Cross-Dehydrogenative Coupling Polymerization via C−H Activation for the Synthesis of Conjugated Polymers
Graphical Abstract
Cross-dehydrogenative coupling (CDC) via C−H activation is the latest addition in the repertoire of synthetic strategies towards conjugated polymers. This article captures the development of this polymerization strategy in the light of background small molecule reactions and highlights how its approach is greener than the previous methods. Arene–arene and arene–alkene couplings via transition metal catalysts have been explored for CDC polymerization.
Abstract
Owing to their versatile (opto)electronic properties, conjugated polymers have found application in several organic electronic devices. Cross-coupling reactions such as Stille, Suzuki, Kumada couplings, and direct arylation reactions have proved to be effective for their synthesis. More atom-efficient oxidative direct arylation polymerization has also been reported for making homopolymers. However, growing interest toward donor-acceptor polymers has led to the recent emergence of cross-dehydrogenative coupling (CDC) polymerization to synthesize alternating copolymers without any prefunctionalization of monomers. Metal-catalyzed cross-coupling of two simple arenes via double C−H activation, or of an arene with an alkene via oxidative Heck-type reaction have been used so far for CDC polymerization. In this article, we discuss the development of CDC polymerization protocols along with the relevant small molecule CDC reactions for an improved understanding of these reactions.
1 Introduction
Extended π-conjugation is one of the highly sought-after features in organic molecules for synthetic chemists involved in both academic and industrial research—this can be attributed significantly to the research on organic electronic materials.1-3 Solution processibility enabling potential low-cost producibility of their devices, the earth abundant nature of the atoms that make the polymer, combined with their ability to be forged into flexible, lightweight, roll-to-roll printable and optoelectronic devices make them a potential alternative to their inorganic solid-state counterparts.4-7 Apart from the cutting-edge innovations of organic photovoltaics (OPV), organic light-emitting diodes (OLED) and organic field-effect transistors (OFET),8-10 conjugated polymers are also being used in drug delivery, bioimaging, bioelectronics and photodynamic therapy.11-16
The journey of conjugated polymers began almost half a century ago with polyacetylenes,17, 18 and has increasingly been diversified, giving rise to complexities in synthesis and application. One of the major advantages offered by these polymers is that the electronic and optical properties of the devices can be tuned by variation and modification of monomer units. Band gap, and absorption and emission wavelengths can be fine-tuned by slightly changing the electronic nature of the repeating unit, and by changing the alkyl side chain as well. The development of donor-acceptor copolymers represents substantial progress in this context.19, 20 An alternating array of electron-rich and electron-deficient π-systems lowers the effective HOMO–LUMO energy difference after hybridization of atomic orbitals of donor and acceptor molecules.21, 22 Hence, efficient strategies for the synthesis of alternating copolymers are highly desirable for this purpose, and for the development of π-conjugated semiconductors altogether. Transition metal-catalyzed cross-coupling reactions like Suzuki-Miyaura coupling,23, 24 Migita-Stille coupling,25 Kumada-Corriu coupling,26, 27 Negishi coupling28 etc. have been successfully used in conjugated polymer synthesis over the years. For copolymerization using them, two monomers contain two organometallic reactive sites and two halogenated reactive sites, respectively (Scheme 1a). These reactions have been and continue to be used for polymerizations owing to their excellent chemo- and regioselectivities, high yields and high degrees of polymerization. However, they require prefunctionalization steps to afford the organometallic and halogenated monomers. For principles of sustainability, the generation of stoichiometric amounts of metalated wastes is also undesirable. Moreover, requirements such as the use of boronic acids or esters for Suzuki coupling, highly toxic stannylated compounds in Stille coupling, air-sensitive organometallic reagents in Stille and Kumada coupling are not ideal for any polymerization reaction. Many of these issues were addressed with the introduction of direct arylation methods in polymer synthesis. Two C−H bonds of a simple arene monomer are activated using transition metal catalyst (mostly Pd) to react with another dihalogenated arene monomer to obtain the alternating copolymer (Scheme 1b). Last decade's research on conjugated polymer synthesis showed big growth in the area of direct arylation polymerization (DArP), reflected in several review articles.29-34 Although avoiding organometallic reagents is good progress, DArP still needs an aryl halide as one of the monomer units. An ideal scenario for a step economic, cost-effective, widely applicable, and green technique for the synthesis of conjugated polymers would be the use of non-functionalized π-units (eg, simple arenes, alkenes) where C−C bond formation would take place simply from C−H bonds of the monomers.
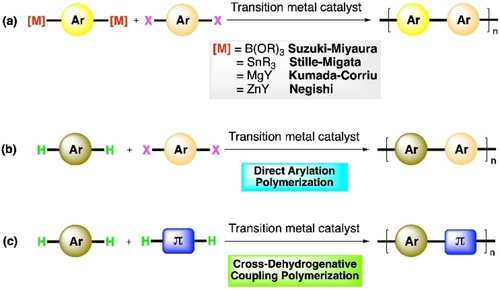
General outline for a) traditional C−M/ C−X cross-coupling polym-erization, b) direct arylation polymerization, c) cross-dehydrogenative coupling polymerization.
Oxidative C−H/C−H coupling or cross-dehydrogenative coupling (CDC) reactions can efficiently lead to C−C bond formation between two different hydrocarbons. This twofold C−H functionalization strategy has been comprehensively demonstrated in small molecule transformations for more than a decade now, which have been documented in various review articles.35-41 It has found application in the synthesis of natural products and pharmaceuticals.42, 43 Direct cross-coupling of two different π-monomeric units without any prefunctionalization of their C−H bonds is presumably an ideal way of constructing π-conjugated alternating copolymers including donor-acceptor polymers, where transition metal-catalyzed cross-dehydrogenative coupling polymerization comes into play (Scheme 1c). While there are many convincing reports on oxidative homopolymerizations, copolymerization using CDC protocol is still at its early stages. Initial attempts surfaced only a few years ago, not always with ideal results.44, 45 Nevertheless, there is slow but steady progress with increasing efforts in this field. Any review article is yet to be written exclusively on CDC polymerization, probably because of the limited body of work. However, we feel it is imperative to capture the sprouting of this new and promising branch of research on conjugated polymer synthesis. Our focus in this article will be on documentation of the early trends of CDC polymerization with the intent of exposing its potential to a wider community of synthetic and material chemists.
