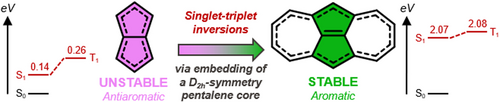
Symmetry-Induced Singlet-Triplet Inversions in Non-Alternant Hydrocarbons**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2022-ghmvg).
Graphical Abstract
Abstract
Molecules with inversion of the singlet and triplet excited-state energies are highly promising for the development of organic light-emitting diodes (OLEDs). To date, azaphenalenes are the only class of molecules where these inversions have been identified. Here, we screen a curated database of organic crystal structures to identify existing compounds for violations of Hund's rule in the lowest excited states. We identify two further classes with this behavior. The first, a class of zwitterions, has limited relevance to molecular emitters as the singlet-triplet inversions occur in the third excited singlet state. The second class consists of two D2h-symmetry non-alternant hydrocarbons, a fused azulene dimer and a bicalicene, whose lowest excited singlet states violate Hund's rule. Due to the connectivity of the polycyclic structure, they achieve this symmetry through aromatic stabilization. These hydrocarbons show promise as the next generation of building blocks for OLED emitters.
Hund's rule stipulates that for a given electronic configuration, the one with the highest spin is the lowest in energy.1, 2 This rule is broadly applicable across organic molecules with closed-shell ground states, where the excited singlet states will have higher energy than the triplets of the same electron configuration (Figure 1a). Given the first excited triplet state (T1) is always thermodynamically favored over the first excited singlet state (S1), Hund's rule constitutes a fundamental limitation for the efficiency of molecular emitters. The energetic inversion of the S1 and T1 excited states illustrated in Figure 1b is a uniquely desirable property for organic light-emitting diode (OLED) materials, as fluorescence from the S1 state is no longer impeded by population transfer to the T1 state. This process has very recently been demonstrated experimentally by Aizawa et al.3 and shows great promise in the design of next-generation thermally-activated delayed fluorescence (TADF) emitters whose fluorescence rates are no longer limited by spin statistics.4-6
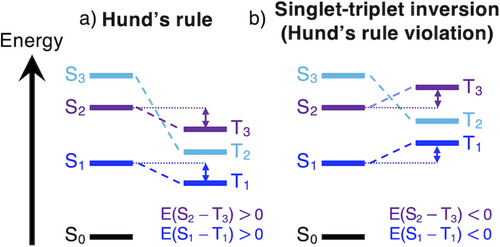
Schematic of three excited states, color-coded to indicate state pairs of identical electron configuration. a) Three pairs of excited states that obey Hund's rule, i.e., each triplet is of lower energy than the singlet of the same configuration. b) The S1 and S2 states violate Hund's rule by having lower energy than the equivalent triplet states, T1 and T3.
Until recently, Hund's rule violations were restricted to unstable diradicals,7, 8 charge-transfer excited-states of large molecules,9, 10 and to cases where strong light-matter coupling is achieved.11, 12 This changed when negative S1-T1 gaps (E(S1−T1)<0) were theoretically identified in cycl[3.3.3]azine and its aza-substituted analogs by Ehrmaier et al.13 and de Silva.14 While a number of analogous azaphenalenes had been studied experimentally since the 1980s due to their photophysical properties15, 16 and later as TADF emitters by Li et al.,17, 18 the possibility of higher-order Hund's rule violations had been overlooked.19-21 The 40 year gap between the discovery of azaphenalenes as inverted gap molecules and their implementation in devices, despite all being in the same molecular family, highlights the scarcity of compounds with singlet-triplet inversions.22
With the prospect of superior emissive properties, it is imperative to find new classes of molecules with singlet-triplet inversions. Given the historic lack of focus on the possible existence of Hund's rule violations among excited states, and because inverted singlet-triplet gaps can only be modelled using high-level quantum chemical methods,14, 23-28 we suggest that there may be many cases of excited state singlet-triplet inversions among already synthesized molecules. Such molecules would provide an excellent starting point for future computational and synthetic efforts in the design of molecular emitters.
Here, we focus on vertical excitations as an initial molecular screening step, while acknowledging that adiabatic relaxation would be required for a complete picture. For a molecule in the S1 state, adiabatic relaxation followed by fluorescence is likely to be a more efficient decay pathway than intersystem crossing if the lowest triplet is thermodynamically disfavored. We mine a dataset of existing crystal structures to identify compounds which exhibit violations of Hund's rule in the lowest excited states through sequential refinement of the geometry and excitation energies with increasingly precise computational methods (Figure 2). We have recently introduced a curated database tailored to materials design applications, the Fragment-Oriented Materials Design (FORMED) dataset,29 consisting of over 117 000 experimentally-reported organic crystal structures and their associated optical properties computed with Tamm-Dancoff-approximated time-dependent density functional theory (TDA-TDDFT) using ωB97X-D/6-31G(d) and geometries with GFN2-xTB30 (Figure 2, top box).
