reactions.58 Based on 1H NMR spectroscopic analysis of the reaction mixture, the authors concluded that the prepared diarylethene-based ligand was an effective inhibitor for the Karstedt's catalyst under visible light. However, the system suffered from a poor photostationary state under UV irradiation. That is, exposure to the appropriate excitation wavelength to induce photoisomerization to the closed form (i.e., without the electron deficient alkene) was not quantitative, resulting in a mixture of photoisomers and noticeable inhibition of the catalyst caused by the residual open photoisomer (Figure 8). Thus, this system achieves the desired inhibition of the catalyst but requires further optimization to obtain complete photoisomerization to the uninhibited form under UV excitation.
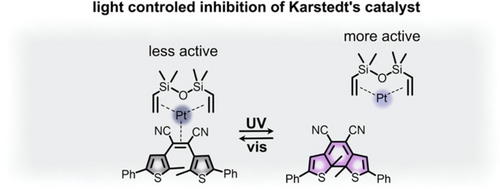
Coordination of the open diarylethene-based ligand (gray) to the platinum center of Karstedt's catalyst (dark blue circle) and photoisomerization to the closed diarylethene-based ligand (purple) which causes ligand dissociation to activate the catalyst toward hydrosilylation reactions. Adapted with permission.58 Copyright 2018 Royal Society of Chemistry.
A different approach to utilizing the change in electronic structure associated with diarylethene photoisomerization was achieved by Wolf and co-workers by modifying a copper complex with a diarylethene-based ligand (Figure 9).59 In this example, extension of conjugation throughout the ligand backbone upon isomerization from the open to closed diarylethene photoisomers resulted in decreased electron density at the copper metal center. As shown in Figure 9, the reduced electron density at the metal center resulted in decreased yields for hydroboration reactions conducted under UV irradiation in comparison with those conducted under visible light.59 Thus, on the example of both hydrosilylation and hydroboration reactions, diarylethene-based ligands are a promising avenue for control of electron density of metal centers and the corresponding reactivity.
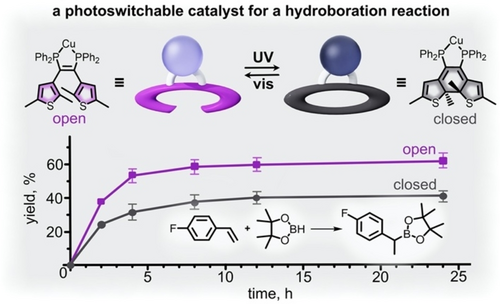
(top) Schematic representation of a copper-containing complex possessing a diarylethene-based ligand (open purple and closed gray ellipses). Photoisomerization from the open (purple) to closed (gray) isomers extends conjugation through the ligand backbone and reduces electron density at the metal center. (bottom) Plot demonstrating the increased yield for hydroboration reactions conducted in the presence of the open-diarylethene-based catalyst. Adapted with permission.59 Copyright 2018 John Wiley and Sons.
In a different example by Chen and co-workers, azobenzene-containing palladium and nickel catalysts were used to control the bulk material properties produced by ethylene polymerization and copolymerization reactions.103 Preparation of azobenzene-functionalized palladium complexes for ethylene polymerization revealed a 12-fold increase in chain transfer rate under UV irradiation to promote trans-to-cis isomerization of azobenzene.103 The authors attribute this increase in chain transfer rate to an increased electrophilicity of the palladium metal center as a result of azobenzene photoisomerization perturbing the electronic structure of the ligand backbone.103 Further, the relative distance between the azobenzene functionalities and the metal center suggest that the changes in reactivity were not due to steric factors. Within the same series of studies, sandwich-type azobenzene-functionalized naphthalenyl amine palladium and nickel complexes were prepared which could affect both the electronic and steric properties of the metal center upon azobenzene photoisomerization. In this case, photoisomerization of the azobenzene moiety directly impacted the steric bulk at the metal center, which coincided with decrease of the catalytic activity under UV irradiation. This sharp difference in behavior between the sandwich complexes and those with azobenzene groups at the outside of the coordination sphere can be attributed to competing electronic and steric factors. These results are in line with commonly used α-diimine nickel catalysts, which exhibit increased polyethylene molecular weight and decreased branching density with increasing ligand steric bulk.103 Thus, these studies show how design of photochromic ligands can alter the mechanism through which switchable catalysis is achieved (i.e., steric or electronic factors). For instance, changes in the electronic and steric properties of the transition metal complexes associated with azobenzene photoisomerization resulted in tunable polymer density, tensile strength, and molecular weight.103 With this in mind, incorporation of photoswitches in polymerization catalysts is an avenue for creating a library of materials from the same starting reagents simply by varying irradiation parameters. Along these lines, Chen and co-workers have also probed ring-opening polymerization by tuning the electronic structure of photoresponsive salicylaldimine ZnII complexes based on azobenzene photoisomerization.97 In each of the described studies by the Chen group, substantial differences in catalytic activity could be achieved through reversible photoisomerization of a ligand.97, 103
While the previous examples focused on a photoswitchable ligand altering the catalytic activity of a metal center, it is feasible to design systems which work in the alternative fashion, i.e., modulation of the catalytic activity of a photoswitchable molecule through coordination to a metal center. For instance, reversible coordination of a catalytically active ligand to a metal cation could perturb, for example, the Lewis basicity of the ligand and alter its chemical properties. In these systems, the metal center does not directly catalyze any organic transformations, but rather modulates the activity of an organic counterpart. One such example utilized an azopyridine photoswitchable ligand in combination with a nickel(II) porphyrin complex to achieve switchable catalytic activity toward nitroaldol reactions (Figure 10).60 Dimethylaminopyridine (DMAP) is known to act as a basic catalyst for nitroaldol reactions and, therefore, changes in the basicity of the nitrogen lone pair in the pyridine group could affect catalytic activity. Functionalization of DMAP with an azobenzene photoresponsive derivative followed by complexation with NiII cations allowed for reversible coordination of DMAP to the Lewis-acidic metal center, resulting in tunable basicity of the DMAP catalyst.60 In particular, irradiation with 530-nm light caused formation of cis-azobenzene and promoted coordination of DMAP to the metal center. Consequently, the catalytic activity was reduced by a factor of 2.2.60 In contrast, irradiation with 435-nm light caused photoisomerization to trans-azobenzene, which promoted de-coordination of DMAP from the metal center and restored the catalytic activity to that of the free catalyst (i.e., uncoordinated DMAP).60