The discussions will be divided in two parts, based on the types of the monomer units being coupled. In the first part, we will focus on CDC polymerization reactions where both monomer units are arenes or heteroarenes. Two successive C−H activation steps are operational in this case, while the inversion of selectivity of two (hetero)arenes towards first and second C−H activation steps is a prerequisite (Scheme 2). Different catalyst systems as well as different electronic natures of the monomers usually control the orthogonal reactivity to furnish the alternating copolymers minimizing the homocoupling defects. The second part of the discussion will be based on cross-dehydrogenative alkenylation polymerizations, where one (hetero)arene monomer unit reacts with an alkene monomer unit. This is primarily based on Fujiwara-Moritani reaction,46, 47 commonly known as dehydro-genative Heck reaction. The reaction starts with the activation of a C−H bond in (hetero)arene to form the metal-(hetero)arene complex, which undergoes insertion to the alkene, followed by β-H elimination to give the cross-coupled product (Scheme 3). PdII catalyst is used as an example in Scheme 2 and Scheme 3, as majority of these polymerization reactions are reported with PdII catalyst. But some other catalysts have also proven to be efficient in CDC polymerizations, which will also be discussed here. In both the sections, discussions on polymerization reactions are preceded by representations of some relevant small molecule reactions. The objective of this minireview is to reflect on the continuity of the process of developing new CDC polymerization techniques so that new ideas can be reciprocated with systematic efforts in this budding field of research.
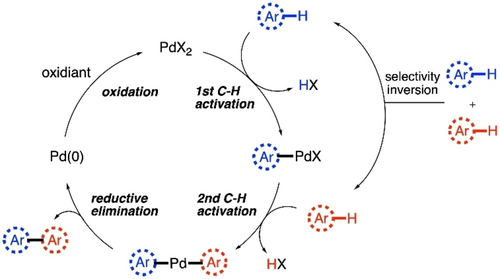
Basic catalytic cycle for CDC of two arenes via double C−H activation.
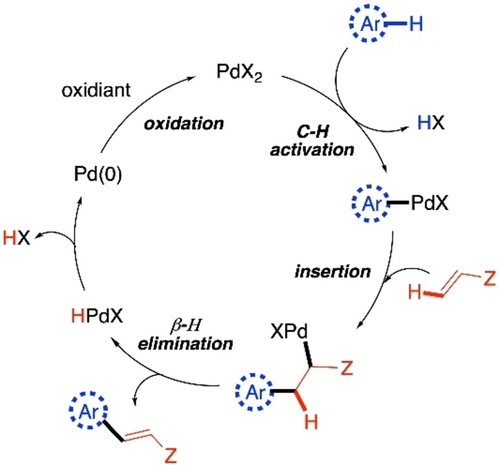
Basic catalytic cycle of cross-dehydrogenative alkenylation of an arene via C−H activation.
2 CDC Polymerization via Arene–Arene Coupling
CDC reactions between two unactivated (hetero)arenes can be perceived as the foundations of existing CDC polymerization methods. Hence, this section starts with some early and relevant examples of CDC reactions involving two-fold C−H activation. Aryl-aryl bond formation has had a long relationship with synthetic chemists due to the important features and applications of biaryl compounds.48-51 However, there was hardly any progress in CDC of two different arenes,52, 53 until Lu et al. reported a Pd(OAc)2-catalyzed cross-coupling of two simple arenes to synthesize unsymmetrical biaryls (Scheme 4).54 An excess amount of benzene compared to anisole, and a substoichiometric amount of trifluoroacetic acid (TFA) were essential for the cross-coupling reaction. With a higher concentration of TFA, or with a benzene:anisole ratio of amounts lower than 10 : 1, homocoupling of benzene, or of anisole became dominant. It was proposed that the electron-poor arene participated in the first C−H activation step, forming an Pd-aryl complex, its excess amount being responsible for the selectivity. Kinetic isotope experiments were also carried out (Scheme 5). A considerable effect in the case of benzene (Scheme 5b) indicates that the C−H activation of a relatively electron-poor arene has a greater influence on the entire reaction rate. Faster C−H activation of the electron-rich arene is likely the second C−H activation step in this reaction, as cross-coupling is favored over homocoupling.
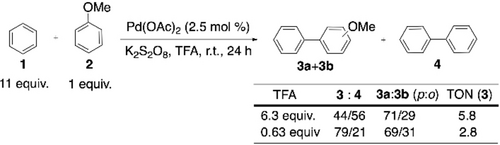
First Pd-catalyzed CDC of two simple arenes.
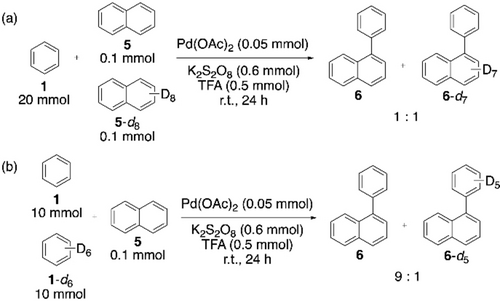
Kinetic isotope experiment for the CDC between benzene and naphthalene.