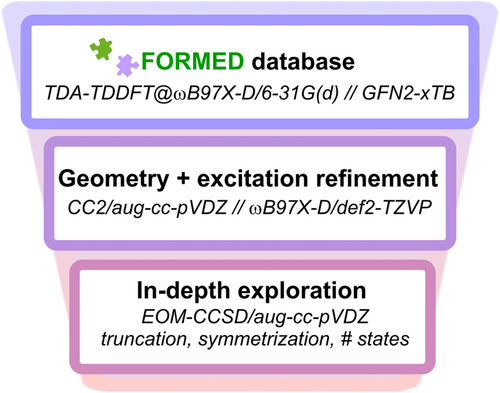
Three-step protocol for screening compounds in the FORMED database (top) for excited-state violations of Hund's rule with increasingly complex methods.
The screening procedure illustrated in Figure 2 starts with a pre-selection of the molecules in the FORMED dataset based on E(S1−T1) (TDA-TDDFT level) and to the extent necessary prioritized by smallest number of atoms in the molecule (Figure S1).31 We optimize the select 1514 geometries at a tighter level (ωB97X-D/def2-TZVP) and recompute the excited states at the second-order approximate coupled-cluster level (CC2/aug-cc-pVDZ); Figure 2, middle box. The states are then paired according to their electronic configuration in order to identify possible Hund's rule violations between the lowest (S1 and T1 in Figure 1b), or alternatively between higher-lying excited states (e.g., S2 and T3 in Figure 1b). The 54 molecules we identify with negative or near-degenerate gaps among the first three singlet excitations are explored at the equations-of-motion coupled-cluster level (EOM-CCSD/aug-cc-pVDZ); Figure 2, bottom box (see Section S1 for details).31
From this virtual screening protocol, we identify three potential classes of materials which exhibit Hund's rule violations among the first three excited state singlets, Figure 3. The first of these belong to the well-established azaphenalene family.3, 13-16, 32-34 TAZCAZ35 and the methyl-substituted analog COSFIZ,36 are penta-azaphenalenes with negative E(S1−T1). State diagrams are shown in Figures S9–S10. The utility of the screening protocol is validated by our ability to recover compounds structurally similar to those for which S1-T1 inversions are known from both experimental and computational studies.25, 26
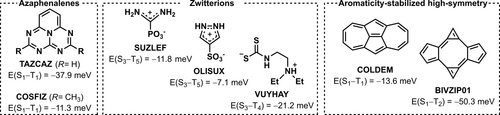
Survey of three classes of compounds exhibiting Hund‘s rule violations identified through screening: azaphenalenes, zwitterions, and polycyclic aromatic hydrocarbons with high symmetry induced by aromaticity. The energy splitting labels correspond to singlet-triplet pairs of the same configuration. Structures are labeled according to their Cambridge Structural Database codes. Excitation energies were obtained with EOM-CCSD/aug-cc-pVDZ using ground state geometries optimized at the ωB97X-D/def2-TZVP level.
The second class comprises small zwitterionic compounds with Hund's rule violations among the higher-lying excited states. SUZLEF37 and OLISUX38 consist of a positive charge delocalized over protonated aminoiminium and pyrazole rings balanced out by phosphonate and sulfonate, respectively, while VUYHAY is the zwitterion of the fully non-conjugated N-(2-diethylaminoethyl)dithiocarbamic acid.39 State diagrams are provided in Figures S11–S13. Furthermore, several molecules in this class are almost gapless with state-paired E(S1−T1)<10 meV in the form of AMMCHC11, ROFJAY, TIRZAX, and VICGOE as listed in Table S1. We note that many zwitterionic compounds exhibit inverted or degenerate state-paired singlet and triplet excitations at the CC2/aug-cc-pVDZ level. These gaps often become slightly positive when the more precise EOM-CCSD/aug-cc-pVDZ method is employed, Table S1.