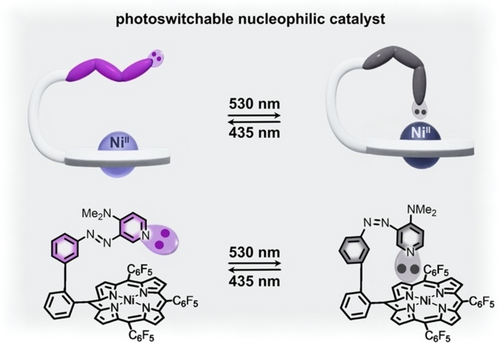
(top) Schematic representation of an azopyridine-containing nickel(II) porphyrin complex. Trans-to-cis isomerization of the azobenzene photoswitch (purple to gray) results in coordination of the pyridine functionality to the metal center, which reduces the basicity and activity of the catalyst. (bottom) Structures of the complex in the catalytically active (purple) and inactive (gray) forms. Adapted with permission under the Creative Commons Attribution License.60
In general, the area of photoswitchable organometallic catalysts is currently dominated by azobenzene-based ligands which can modulate the geometry around the metal center.55-57, 60, 97, 101, 103, 106 While these efforts have certainly opened the door for many exciting studies, the scope of reactions catalyzed by the prepared complexes could be expanded through in-depth mechanistic studies of the stimuli-responsive catalyst behavior and application of a broader variety of photoswitch motifs. Furthermore, switchable enantiodivergent catalysis (i.e., catalysts which can produce either enantiomer of a product based on photoisomerization of a ligand) could also be an attractive direction that requires rational design of photoresponsive ligands with specific geometric arrangements around a metal center to promote stereoselective synthesis.99
2.3 Extended Structures and Biomimetic Assemblies
In addition to organic or organometallic photoresponsive molecular compounds, much effort has been devoted to biomimetic systems or extended structures comprised of many photoswitchable molecules which are synergistically combined to affect catalytic outcomes.61-66, 113-128 There are several platforms, such as proteins, nanoparticles, and periodic structures which offer distinct opportunities for integration of photoswitches into different complex assemblies.61-66, 113-128 For example, photoresponsive molecules with azobenzene cores could be covalently embedded into a protein structure to affect its folding upon photoisomerization.62 As a result, photoswitches represent an avenue toward control of enzyme activity through alteration of active site geometry.62 A conceptually different approach to modulating larger systems is through coupling of photoswitches through formation of extended periodic structures, such as covalent- and metal–organic frameworks (COFs and MOFs, respectively).64, 121-126 Choice of inorganic, organic, or hybrid materials as platforms allows for strategic engineering of distances and angles between catalytically active sites and confirmation of their location using single-crystal X-ray diffraction analysis. Finally, catalytic activity of nanoparticles (NPs) could be controlled through an external stimulus by addition of a layer of photoswitchable ligands at the NP surface to regulate access to the catalytically active core.64, 121-126
2.3.1 Self-assembly of catalyst systems as a function of light
One of the strategies for controlling the catalytic activity of extended structures is to incorporate photoswitches for which isomerization is accompanied by significant changes in polarity (e.g., spiropyran, Scheme 2) to affect self-assembly processes. For example, assembly of micelles or nanotubes, which often depends on organization of amphiphilic molecules, could be controlled through switching between hydrophilic and hydrophobic forms of a photoresponsive molecule (e.g., switching from merocyanine to spiropyran).61, 127 Systems for which catalysis occurs only through the assembled supramolecular structure can, therefore, be modulated through isomerization of the photochromic fragment.124 For example, Tan and co-workers achieved catalysis of asymmetric sulfoxidation reactions in aqueous media through the light-activated self-assembly of spiropyran-containing metallomicelles (Figure 11).61 In these studies, a series of spiropyran-based chiral diblock salen TiIV copolymers were used to form metallomicelles capable of catalyzing the asymmetric sulfoxidation of aryl alkyl sulfides.61 In contrast to the majority of studies that have utilized spiropyran derivatives in organic solvents, the mentioned work was conducted in an aqueous environment, which preferentially stabilized the charge-separated merocyanine form of this photoresponsive molecule even in the dark. Thus, exposure to visible light induced isomerization from the hydrophilic merocyanine isomer to the hydrophobic spiropyran form. As a result, the metallomicelles underwent self-assembly to the catalytically-active organized structure in the dark, followed by disassembly and precipitation from aqueous solution under exposure to visible light.61 The catalytic activity of this assembled metallomicelle was first evaluated through probing the asymmetric oxidation of phenyl methyl sulfide in the dark. After one hour, >97 % yield with 99 % enantioselectivity was detected.61 Further studies using the same metallomicelles showed promising yields for a wider variety of aryl alkyl sulfide substrates, including p-methoxyphenyl methyl sulfide, o-methoxyphenyl methyl sulfide, phenyl n-butyl sulfoxide, and phenyl n-hexyl sulfoxide. The studied spiropyran-containing metallomicelles not only allowed for control of reaction yields for several sulfoxidation reactions, but they also demonstrated a pathway for facile recycling of catalysts. In particular, irradiation at 550 nm caused photoisomerization to the hydrophobic form of the metallomicelle, which precipitated from the reaction mixture and could be recycled through filtration.