In 2007, the Fagnou group developed a Pd-catalyzed cross-coupling between N-acetylindoles and benzene derivatives using Cu(OAc)2 as an oxidant, where C3-arylation was major (Scheme 6).55 The homocoupling-free reaction led them to hypothesize a mechanistic duality of activation for two different arenes for the inversion of selectivity. They went on to establish regiocontrol for this reaction with proper choice of the oxidant.56 When AgOAc was used instead of Cu(OAc)2 as oxidant, with N-acetyl group changed to N-pivaloyl, C2-arylation predominantly took place. Based on the experimental results and earlier literature,57-59 a rationale for this regioselectivity switch was provided in this article. The group of DeBoef also published similar outcomes around the same time.60, 61
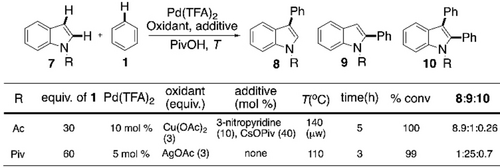
Pd-catalyzed regioselective CDC of indole and benzene.
This initial momentum was reflected in several reports on CDC reactions in the following years.62-65 Miura et al., in 2011, published a palladium-free method for coupling 2-arylazines with different azoles, using 5 equiv of Cu(OAc)2.66 In the next year, You et al. reported a Cu-catalyzed CDC of two different azoles.67 They found the use of 20 mol % Cu(OAc)2 along with Ag2CO3 and pyridine as optimum for this reaction. The reaction between benzothiazole and 1-methyl-1H-benzimidazole yielded 67 % of the cross-coupled product, even without using any one of the azoles in excess amount (Scheme 7a). This CDC strategy was applied in the synthesis of π-conjugated polymers.43 Apart from succeeding in the homopolymerization of benzodiimidazoles, they also synthesized copolymer P1 by CDC polymerization of two regioisomeric benzodiimidazoles, with even lower amount of Ag2CO3 (0.5 equiv) under O2 atmosphere (Scheme 7b). The copolymer was regiorandom with a low molecular weight (Mn=4100). The lack of regioregularity in P1 was also reflected in its low thermal stability and blue-shifted emission in polystyrene film. Nevertheless, it marked the entry of CDC polymerization protocol into the domain of conjugated polymer synthesis.
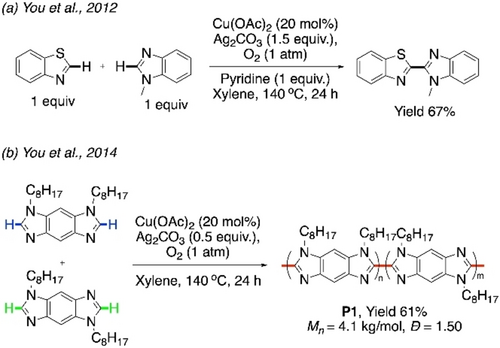
Cu-catalyzed a) CDC of benzothiazole and benzimidazole, b) CDC polymerization between two regioisomeric benzodiimidazoles.
The issue of regioregularity in CDC copolymerization was addressed by the group of Thompson in 2017.44 Two sets of random copolymers of hexyl thiophene-3-carboxylate (3HET) were synthesized with thieno[3,4-c]pyrrole-4,6-dione (TPD) and 4,4′-dimethyl-2,2′-bithiazole (BTz) monomers respectively (Scheme 8). High regioregularity could be accessed with low catalyst loading. At the same time, decreasing the catalyst load resulted in lower molecular weight of the polymer. An optimum of 3 mol % of PdCl2(PPh3)2 with the ligand PCy3HBF4 and oxidant Ag2CO3 was found to work the best in the solvent N,N-dimethylacetamide (DMA). Neodecanoic acid was effective in hindering the β-defect. Three different feed ratios of the primary monomer (3HET) to the secondary monomer (BTz or TPD) were tried for the polymerization (11:12 in Scheme 8). All the feed ratios were reflected in the polymer compositions without much deviation (m:n in Scheme 8). Molecular weight of the polymer decreased with the increasing proportion of the secondary monomer, probably due to the lower solubility of BTz and TPD in DMA. The NMR data indicated high regioregularities and good extents of cross-coupling in these polymerizations, which could be two takeaways from this report at that point of time.
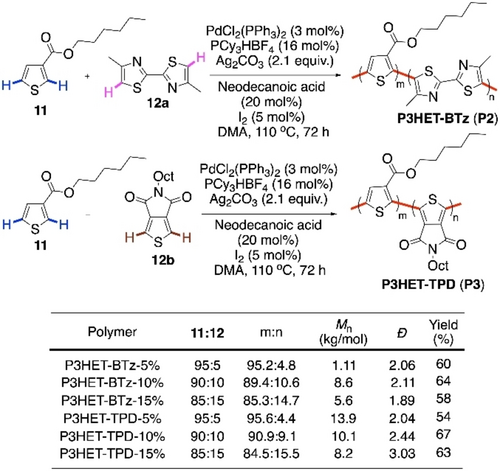
Ester-directed CDC polymerization of thiophene with bithiazole and thienopyrroledione.
Stronger sulfonyl directing group was used by the group of Chen.68 Pd-catalyzed direct C−H/ C−H coupling reactions were performed with four different monomers containing thiophene moieties to synthesize regioregular alternating copolymers P4, P5, P6, P7 without homocoupling defects (Scheme 9). One of the two monomer units in each case contained two sulfonyl ester groups which were useful for their electronic as well as steric properties. While the first C−H palladation was assisted by the sulfonyl group, another molecule of the same monomer was not favored for the second palladation due to the steric bulk of the sulfonyl group. Hence, the monomer without the bulky sulfonyl group was favored in the second step, which explains the exclusive cross-selectivity of this method.
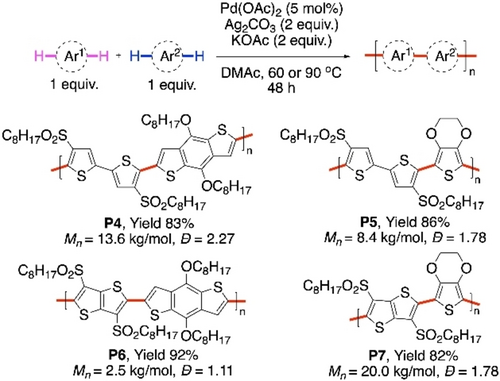
Sulfonyl group-directed CDC polymerization of different thiophene-containing monomers.