The third, and most promising class of compounds consists of fused polycyclic hydrocarbons containing odd-membered rings. We identify the dicyclohepta[cd,gh]pentalene COLDEM,40 a fused azulene dimer with negative E(S1−T1) of −14 meV with D2h symmetry, the state diagram of which is given in Figure S14. As with the azaphenalenes,13, 14 the S1 and T1 states are both described predominantly by HOMO→LUMO configurations. The highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbitals of COLDEM are spatially proximal, though non-overlapping: the HOMO is centered on the pentalene-like core, and the LUMO on the tropylium rings (Figure 4), thus minimizing the exchange interactions necessary for inverted singlet-triplet splitting. Both the 5- and 7-membered rings of COLDEM are aromatic, as evidenced by the respective nucleus-independent chemical shifts (NICS(1)zz) of −36 and −32 ppm, see Section S1 for details.41 While the 5-membered rings of COLDEM are slightly less aromatic than the equivalent rings in the parent azulene (Figure S3), the 7-membered rings are more aromatic than that of azulene. Due to the aromatic nature of the rings, COLDEM exhibits D2h symmetry with almost no bond-length alternation.
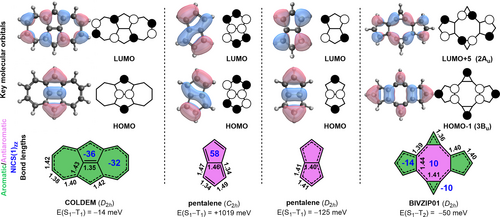
Key molecular orbitals, ground-state ring aromaticity (aromatic in green, antiaromatic in magenta) as determined from nucleus-independent chemical shifts (blue), and bond lengths (in Å, black) of ground-state COLDEM, the ground-state (C2h) and high-symmetry (D2h) geometries of pentalene, and ground-state BIVZIP01. Geometries were optimized at the ωB97X-D/def2-TZVP level; SCF orbitals and excitation energies were obtained at the EOM-CCSD/aug-cc-pVDZ level.
Given the unique electronic behavior of COLDEM, we examined the pentalene core to understand the origin of this inverted singlet-triplet splitting (Figure 4).42 Pentalene is a textbook Hückel antiaromatic system.43 Its C2h ground-state structure is a frequently-studied antiaromatic molecule (NICS(1)zz=58 ppm) with significant bond-length alternation,43-45 and a strongly positive E(S1−T1)=1018 meV. If the D2h symmetry of COLDEM is imposed on pentalene it achieves an inverted E(S1−T1)=−125 meV, but becomes strongly antiaromatic owing to its 8 π-electrons distributed in two symmetric rings.44 We do not assess D2h-pentalene further due to its inherent multireference character, see Figure S5. Pentalene sub-units in larger molecules often cause them to distort into lower symmetry through bond-length alternation to escape antiaromaticity. By virtue of aromatic stabilization due to the presence of the fused 7-membered rings, the pentalene core of COLDEM retains the highest possible symmetry. The similarity between the two is evident from the D2h symmetry, the alternating form of the HOMO and LUMO (Figure 4), and the concomitant singlet-triplet inversion (E(S1−T1)=−14 meV). And yet, unlike D2h-pentalene, COLDEM is not a sensitive case for static correlation, as revealed by fractional occupation number weighted electron density analysis (FOD, see Figure S5).46 With the pentalene core embedded within an aromatic ring system, COLDEM is a stable molecule that has been synthesized with47 and without substituents,40 and more recently in an annulated form.48
The high symmetry and non-overlapping nature of the orbitals involved in the S0→S1 excitation of COLDEM results in null oscillator strength ( ). For COLDEM derivatives to work as emitters, such functionalized molecules must achieve appreciable oscillator strengths. As an initial assessment, we carry out a cursory screening with amino (−NH2) and cyano (−CN) substituents on the available C−H sites of the COLDEM core. Both excitation energy and can be modulated through substitution effects while retaining the negative E(S1−T1) values (see Figure S6 and S7). These initial results reveal the potential for chemical optimization of the emissive properties of COLDEM. A combined computational and experimental approach was recently successfully applied by Aizawa et al.3 in the design of azaphenalenes-based emitters. To guide future synthetic efforts based on the COLDEM motif, a large-scale screening of more diverse substituents is needed to identify targets with appreciable fluorescence rates.
The excited state singlet-triplet inversion in COLDEM is driven by the D2h symmetry of the central pentalene unit, which is achieved by an aromatic stabilization of the pentalene core by the peripheral tropylium rings. Here, we have identified the singlet-triplet inversion in COLDEM through high-throughput screening of experimental molecules. However, COLDEM and pentalene were among several conjugated hydrocarbons proposed by Toyota and co-workers as possible Hund's rule violations in the 1980s,49-51 which have been overlooked in the recent literature. Given that this prior work was based on symmetry analysis and HF/STO-3G level calculations with a simplified configuration interaction scheme, we surveyed all proposed molecules from this earlier work at the EOM-CCSD/aug-cc-pVDZ level (Figure S16). Out of those suggested by Toyota and co-workers, the only other Hund's rule violation is bicalicene (see Figure 4). This structure also exists in our database in bare52, 53 and functionalized53 forms (Cambridge Structural Database codes BIVZIP01 and DUWXIC, respectively). These were beyond the cutoff of molecule sizes yet considered in our screening protocol.