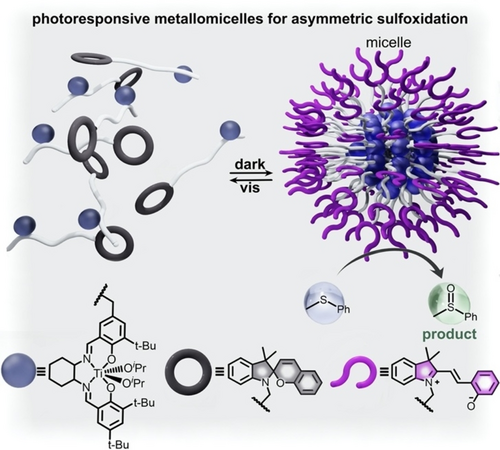
(top) Schematic representation of metallomicelle self-assembly upon conversion from spiropyran to merocyanine in the dark. The substrate for asymmetric sulfoxidation catalyzed by the metallomicelle is shown as the light blue sphere, and the product is shown as a light green sphere. (bottom) Structures of the chiral TiIV salen metal complex (dark blue sphere), as well as the closed and open forms of the spiropyran functionality (gray and purple rings, respectively). Adapted with permission.61 Copyright 2019 American Chemical Society.
Rather than self-assembly occurring due to light-induced changes in polarity as described above, a different approach is to utilize changes in geometric configurations to promote self-assembly of catalytically-active systems. Functionalization of a peptide chain with an azobenzene moiety, for instance, has been used to control the self-assembly of peptide fibrils through promotion of π-π stacking interactions for the trans-azobenzene-containing peptide chain.62 In this system, the trans-isomer of the azobenzene functionality allowed for noncovalent interactions, such as the mentioned π-π stacking in combination with other hydrophobic interactions. As a result, the trans-azobenzene-based peptide chains underwent self-assembly into an organized peptide fibril (Figure 12). In contrast, exposure to UV irradiation resulted in formation of the cis-azobenzene photoisomer. The significant geometric rearrangement upon photoisomerization broke the noncovalent interactions between the assembled peptide subunits and resulted in destruction of the organized fibril system. As shown in Figure 12, the assembled fibril could promote hydrolysis of p-nitrophenyl acetate as an artificial hydrolase enzyme mimic, but the disassembled state was unable to do so. The mechanism for the hydrolysis reaction promoted by the assembled peptide fibril relies on catalytically active histidine residues which facilitate deprotonation of water molecules to initiate hydrolysis.62 He and co-workers attributed the detected higher reaction rate for the trans-azobenzene-containing system to cooperation between the active histidine residues located in proximity to each other in the self-assembled structure.62 That is, the aligned histidine residues could cooperatively lower the pKa of bound water molecules to promote deprotonation. As a result, the assembled hydrolase mimic exhibited a catalytic activity of 95.87 μM min−1, while irradiation with UV light to cause disassembly reduced the catalytic activity to 65.56 μM min−1.62 Thus, control of the catalytic activity and of the self-assembly process was possible through irradiation of the system to cause azobenzene isomerization. Further studies from Bandyopadhyay and co-workers have also illustrated that integration of azobenzene in enzyme mimics allows for precise tuning of the reaction outcomes.129 Rather than light-activated self-assembly of protein structures, azobenzene photoisomerization could also cause relatively small geometric changes in the distance between amino acid residues in a racemase mimic, which modulated the kinetics of the racemization reaction converting L-alanine to D-alanine.129 Similarly, modulation of the size of the catalytic pocket for peptide-based catalysts through azobenzene photoisomerization has been implemented to control the site-selectivity in biomimetic acetylation of sugars.68 Advances in biomimetic catalysts are not limited to azobenzene-containing systems, and the binding affinity of substrates and peptides has also been successfully regulated using diarylethene130 and spiropyran127 derivatives.
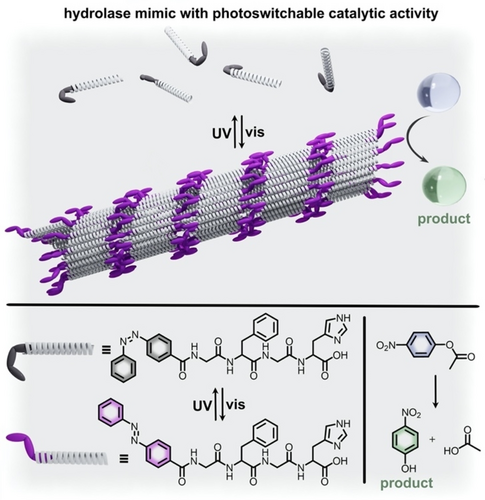
(top) Schematic representation of the assembled peptide fibril capable of catalyzing the hydrolysis of p-nitrophenyl acetate as a hydrolase enzyme mimic. The substrate is represented by the light blue sphere, and the product is represented by the light green sphere. (bottom) Structures of an azobenzene-containing peptide chain under irradiation with visible (purple) and UV (gray) light. Adapted with permission.62 Copyright 2018 Royal Society of Chemistry.
2.3.2 Tailoring membrane permittivity as a function of light
In addition to modulation of geometric parameters and self-assembly processes, incorporation of light-responsive molecules in micelles or vesicles has also allowed for tailorable membrane permittivity which could be altered to regulate flow of reactants through a membrane (Figure 13).63, 131 In this direction, Bruns and co-workers utilized photoresponsive donor-acceptor Stenhouse adducts (DASAs) to construct nanoreactors with membranes possessing switchable permeability.63 In this case, the DASAs could be converted between nonpolar triene-enol and polar cyclopentanone forms upon irradiation with visible light.63 As illustrated in Figure 13, embedding DASAs in self-assembled vesicles prepared from amphiphilic block copolymers provided an opportunity to switch between a nonpolar and polar membrane surrounding a horseradish peroxidase enzyme. Upon irradiation with visible light, the prepared nanoreactors exhibited increased permeability, allowing for diffusion of reactants into the vesicle which housed the enzyme.63 Specifically, diffusion of pyrogallol to horseradish peroxidase in the presence of hydrogen peroxide resulted in catalytic formation of purpurogallin. Notably, the reaction was only effective under constant irradiation with visible light, indicating that diffusion of reactants toward a catalytic site could be controlled via irradiation with an appropriate excitation wavelength. Moreover, design of two nanoreactors responsive to different excitation wavelengths (e.g., green or red light) allowed for control of cascade catalysis in which the product formed by one reaction was allowed to diffuse into a second nanoreactor with high spatiotemporal control.63 Notably, the concept of photoswitchable nanoreactors has also been successfully demonstrated using spiropyran as the photochromic moiety, and the resulting system was capable of regulating pathways for incompatible tandem catalysis.127 For example, multi-step synthesis requiring more than one catalyst which could interact unfavorably (i.e., catalyst poisoning or increased formation of byproducts), could be achieved in a one-pot procedure if the catalytically active species were separated by micelles.127 Thus, construction of photochromic micelles, which behave as photoresponsive nanoreactors, represents a pathway to synergistically catalyze multiple organic transformations in an efficient manner.