These early examples of CDC copolymerizations were far from being ideal. Accesing perfectly alternating copolymer without using directing group would be a more challenging and desirable goal. Promising results in that direction have indeed been obtained with the copolymerization of various thiophenes with perfluoroarenes as donors and acceptors respectively. To bring more clarity into the proceeding, early CDC reactions of polyfluoroarenes and thiophenes may be looked briefly. Su and co-workers, in 2010, reported an oxidative cross-coupling reaction between polyfluoroarenes and simple arenes using 10 mol % Pd(OAc)2 (Scheme 10, method A).69 Along with Cu(OAc)2 as oxidant, a combination of Na2CO3 as base and PivOH as the additive turned out to be optimum for the reaction. Substrate scopes suggested that steric factor mainly controlled the regioselectivity of the reaction. Contrary to earlier reports of electron-poor arenes used in large excess, comparatively electron-rich arene is used in excess for this method. Almost around the same time, Shi and co-workers reported a similar protocol for the same reaction, where they used Ag2CO3 as the oxidant and AcOH as the acid additive (Scheme 10, method B).70 Surprisingly, addition of diisopropyl sulfide, known as poison for Pd-catalysts, drastically improved the yield to 91 %. Acidity of C−H bonds in fluoroarenes were found to be crucial for the reactivity.
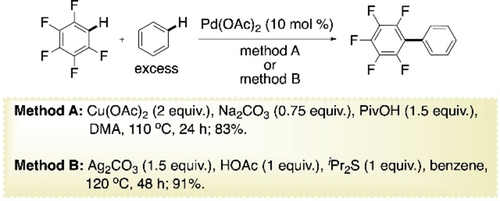
Pd-catalyzed CDC of benzene with pentafluorobenzene using Cu-oxidant (method A) and Ag-oxidant (method B).
Shi et al. carried out a set of control experiments including kinetic isotope experiments and H/D exchange experiment to elucidate the mechanism of this cross-coupling. Finally, they proposed a catalytic cycle for this CDC reaction (Scheme 11), according to which Ag2CO3-mediated deprotonation of the acidic C−H bond of the polyfluoroarene leads to the anion 12. Subsequent palladation forms the fluoroarylpalladium complex 13. Then, concerted metalation-deprotonation (CMD) pathway via transition state 15 was proposed for the C−H activation of the simple arene 14. The biarylpalladium complex 16 hence formed undergoes reductive elimination to furnish the cross-coupled product 17. Pd0-species generated gets oxidized by Ag-oxidant back to the PdII-species.
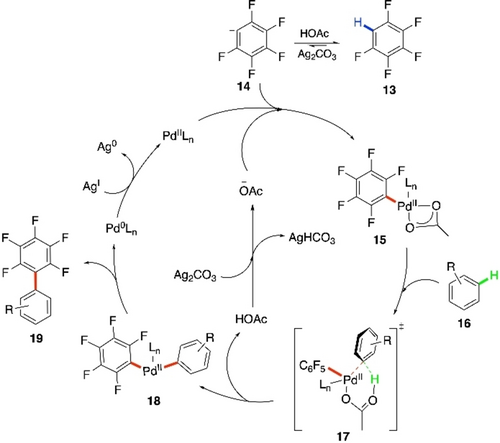
Proposed mechanism of CDC between pentafluorobenzene and simple arenes.
Zhang and co-workers reported a more efficient cross-coupling between pentafluorobenzene and 2-substituted thiophene (Scheme 12, method A).71 2.5 mol % Pd(OAc)2 was sufficient in presence of Ag2CO3 and AcOH. Little amount of dimethylsulfoxide (DMSO) present in dimethylformamide (DMF) (1 : 20) proved to be essential for a better conversion. It was encouraging in the prospect of polymerization to see the diarylation happening if both 2- and 5-poistions of thiophene were free. Less acidic 1,3,5-trifluorobenzene gave inferior results. This method was improvised using just 5 mol % of Ag2O in O2 atmosphere, replacing 1.5 equiv of Ag2CO3 (Scheme 12, method B).72 Various control experiments indicated a mechanism similar to Scheme 11. Homocoupling of either of the two arenes was not favorable under this condition.
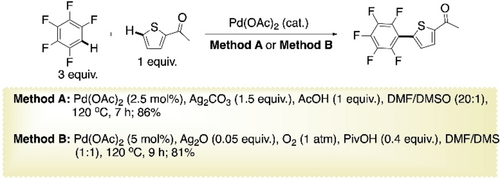
Pd-catalyzed CDC of pentafluorobenzene with 2-acetylthiophene.
The above reaction protocol was adopted by Kanbara et al. in 2018 for arguably the first highly efficient synthesis of donor-acceptor polymers via CDC polymerization without directing groups.73 Conditions in Scheme 12, method A were slightly modified for the copolymerization of octafluorobiphenyl with a bithiophene to prepare the donor-acceptor polymer P8 with a significantly high Mn of 53 400 (Scheme 13a, condition A). On addition of K2CO3, only 4 % homocoupling of bithiophene, with no homocoupled fluoroarene, was detected. The aerobic condition of Scheme 12, method B was also conducive to this polymerization, but with a lower molecular weight (Scheme 13a, condition B). However, this polymerization strategy was not effective in case of tetrafluorobenzene due to its volatility under the reaction temperature. Very recently, the same group reported a one pot-two step method to solve this problem.74 Ag-tetrafluorobenzene complex was prepared at room temperature in the first step. Then the bithiophene and the Pd-catalyst was added to the reaction mixture. Negligible amount of cross-coupling was observed. When the donor monomer was changed to a cyclopentadithiophene, the alternating copolymer P9 was afforded with a lower yield of 28 % and molecular weight of 14 600 (Scheme 13b). Polymer P8 exhibited light green photoluminescence and performed as emitting material in an OLED device. However, eight fluorine atoms in the acceptor units probably caused restricted conjugation, leading to relatively large optical band gaps in both P8 and P9.