The D2h-symmetry bicalicene BIVZIP01 has a positive gap between the lowest excited singlet and triplet states, but the configuration-paired E(S1−T1)=−50 meV constitutes a Hund's rule violation, as shown by the state diagram in Figure S15. BIVZIP01 consists of a calicene dimer forming a central 8-membered ring structurally reminiscent of cyclooctatetraene (COT) that is stabilized by the aromaticity of the peripheral cyclopentadienyl and cyclopropenium rings through a push-pull mechanism.54-56 The NICS-values and bond lengths of the 5- and 3-membered rings in the dimer are almost equivalent to those of the corresponding rings in the parent calicene (see Figure S3). The central COT-ring of BIVZIP01 is only weakly anti-aromatic (NICS(1)zz=10 ppm, Figure 4). As with COLDEM discussed above, the non-overlapping orbitals describing the S1/T2 states coincide with the HOMO and LUMO of D2h-pentalene, albeit separated from one another by the central COT-like ring and with additional features on the latter. The presence of the high-symmetry pentalene-like core of COLDEM and BIVZIP01 is the necessary requirement for the existence of the negative singlet-triplet gap in this class of molecules. We further assess the gaps of simplified analogs of the substituted bicalicenes, which all have inversion of the S1-T2 gap similar to BIVZIP01 (Figure S8). As with the COLDEM core, the Hund's rule violation in bicalicene is persistent under structural modification, and it is therefore a parent unit within the same D2h-pentalenic class as COLDEM. Finally, we note that OLISUX, in the zwitterionic class discussed above, is also highly symmetric as a result of its aromatic ring structure (see Figure S3).
COLDEM and BIVZIP01 are Hückel aromatic molecules in the ground state. Future work must address their fluorescent properties and excited state dynamics, which are potentially governed by antiaromaticity in the excited state.57, 58 Using excited-state aromaticity as a strategy to tune the energies has recently been applied successfully, for instance in the context of singlet fission.45, 59 We briefly assess the aromaticity of the T1 excited-state structures (Figure S4). As expected for a planar hydrocarbon with a Hückel aromatic ground state, COLDEM is Baird antiaromatic in the T1 excited state.60, 61 BIVZIP01 distorts into C2v symmetry and only one of the five-membered rings becomes Baird antiaromatic in the T1 state (Figure S4). A strategy based on excited-state aromaticity which lowers the state inversion to being between S1 and T1 may enable rational chemical design. In future screening efforts, it is also clear that the initial step (Figure 2, top) must consider higher-order Hund's rule violations than simply the S1-T1 gap, which is the subject of ongoing work.
In conclusion, we have reported two classes of molecules that violate Hund's rule in the excited state. The first class consist of zwitterions that exhibit violations in the higher excited states, and are therefore unlikely to be of practical use for OLED emitters. The second class of D2h-pentalenic molecules is comprised of high-symmetry non-alternant polycyclic hydrocarbons. These consist of the fused azulene dimer COLDEM and bicaclicene BIVZIP01 and their derivatives, which demonstrate significant potential for materials applications. Both these molecules have D2h-symmetry ground-state structures due to aromatic stabilization, which enables the higher symmetry pentalene moieties that are key to achieving singlet-triplet inversion. The S1-T1 states are inverted in COLDEM, and we show that the energies and oscillator strength of this compound are tunable via functionalization while retaining the inverted gap. We highlight that all of the parent compounds discussed here have been synthesized, and therefore constitute an excellent starting point for the synthetic design of new OLED emitters beyond azaphenalenes. We are currently applying our screening protocol to a broader range of compounds, and applying these symmetry-based design rules to new core motifs exhibiting excited-state singlet-triplet inversions.
Acknowledgments
The authors are grateful to the EPFL for financial support and the allocation of computational resources. J. T. B. and M. H. G. thank Dr. Daniel Jana for technical assistance. M. H. G. is grateful for funding from the Carlsberg Foundation (CF21-0202). We dedicate this paper to August Garner. Open Access funding provided by École Polytechnique Fédérale de Lausanne.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Materials Cloud at https://archive.materialscloud.org/record/2022.162, reference number 2022162.