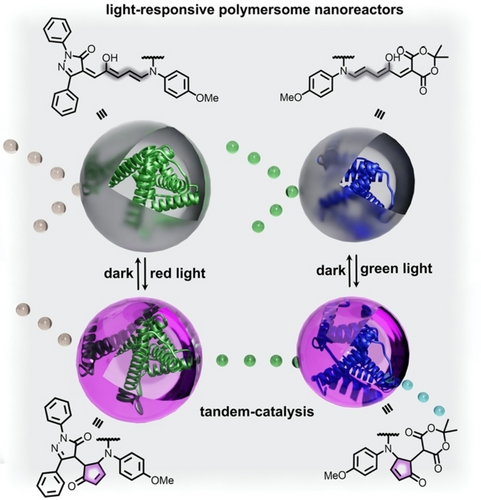
Schematic representation of two nanoreactors containing horseradish peroxidase enzymes (green and blue helices). Embedding photoswitchable DASAs in the membrane of the nanoreactor allows for control of membrane permeability. As a result, tandem catalysis can be initiated by exposure to red light, which “opens” the first nanoreactor, followed by exposure to green light to “open” the second nanoreactor. Adapted with permission.63 Copyright 2018 American Chemical Society.
2.3.3 Nanoparticle catalytic activity as a function of light
A different direction which has gained significant attention in recent years is coupling inorganic NPs with organic photochromic ligands because the catalytic behavior of the resulting NPs could be tuned using light.64, 65, 122-126, 132, 133 The catalytic behavior and stability of many NPs (e.g., gold NPs) are affected by several factors, including the nature and conformation of organic ligands.64, 65, 122-126, 132, 133 For example, a passivating layer of organic ligands is necessary to prevent NP aggregation and precipitation, but the presence of organic ligands also usually reduces the accessible surface area and catalytic activity.122, 125 Thus, photochromic ligands, which could reversibly change their configuration on the surface of the NP, is one of the avenues for regulating the activity and recyclability of NP-based catalysts.64, 121-123, 126, 133 For instance, Knecht and co-workers demonstrated that switching of azobenzene-containing peptide ligands bound to the surface of gold NPs could alter the rate of the catalytic reduction of 4-nitrophenol to 4-aminophenol.122 These studies probed the possibility of alternation of binding affinity of material-binding peptides as a function of azobenzene photoisomerization. While the peptide ligands exhibit strong binding to the NP surface through noncovalent interactions, these interactions can be weakened by small changes (e.g., point mutations affecting residue sequence) which alter the conformation of the peptide with respect to the NP surface.122 With this in mind, integration of an azobenzene moiety within the peptide sequence could allow for light-induced “mutations” that alter the peptide ligand binding strength. In fact, the presence of trans-azobenzene coincided with an increased rate (10.5±0.2×10−2 s−1) for the reduction of 4-nitrophenol to 4-aminophenol, which could be attributed to weaker binding and dissociation of some ligands to expose a larger portion of the catalytically active NP surface. In contrast, irradiation with 365-nm light to yield cis-azobenzene was accompanied with stronger binding of the peptide ligands and reduced reaction rate (9.4±0.7×10−2 s−1).122 A different example coupled a proline organocatalyst with gold NPs by covalently bonding the proline functionality to an aliphatic tail which could be coordinated to the metal surface by a thiol end group.64 An azobenzene spacer was used within the aliphatic chain, allowing for modulation of the distance between the proline catalyst and NP surface (Figure 14). For instance, irradiation with 365-nm light caused photoisomerization to form cis-azobenzene, which brought the catalyst close to the NP surface and suppressed catalytic activity by preventing close interactions between catalyst and substrate through steric hindrance.64 These latter examples64 illustrate how incorporation of photoresponsive ligands on the surface of inorganic NPs is a pathway to control catalytic activity of either the metal NP or an organic catalyst depending on the system design.
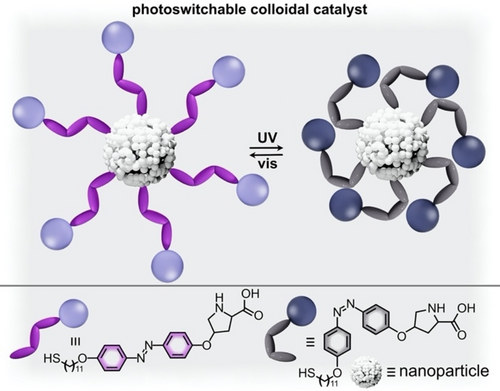
(top) Schematic representation of gold nanoparticles (white sphere) capped with azobenzene-containing organic ligands. Trans-to-cis photoisomerization of the azobenzene derivative blocks the catalytically active sites. (bottom) Structures of the azobenzene-based ligands in the catalytically active (trans, purple) and inactive (cis, gray) forms. Adapted with permission.64 Copyright 2018 American Chemical Society.