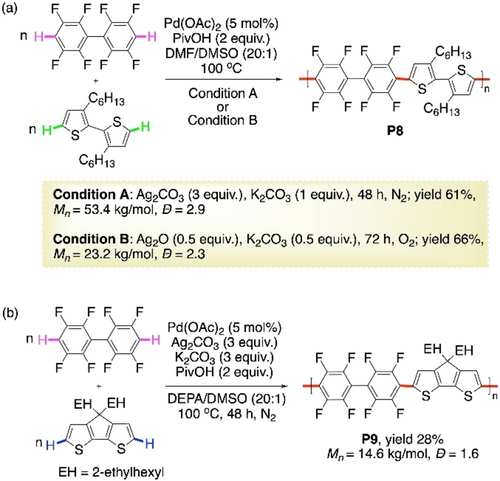
Pd-catalyzed CDC polymerization of octafluorobiphenyl with alkylated a) bithiophene and b) cyclopentadithiophene.
The problem of restricted conjugation was addressed in a follow up work by the group of Kanbara next year.75 A planar aromatic unit was inserted between two tetrafluorophenyl units, eventually furnishing an alternating copolymer with an acceptor and two different donor units (Scheme 14). A two-step CDC strategy was employed, the first step being the synthesis of monomer 20 and the polycondensation as the second step. The conditions of Scheme 12, method A were applied for the cross-coupling, changing the solvent to only DMSO, to afford the monomer 20. Then the protocol of Scheme 13, condition A was applied for the polycondensation between 20 and 3,3′-dihexyl-2,2′-bithiophene. The polymer with three different aryl units, P10, was afforded with less than 3 % homocoupling of bithiophenes. An increased coplanarity led to a reduced optical band gap in P10 than in P8.
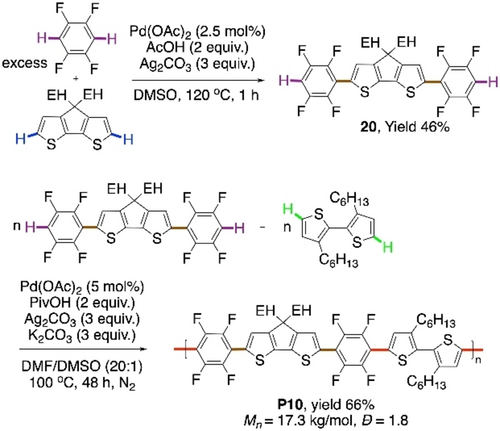
CDC polymerization with two donor and one acceptor units
Intriguing results of their CDC polymerizations led them to the detailed mechanistic study of the CDC reaction between fluoroarene and thiophene.76 Based on various model reactions and kinetic analyses, an updated Pd/Ag-dual catalytic cycle was proposed (Scheme 15). It states that Ag-mediated C−H activation of perfluoroarene 21 is the first step, where Ag-intermediate 22 enters the PdII/Pd0 catalytic cycle. Then Pd-complex 23 is formed via transmetalation, which is supposedly in equilibrium with the homo-biaryl-palladium(II) complex 24. This biaryl complex is perceived to be the responsible resting state for preventing the homocoupling of perfluoroarene. It can get back to monoaryl-Pd complex 23 through Ag-mediated ligand exchange. Complex 23 then activates the C−H bond of thiophene 25 through a CMD pathway to form the hetero-biaryl-palladium(II) intermediate 26, which on reductive elimination leads to the cross-coupled product 27. Pd0-species is reoxidized to PdII-species by Ag-salt to carry on the catalytic cycle. C−H activation of the thiophene was found to be the rate-determining step in this reaction.
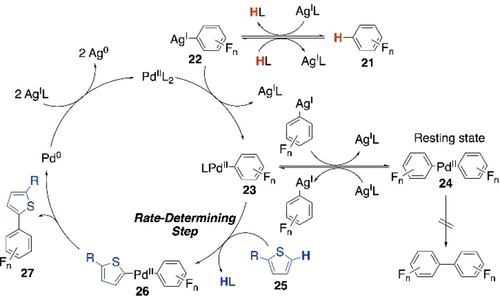
Plausible Pd/Ag-dual catalytic cycle for the CDC of thophene and perfluoroarene.
Although it was a big disclosure by Kanbara's group, it only dealt with the first cross-coupling reaction during the polymerization. But the chain extension steps can also behave differently from the first step in polymerizations.77-81 Our recent studies on the above polymerization reaction confirmed that it violates the Carothers equation due to a rate acceleration in the chain extension step.82 We started with a set of small molecule model reactions to follow the reaction patterns for the first and the second cross-coupling reactions of the polymerization (Scheme 16). Unlike the poor outcome of the first cross-coupling (Scheme 16a), the second cross-coupling of the dimer 32 with 2-methylthiophene showed outstanding reactivity and cross-selectivity (Scheme 16b). Similar patterns were observed when the monomer 35 reacted with 2-methylthiophene, the trimer 33 was preferentially formed over the dimer 32 and the homocoupled thiophene 31 (Scheme 16d). Ineffectiveness of the pentafluoroaryl-substituted thiophene dimer is clear from Scheme 16c and Scheme 16e. Hence, this model reactions suggest that the chain extension on the thiophene-substituted polyfluoroarene is accelerated compared to the first cross-coupling reaction in this CDC polymerization. Stepwise sampling experiments indicated a silver-mediated C−H activation of the dimer 32, a transmetalation from Ag to Pd and formation of Pd-dimer complexes, Pd-(32)2 being the major one (Scheme 17).
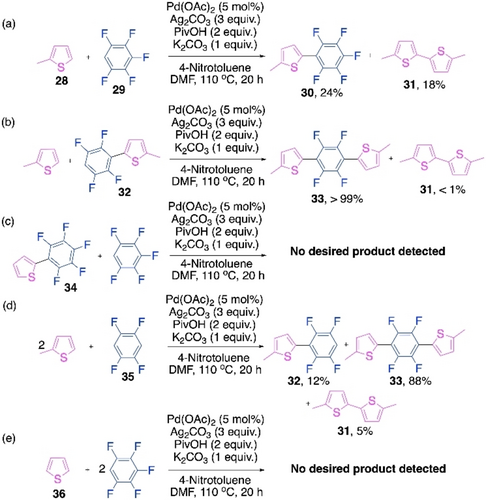
Small molecule model reactions for Pd/ Ag-cocatalyzed CDC polymerization of thiophene and perfluoroarene.
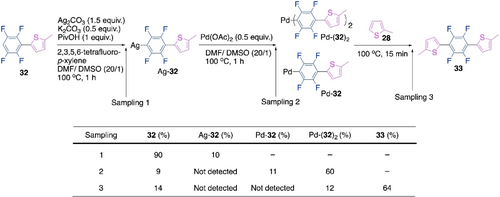
Stepwise sampling experiments to determine the mechanistic sequence of the chain extension CDC in the Pd/Ag-cocatalyzed polymerizations.
Further, controlled stepwise sampling experiments were performed which validated the mechanistic sequencing of Scheme 17. Although, C−H activation of thiophene was rate-limiting in the first CDC reaction (Scheme 15), kinetic analyses showed that Ag-mediated oxidation of Pd0 to PdII was rate-limiting in the chain extension step. Moreover, Density Functional Theory (DFT) calculations suggested that the activation energy barrier for the C−H activation of thiophene 28 was lowered due to its stronger interaction with Pd of the Pd-complex Pd-32 in presence of a thienyl substituent on the polyfluoroaryl ring. This is the probable reason for the accelerated chain extension step. Based on all these observations, we proposed the plausible mechanism of the chain extension step for this CDC polymerization (Scheme 18). This mechanistic sequence is similar to Scheme 15. However, this finding on rate-accelerated chain extension CDC was crucial in the explanation of the high molecular weight and perfectly alternating nature of the synthesized polymer.
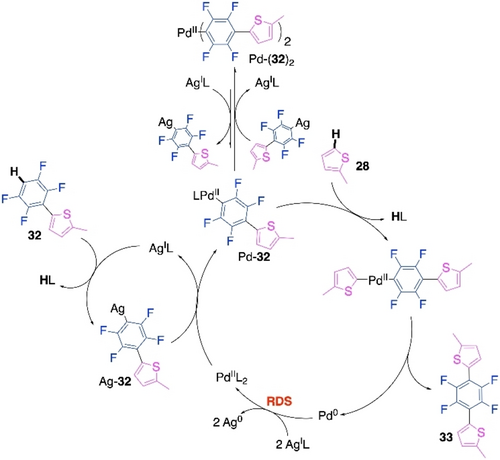
Proposed mechanism of the chain extension CDC of the Pd/Ag-cocatalyzed polymerization.
Donor-acceptor conjugated polymers made of thiophene and benzotriazole are known to have useful semiconducting properties.83-86 Recently, Lu and co-workers have reported CDC polycondensation of 5,6-difluorobenzotriazole with different thienyl donor units to synthesize a series of alternating copolymers P11-17 (Scheme 19).87, 88 The highest number average molecular weight was obtained for P13 (Mn=28.1 kg mol−1) with 82 % yield. The same polymer synthesized via Stille coupling had a lower molecular weight (Mn=23.3 kg mol−1). High cross-coupling selectivities were also obtained. Unwanted cross-linking was suspected by the authors as the reason for poor yield of P14, while insoluble oligomeric solids were obtained as P16 and P17.
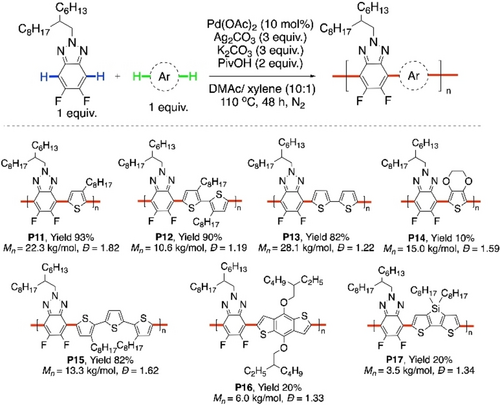
Pd-catalyzed CDC polymerization of 5,6-difluorobenzotriazole with various thiophenes.
In 2015, Larrosa et al. reported the first Au-catalyzed CDC reaction of between two different (hetero)arenes.89 There was an example of CDC reaction between pentafluoroarene with 2-methylthiophene using 5 mol % PPh3AuCl in that report (Scheme 20). Concept of this CDC was adopted by our group to perform the first Au-catalyzed CDC polymerization reaction.79 Dihexylbithiophene was reacted with tetraflurobenzene or octafluorobiphenyl to get the polymers P18 or P19, respectively (Scheme 21a and 21b, respectively). Amount of AgOPiv used was changed to 2 equiv from 35 mol % in Larrosa's report for a better result. Yields and the molecular weights were better for P19. However, homocoupling defect was quite prominent, and the percentages of alternating units were only 69 % in both the cases.
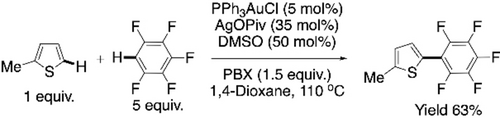
Au-catalyzed CDC between 2-methylthiophene and pentafluorobenzene.