Combining the areas of switchable catalysis and bioorthogonal chemistry has resulted in a pathway for noninvasive control of catalytic processes even in living cells.65 In this direction, Qu and co-workers designed heterogeneous palladium catalysts by grafting of Pd0 NPs to a macroporous silica support (Figure 15).65 While the prepared NPs were capable of catalyzing Suzuki-Miyaura cross-coupling reactions by themselves, modification with supramolecular azobenzene and β-cyclodextrin functionalities allowed the system to be controlled by light. First, the Pd0 NPs were embedded inside the pores of the silica support, and the support was then functionalized with photoresponsive azobenzene groups. As shown in Figure 15, trans-azobenzene promoted interactions with β-cyclodextrin, which served to block the pores of the silica support and prevent catalysis from occurring at the Pd0 NPs.65 Exposing the system to UV light promoted the trans-to-cis isomerization of azobenzene, which broke the interactions with β-cyclodextrin and exposed the Pd0 catalysts inside the pores. As a result, higher catalytic activity was detected under UV irradiation than visible light. To advance these studies further, living cancer cells were incubated in a suspension of the prepared NPs embedded in the silica support. Then, the NP-containing cells were exposed to a fresh solution of non-fluorescent starting materials for a Pd-catalyzed cross-coupling reaction. The product of the cross-coupling reaction was a mitochondria-specific fluorescent probe. Only under UV light did the cross-coupling reaction occur, and the reaction progress could be tracked using fluorescence imaging of the cells. Control experiments utilizing a commercially available mitochondrial dye revealed that the light-gated Suzuki-Miyaura cross-coupling reaction was capable of producing fluorescent probes for cellular imaging in vitro. Thus, these studies foreshadow new pathways for the development of noninvasive imaging, diagnosis, and treatment using photoresponsive heterogeneous catalysis.
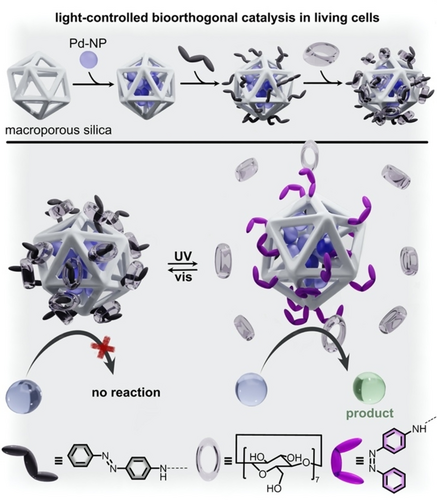
Schematic representation of palladium NPs (dark purple spheres) embedded in a porous silica support (white cage). Functionalization of the silica support with azobenzene groups (gray or purple) allowed for control of β-cyclodextrin (transparent rings) binding to the support. Upon irradiation with UV light, cis-azobenzene expels β-cyclodextrin to expose the NP active sites and catalyze Suzuki-Miyaura cross-coupling reactions. The substrate and product are represented by blue and green spheres, respectively. Adapted with permission under the Creative Commons CC BY License.65
2.3.4 Catalytic activity of extended structures as a function of light
While the previously described study utilized macroporous silica to support the catalyst, many studies in the area of heterogeneous catalysis have focused on COFs and MOFs as other suitable platforms due to their high surface area and tailorable porosity, which promotes mass transport of the reactants throughout the catalytic framework. Additionally, these frameworks could be tailored to include either active metals within the metal nodes or organocatalysts as parts of linkers connecting secondary building units.66, 113-120 Given the several successful examples of COF- or MOF-based heterogeneous catalysis, integration of photochromic moieties into these systems49, 134, 135 is a logical approach to expand the role of COFs and MOFs in the field of switchable catalysis. For example, switchable generation of 1O2 for oxidation of various organic substrates66 or for photodynamic therapy10 is one area where COFs and MOFs have been implemented successfully. For instance, a COF constructed from porphyrin- and diarylethene-based linkers could be used to modulate the photocatalytic oxidation of amines through selective 1O2 generation (Figure 16).66 In this system, the triplet excited state of the porphyrin linker by itself promotes conversion of 3O2 to 1O2; however, incorporation of a diarylethene-based linker creates a competitive energy transfer pathway that can suppress generation of 1O2.66, 136 In particular, the lowest triplet energy of the open form of the diarylethene-based linker exceeded that of the porphyrin linker, which prevents energy transfer from occurring in the presence of the open diarylethene photoisomer. At the same time, the lowest triplet energy of the porphyrin linker lies above that of 3O2, allowing for efficient conversion of 3O2 to 1O2. In contrast, photoisomerization of diarylethene from the open to closed form results in a lower energy triplet excited state that is compatible with energy transfer from the porphyrin derivative to the diarylethene linker. As such, switching from the open to closed form of the diarylethene linker upon exposure to blue light caused the 1O2 generation pathway to be suppressed by the favored energy transfer pathway. This concept was demonstrated experimentally through the photocatalytic oxidative coupling of amines to yield imines under various irradiation conditions. Indeed, enhanced generation of 1O2 associated with the open diarylethene photoisomer resulted in reaction conversions of up to 99 % for oxidative coupling of benzylamine in comparison to 63 % conversion detected for the COF containing closed diarylethene.
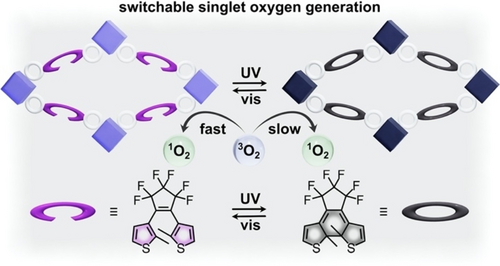
Schematic representation of a COF possessing porphyrin- (light purple or dark gray squares) and diarylethene-based (purple or gray ovals) linkers. Generation of 1O2 by the porphyrin functionality is suppressed upon irradiation with UV light to form the closed diarylethene linker because of the possible energy transfer pathway between porphyrin and closed diarylethene. Adapted with permission.66 Copyright 2022 American Chemical Society.