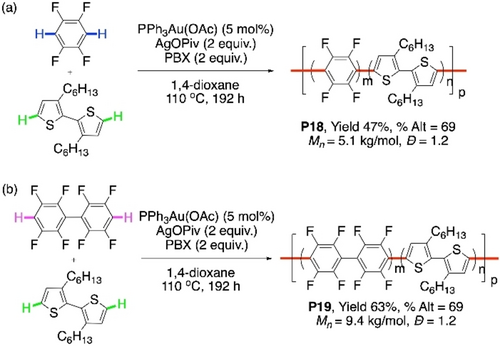
Au/Ag-cocatalyzed CDC polymerization of bithiophene with a) tetrafluorobenzene and b) octafluorobiphenyl.
Based on the results of small molecule model reactions and control experiments, a unique Au/Ag-dual catalytic pathway was proposed for the model CDC reaction of pentafluorobenzene with 2-methylthiophene (Scheme 22). It was proposed that C−H bond of pentafluorobenzene is activated by AgI species, while AuIII species activates the thiophene C−H bond. This Au/Ag-dual catalytic pathway was validated by a detailed mechanistic study a year later.90
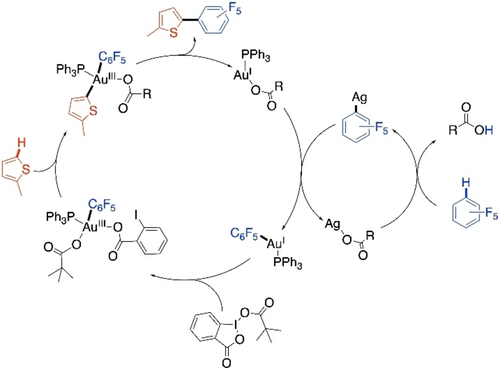
Proposed Au/Ag-dual catalytic cycle for the CDC of 2-methylthiophene and pentafluorobenzene.
3 CDC Polymerization via Arene–Alkene coupling
Poly(arylenevinylene)s are another class of π-conjugated polymers with promising semiconducting properties.91-93 Synthesis of these polymers can also be realized using cross dehydrogenative coupling between a simple arene monomer and an alkene monomer. In this regard, the rhodium-catalyzed dialkenylation reactions reported by Miura et al. in 2009 and Huang et al. in 2014 were among the early inspirations.94, 95 Pyrazole and pyrimidine were used as the directing groups for activating two ortho C−H bonds of benzene and pyrrole respectively (Scheme 23a and b, respectively). In 2016, Kanbara and co-workers utilized this strategy for the synthesis of poly(arylenevinylene)s via CDC polymerization.96 1-(2-Pyrimidinyl)pyrrole reacted with diethynylated fluorene to give the regioregular alternating copolymer P20 (Scheme 24). Other combinations of monomers were used, including 2-pyridinyl as directing group, to synthesize the polymers P21, P22, P23 and P24. Notably, a high number average molecular weight of 55800 was obtained for P21.
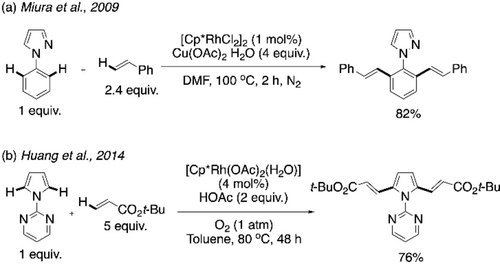
Directing group-assisted Rh-catalyzed cross-dehydrogenative dialkenylation of a) benzene and b) pyrrole.
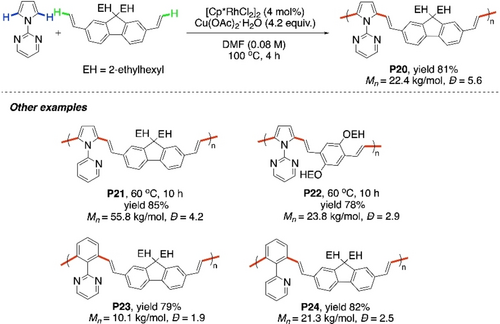
Rh-catalyzed CDC polymerization of pyrimidinylpyrrole with diethynylated fluorene.
In 2010, Zhang et al. reported alkenylation of arenes without using directing groups.97 C−H acidity of pentafluorobenzene was utilized for the CDC with styrene under the catalysis of Pd(OAc)2 (Scheme 25a). This strategy devoid of the use of directing group is obviously more attractive for making a polymer. The group of Kanbara reported similar a CDC protocol to synthesize the polymer P25 with modest yield (Scheme 25b).98 Reagents and conditions of Scheme 25a were modified. An alternating copolymer with trans-configuration was obtained with less than 3 % homocoupling defect. P25 showed strong light-blue photoluminescence in thin film with high quantum yield.
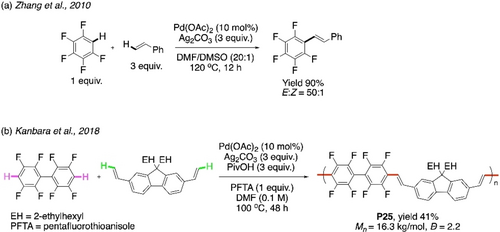
Directing group-free a) CDC between pentafluorobenzene and styrene, b) CDC polymerization of octafluorobiphenyl with diethynylated fluorene.
CDC alkenylations between dithiophenyldiketopyrrolopyrrole (DTDPP) and divinylateddialkoxybenzenes were carried out by using 10 mol % Pd(OAc)2 in the presence of Cu(OAc)2⋅H2O and pyridine to furnish the polymers P26 and P27 (Scheme 26).99 Relatively higher Mn of P27 is due to its higher solubility caused by the branched alkyl chain. Low optical energy band gaps (1.38 eV and 1.36 eV for P26 and P27, respectively), are quite promising for their usefulness.