Other approaches to synergistic coupling of diarylethene and porphyrin derivatives in COFs have involved oxidation of N-methylpyridinium salts under alternating irradiation conditions.118 In this case, the open form of the diarylethene linker promoted catalytic oxidation of 1-methylquinoline-1-ium iodide to 1-methylquinolin-2(1H)-one by molecular oxygen, and the detected conversion was 99 %, which the authors attribute to both higher specific surface area and changes in electronic structure due to photoisomerization. Irradiation with UV light to induce photoisomerization to the closed form of the diarylethene linker resulted in a reduced conversion of 61 % on average under the same reaction conditions.118 Further studies are necessary to fully uncover the mechanism for the enhanced catalytic activity of the open diarylethene derivative in comparison with the closed photoisomer. One of the hypotheses for the observed behavior could be attributed to the fact that the closed isomer suppresses electron transfer from the porphyrin derivative to the substrates.118
In addition to the described studies focusing on photoresponsive COFs, it is worth mentioning that hydrazone-based COFs have been utilized as efficient photocatalysts for several types of organic transformations, but the role of the hydrazone derivative has mainly been limited to a light-harvesting unit rather than a “knob” for control of catalytic activity.105, 116 Thus, future studies involving the photoswitching ability of hydrazone-based COFs in conjunction with photocatalysis will undoubtedly yield valuable insights for the development of new switchable catalytic materials.
Integration of azobenzene-based linkers in MOFs has also been employed to modulate the response of heterogeneous catalysts.120 For example, an azobenzene-functionalized MOF was evaluated in its ability to catalyze several Knoevenagel condensation reactions, which are typically base-catalyzed processes.137-140 The azobenzene photoswitch could then serve a dual role. First, the basicity of the N=N bond of the azobenzene was probed for promotion of the condensation reaction, and E/Z photoisomerization could change the available pore space and control diffusion of the reactants through the catalyst. For instance, the adsorption capacity of the azobenzene-MOF was detected to decrease under UV irradiation and increase when the MOF was stored in the dark, indicating that photoisomerization of the azobenzene group affected the pore volume. These results were supported by the catalytic yields, which were elevated when the reactions were conducted in the dark and suppressed under UV irradiation. For example, the condensation of salicylaldehyde proved to be extremely sensitive to azobenzene photoisomerization as the conversion was determined to be 93 % in the dark and only 6 % under UV excitation. Further, the reaction conversion was dependent on substrate size because it is a limiting factor in diffusion rate.120 Thus, photochromic MOFs offer the ability to not only modulate catalytic activity, but also selectivity using a single platform.
3 Stimuli Beyond Light
Although many studies have selected light as an external stimulus due to its high spatiotemporal resolution, challenges associated with light-activated systems (e.g., limited penetration depth) could be addressed using a combination of alternative stimuli, including pH, electric current, pressure, or heat.141-154 For example, spiropyran- and hydrazone-based photoswitches, which were described above, could also undergo isomerization upon pH modulation.148, 149 Interrogation of the system by multiple stimuli not only provides continuous control of its properties, but it also offers the possibility to tune properties simultaneously, for instance, toward desired applications. Therefore, studies of catalysts activated by other external stimuli will play a vital role in the development of new synthetic industrial processes, including logic-gated systems for synthesis of a series of organic molecules promoted by a single switchable catalyst. Notably, catalytic systems which can be switched based on an applied potential have received significant attention deserving of several recent reviews elsewhere.8, 146, 155 With this in mind, we discuss recent advancements in switchable catalysis that utilize external stimuli beyond light, including pH- and thermally-switchable catalysts.141-154
3.1 pH-Switchable Catalysis
Considering pH-switchable catalysis, it is important to differentiate between acid- or base-catalyzed reactions and reactions for which the activity of a known catalyst can be altered by changes in pH. For instance, many reactions can be catalyzed by addition of acid or base, and failure to achieve the appropriate pH could result in an observed loss of catalytic activity; however, in this scenario, the acid or base acts only as a catalyst and not as a stimulus which alters the behavior of an independent catalyst. Thus, we choose to limit our discussion of pH-switchable catalysts to systems in which addition of acid or base alters the structure and, as a result, the behavior of a separate catalyst (e.g., protonation or deprotonation of the catalyst). Modulation of catalytic activity through changes in pH offers the advantage of near quantitative switching between catalytic states through even relatively small changes in reaction conditions, which has provided access to several studies that achieved efficient switching and recycling of molecular catalysts.
Inspired by the switchable activity of enzymes, there have been several approaches using changes in pH to affect the structure and, as a result, activity of biomimetic catalysts.128 Moyano and co-workers have utilized pH-dependent aggregation of secondary amine-porphyrin hybrids to not only switch between “on” and “off” catalytic states, but also for facile recovery and separation of the catalyst from the product mixture.148 Specifically, preparation of a series of 5-(cyclic-secondary-amine)-10,15,20-tris(4-sulfonatophenyl)porphyrins provided a pathway to control aggregation of the catalytically active system (Figure 17 and Table 1, #30).148 In this case, protonation of the pyrroleninic nitrogen atoms of the prepared 5-(isoindolin-2-ium-5-yl)-10,15,20-tris(4-sulfonato-phenyl)porphyrin disodium salt allowed for electrostatic interactions with the peripheral anionic sulfonate groups. These interactions promoted the formation of J-aggregates, which limited solubility of the catalyst and reduced its activity. The prepared amine-porphyrin hybrids were evaluated in their ability to catalyze the aldol addition of cyclohexanone to 4-nitrobenzaldehyde, and they exhibited 99 % yield at neutral pH and no detectable conversion in acidic conditions (pH 3.6) due to their aggregation. Notably, control experiments supported that the reaction would progress in acidic conditions using catalysts insensitive to pH-induced aggregation. This strategic design was not limited to only control of catalytic activity but also can be used to efficiently separate the catalyst aggregates from the product mixture by acidification and centrifugation.148
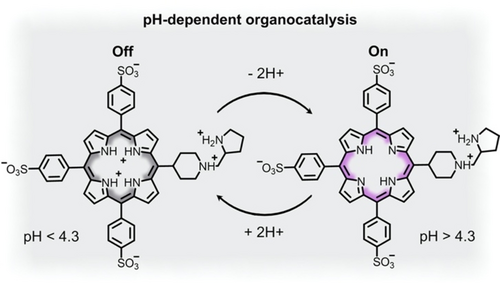
Representation of the pH-dependent supramolecular behavior of a chiral proline-derived amphiphilic 5-substituted-10,15,20-tris(4-sulfonatophenyl)porphyrins. Above a pH of 4.3, the organocatalyst becomes soluble in aqueous solutions allowing enamine-based catalysis (purple). Below a pH of 4.3 the protonated porphyrin core leads to the formation of J-aggregates rendering the catalyst inactive (gray). Adapted with permission under the Creative Commons Attribution CC BY License.148
Rather than changing pH to switch between active and inactive catalysts, pH-activated systems are also capable of switching between two different active states.149 That is, either form of the catalyst is active, but the organic transformations capable of being catalyzed depends on the form of the catalyst present in the reaction mixture. For example, conversion between imino- and amido-containing forms of a ruthenium-based catalyst allowed for switching between hydrogenation and dehydrogenation reactions through addition of acid or base.149 Specifically, this system features a RuII-benzimidazole (i.e., neutral, σ-donating ligand) complex which could be deprotonated in basic conditions to form a RuII-benzimidazolate (i.e., anionic, σ- and π-donating ligand) complex. In an acidic environment, the RuII-benzimidazole complex promoted hydrogenation of amine substrates, while addition of base to form the RuII-benzimidazolate complex could promote the dehydrogenative coupling of amines. Remarkably, this system could be applied for tandem catalysis in which sequential dehydrogenative coupling of amine substrates and hydrogenation of the resulting imine could be achieved in a “one-pot” synthetic method by stepwise addition of base and acid.
3.2 Thermally-Switchable Catalysis
In contrast to the pH-switchable systems mentioned above, choice of temperature as a stimulus offers the advantage of introducing no exogenous reagents to the reaction mixture and can be finely tuned with accessible laboratory equipment. In this section, we will discuss recent works in which a change in temperature could be utilized to selectively alter the observed catalytic activity of a system. Several successful directions, including the use of thermosensitive polymers and nanoparticles, have illustrated that temperature can play an active role in catalysis beyond simply reaching a particular reaction energy barrier. Unlike the other stimuli discussed above, which serve to switch the catalyst between two distinct states, changes in temperature have generally been used to modulate the pathways for diffusion of reactants to catalytic sites. For example, gold nanorods (AuNRs) layered with a silver shell (AuNR@Ag) could be grafted with thermally-sensitive polymers, such as poly(N-isopropylacrylamide).125 In these studies, AuNR@Ag was known to catalyze the reduction of 4-nitrophenol. Without grafting of the thermally-sensitive polymer layer, AuNR@Ag displayed typical Arrhenius behavior, meaning that the apparent rate constant for the reduction reaction increased with temperature; however, addition of the polymer layer resulted in self-inhibition of the AuNR@Ag catalyst. That is, the apparent rate decreased with temperature when the reaction was heated above 30 °C.125 Li and co-workers attributed this behavior to shrinkage of the polymer ligands toward the surface of the silver shell, which would decrease the effective surface area and limit diffusion of the reactants to the catalytically active surface. Thus, the pathway for reactant diffusion could be turned off by increasing the reaction temperature. In essence, the described system represents a mechanism for stopping the reaction progress in the case of overheating. For example, large-scale industrial reactions could benefit from automatically adjusted reaction rates for highly exothermic and potentially dangerous processes. Thus, the area of self-inhibition of thermally-switchable catalysts warrants further work which could improve safe handling of highly exothermic and spontaneous reactions.125
A conceptually different approach to thermally-switchable catalysis involves integration of organocatalysts within porous polymer microgels.150 Well-dispersed polymer microgels (i.e., high solubility) allow for rapid diffusion of reactants to the catalytically active sites, whereas aggregated microgels exhibit limited catalytic activity due to diffusion and solubility limitations. Thus, Rueping and co-workers integrated a catalyst for asymmetric alcoholysis of cis-tetrahydrophthalic anhydride into porous polymer microgels which exhibited temperature-dependent aggregation (Figure 18 and Table 1, #35).150 For this, a quinine-derived sulfonamide organocatalyst with a polymerizable styrene moiety was used to prepare catalytically-active microgels. At increased temperatures, the microgels exhibited swelling and high colloidal stability, which significantly increased the reaction rate. In contrast, reducing the temperature resulted in the collapse of the microgels to form insoluble aggregates. As a result, the catalyst-microgel hybrid precipitated from solution and ceased to catalyze the reaction. The catalyst could then be separated from the reaction mixture by centrifugation and filtration, followed by redispersion in fresh isopropanol for the next catalytic cycle. Notably, the use of the catalytically-active microgels resulted in high yields and enantiomeric ratios even for the recycled catalysts. In fact, under optimized conditions, the catalysts could be recycled 10 times with yields from 94 % to 97 %.150 The mentioned studies demonstrate how thermally-switchable polymers could be utilized to unite the advantages of homogeneous and heterogeneous catalysis, including high reactivity and selectivity, as well as facile recovery of the catalyst.150
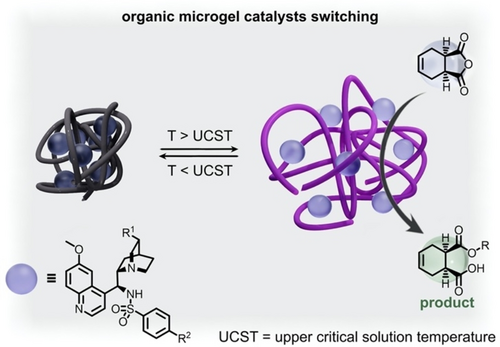
Schematic representation of a temperature-responsive polymer microgel switching upon exceeding the upper critical solution temperature (UCST) from an insoluble (black) to a soluble (purple) form. The catalytic sites are inaccessible in the insoluble form of the polymer (dark blue spheres) but are accessible in the soluble form of the polymer (light blue spheres) for the alcoholysis of cis-tetrahydrophtalic anhydride (light blue to light green). Adapted with permission.150 Copyright 2018 American Chemical Society.