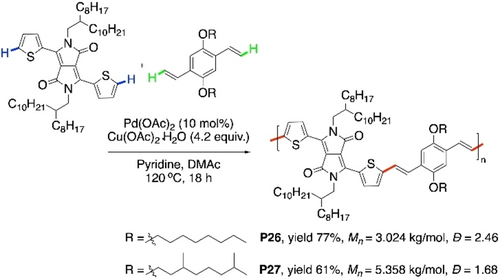
Pd-catalyzed CDC polymerization of DTDPP with divinylated arenes.
A Rh-catalyzed redox-neutral CDC polymerization of phenylhydrazine 40 with azobenzene acrylate 41 to furnish the polymer P28 (Scheme 27a) was recently reported.100 Axially chiral monomers R-42 and S-42 were also used to get the polymers R-P29 and S-P29 with retaining chiral identities (Scheme 27b). N-amino groups in 40 play the roles of both directing group and internal oxidant.
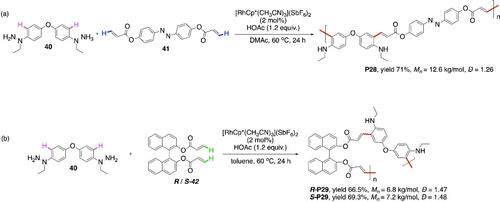
Rh-catalyzed CDC polymerization of phenylhydrazine 40 with a) diacrylate 41 and b) chiral acrylate R- or S-42.
Recently, Nakamura et al. reported an iron-catalyzed strategy for copolymerization of various aromatic bisthiophenes and bisenamines via Fujiwara–Moritani type reaction. (Scheme 28).101 Milder oxidant diethyloxalate was sufficient, perhaps due to lower redox potential of FeIII/FeI system, compared to PdII/Pd0 cycle. Unique enamine-containing conjugated polymers P30–35 were synthesized in good to excellent yields. FeIII-catalyzed thienyl C−H activation via σ-bond metathesis was proposed for the CDC.
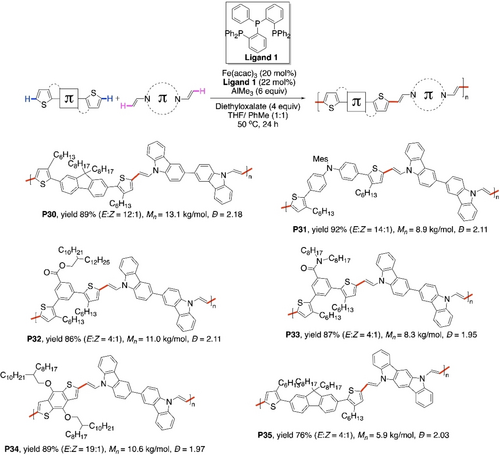
Fe-catalyzed CDC polymerization of thiophenes with enamines.
4 Summary and Outlook
In the quest for new and efficient methods for synthesizing semiconducting π-conjugated polymers, CDC polymerization via C−H activation is the latest venture. Additional steps of prefunctionalization in traditional cross-coupling and DArP methods have been circumvented. Use of simple arenes and alkenes as monomers renders the possibility of accessing wider varieties of conjugated polymeric structures via CDC polymerization, avoiding unwanted end-groups affecting the properties of the polymers. Although it took a while to adapt the metal-catalyzed CDC strategy in polymerization reactions, it has gradually thrived in the past few years.
Majority of the reports highlight the use of Pd-catalysts and Ag-oxidants. Limited examples of Cu-, Au-, Rh- and Fe-catalysis are there as well. Mechanistic analyses established the catalytic role of Ag-salts in the C−H activation of electron-poor arenes as well. Selectivity of the C−H bond is guided either by the intrinsic electronic and steric nature of the monomer or by using directing groups. Orthogonal reactivity of two arene monomers towards the catalyst system is utilized for cross-selectivity prerequisite for a donor-acceptor polymer. On the other hand, poly(arylenevinylene)s are afforded via Fujiwara–Moritani type CDC polymerization reactions.
Although Mn up to 55 800 has been achieved, inconsistencies in making high molecular weight polymers remain in CDC polymerizations. Homocoupling defects are prevalent in many of the reports. Overcoming these limitations requires more careful studies on the roles of different ligands and solvents in controlling reactivity and selectivity, which have not been well explored unlike in DArP. The requirement of more than stoichiometric amounts of Ag- or Cu-salts is a drawback in an otherwise atom-economic process such as this, and future studies should focus on the use of alternative oxidants such as air. Comparative studies on the structure-function relationships of semiconducting polymers prepared via CDC polymerization with those made by traditional cross-couplings or DArP are not there yet. Reports of Cu- and Fe-catalyzed CDC polycondensations are promising in moving toward more sustainable catalytic systems beyond Pd. We believe focused review articles like this will help in expanding the research on CDC polymerization beyond only a few groups working on it at present. With the exploration of new monomers, greener and more efficient solvents, and catalysts, this highly atom- and step-economic polymerization technique may take center stage in the production of semiconducting polymeric materials.
Acknowledgments
We thank Olivier Fernandez (Okinawa Institute of Science and Technology) for his help in designing the frontispiece. Funding from the Okinawa Institute of Science and Technology and JSPS KAKENHI Grant Number JP22K20547 is gratefully acknowledged.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Christine Luscombe was born in Kobe, Japan. She completed her PhD in Chemistry at the University of Cambridge and became a post-doctoral researcher at UC Berkeley. She was a faculty member at the University of Washington for 15 years and moved to the Okinawa Institute of Science and Technology, Japan, in 2021. Her research focuses on the development of conjugated polymers.
Biographical Information
Baitan Chakraborty was born in Kolkata, India. He completed his PhD in Chemistry from Jadavpur University, India in 2021 under the supervision of Prof. Umasish Jana. He is currently working as postdoctoral researcher with Prof. Christine K. Luscombe at Okinawa Institute of Science and Technology, Japan. He is currently working on cross-dehydrogenative polymerization for conjugated polymer synthesis.