While the previously described studies mainly focus on implementation of one stimulus at a time, development of logic-gated systems in which multiple stimuli could be used to control the outcome of a given reaction are the next step in the field of switchable catalysis. Progress in this area has been demonstrated by Johnson and co-workers, for example, with a thermally-responsive photoredox catalyst based on 10-phenylphenothiazine used to promote block copolymer synthesis.85 In this example, a single light- and heat-sensitive catalyst was used to control the radical polymerization of a wide variety of monomers, including acrylates, acrylamides, and vinyl esters, among others.85 Monomer conversion and polymer molar mass distribution of poly(tert-butyl acrylate) and poly(N,N-dimethylacrylamide), among other polymer products, could be controlled using three stimuli, presence or absence of catalyst, irradiation with light, and temperature, for both reversible addition-fragmentation chain-transfer and atom transfer radical polymerization reactions, which demonstrates the potential applications of logic-gated systems to a broad range of materials synthesis.
4 Conclusions and Perspectives
As demonstrated by the presented studies, the field of switchable catalysis has seen substantial growth within the last five years through the combined efforts of the organic, inorganic, and physical chemistry, as well as materials science communities. In particular, the expansion of the mechanisms by which catalytic activity can be tuned has resulted in a wealth of switchable catalysts utilizing several classes of photochromic molecules. For instance, the widely-used class of azobenzene photoswitches has not been limited to only blocking or exposing of active sites, but has been shown to affect parameters like binding affinity106 and nucleophilicity60 as well. Likewise, hydrazone, diarylethene, and spiropyran derivatives have all played distinct roles in controlling a variety of organic transformations, including Sonogashira and Suzuki coupling, hydroboration, Michael addition, Friedel-Crafts, and nitroaldol reactions, as well as a variety of olefin metathesis and polymerization reactions.51-53, 66, 127 Further, incorporation of photoswitches in extended structures, micelles, and nanoparticles has resulted in biomimetic systems modeling enzyme behavior.61-66, 113-128 In addition to photoswitchable catalysis, notable work utilizing alternative stimuli, such as pH and temperature, has improved catalyst recyclability.148, 150 The mentioned studies also demonstrate the importance of the stimulus choice for tailoring catalytic activity of a particular system. For example, systems which are prone to degradation outside of a given pH or temperature regime could benefit from light as the stimulus because it offers the advantage of noninvasive control of catalyst behavior. At the same time, light-activated systems must be designed with the PSS of the photoswitch and the penetration depth of the excitation wavelength in mind. The benefits of using pH or temperature as the stimulus should not be overlooked as these systems can undergo near-quantitative switching upon addition of enough acid or base or upon heating to a critical temperature. Although quantitative switching is an attractive direction, not all catalytic reactions may be compatible with such conditions. Thus, choice of a pH- or temperature-activated catalyst is limited by the sensitivity of the targeted organic transformation. Taken together, the last five years of research represent exceptional progress in broadening the scope of organic transformations which can be affected by switchable catalysts as well as in the development of more sustainable approaches to critical synthetic steps.
Progress in the field has also uncovered challenges which invite creative solutions and fundamental studies of the stimuli-responsive behavior of catalysts. In particular, the majority of the presented studies provide proof-of-concept results demonstrating the possibility to switch between two states of catalyst behavior. While promising, further optimization and characterization will be required to compete with state-of-the-art catalysts. For example, very few studies in this field report turn over number or frequency for the prepared switchable catalysts, which would definitely provide a benchmark for comparison and improvement of catalyst design. Further, detailed mechanistic studies are necessary for many systems in order to optimize catalyst performance and apply the obtained knowledge to new organic transformations. Given that the research conducted in the last five years alone has uncovered several unprecedented directions and approaches to switchable catalysis, one can only assume that the coming years will build on these studies to realize switchable catalysis as a common sustainable laboratory and industrial practice.
Acknowledgments
The authors are grateful for support from the NSF Award (DMR-2103722) and SC EPSCoR GEAR. N.B.S. also acknowledges support from the Dreyfus Teaching-Scholar Award supported by the Dreyfus Foundation, the Hans Fischer Fellowship, and ASPIRE-II awards granted through the USC Office of the Vice President for Research. N.B.S. and J.H. thank the TUM Institute for Advanced Study (IAS) for their support. G.C.T. acknowledges support from the National Science Foundation Graduate Research Fellowship under Grant No. DGE-2034711.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Grace C. Thaggard is currently pursuing her PhD degree in inorganic chemistry at the University of South Carolina after completing her B.Sc. degree in chemistry from the University of Alabama at Birmingham in 2021. Her research interests include the design of novel stimuli-responsive materials exhibiting directional energy transfer. She was selected as an NSF Graduate Research Fellow.
Biographical Information
Johanna Haimerl is currently pursuing her PhD degree in inorganic chemistry at the Technical University of Munich after receiving her B.Sc. degree from the University of Bremen in 2018 and her M.Sc. degree from the Technical University of Munich in 2020. Her research interests include carbon dioxide reduction and artificial photosynthesis in metal-organic frameworks.
Biographical Information
Roland A. Fischer holds the Chair of Inorganic and Metal-Organic Chemistry at the Technical University of Munich (TUM) and is the Director of the TUM Catalysis Research Centre. Previously he was a Professor at Ruhr-University Bochum and Heidelberg University. He is a member of the European Academy of Sciences. His research interest focuses on functional molecular materials, in particular on metal-organic frameworks and on superatomic clusters for applications in energy conversion, catalysis, gas storage and separation, photonics, and microelectronics.
Biographical Information
Kyoung Chul Park is currently pursuing his PhD degree in inorganic chemistry at the University of South Carolina after completing his B.Eng. and M.Eng. degrees from Incheon National University in 2016 and 2018, respectively. His research focuses on the development of stimuli-responsive frameworks and metal-organic materials for nuclear waste administration.
Biographical Information
Natalia B. Shustova is a Professor of Chemistry at the University of South Carolina and an Associate Editor of ACS Materials Letters. She received her M.Sc. degree in materials science and two PhD degrees in physical and inorganic chemistry. Her research program focuses on materials for sustainable energy conversion, actinide-based platforms, and stimuli-responsive frameworks. Her research accolades include the Fischer, McCausland, and Sloan Fellowships and Dreyfus Teaching-Scholar, Cottrell Scholar, Breakthrough, and NSF Career Awards.