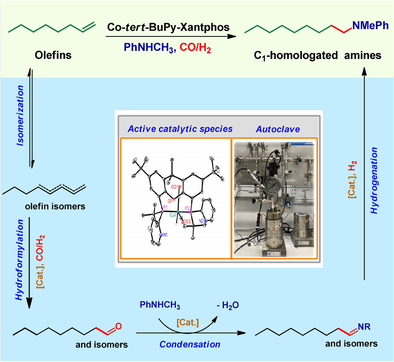
A Selective and General Cobalt-Catalyzed Hydroaminomethylation of Olefins to Amines
Graphical Abstract
Abstract
A new cobalt catalyst is presented for the domino hydroformylation-reductive amination reaction of olefins. The optimal Co-tert-BuPy-Xantphos catalyst shows good to excellent linear-to-branched (n/iso) regioselectivity for the reactions of aliphatic alkenes with aromatic amines under mild conditions. This system is far more selective than traditional cobalt(I) catalysts and even better than most known rhodium catalysts.
Carbonylation reactions of olefins constitute a versatile synthetic toolbox to synthesize aldehydes, carboxylic acid derivatives, alcohols, and amines in a straightforward and atom-efficient manner. The originally applied catalysts in these reactions were based on cobalt and nickel complexes, but nowadays more expensive rhodium and palladium catalysts prevail in industry for such transformations, which work at milder conditions and allow for much higher chemo- and regioselectivities. Among the different carbonylation reactions, the so-called hydroaminomethylation (HAM), which consists of a hydroformylation1-reductive amination domino process, allows for synthesizing amines in one step from olefins. Hence, this domino transformation aroused significant interest in industry to produce adhesives, coatings, paints, rubber chemicals, detergents, pharmaceuticals, crop protection agents and many more.2 Traditional approaches for the preparation of amines include reductive amination of carbonyl compounds,3 hydrogenation of nitriles or nitro derivatives,4 and classic nucleophilic substitution reactions of alkyl halides and similar substrates (Figure 1 a).5 Despite all these methods, the environmentally benign, atom-efficient, and selective synthesis of functionalized amines from easily available olefins continues to attract the interest of many researchers.6 Notably, the apparently simple direct addition of amines onto olefins is restricted to specific substrates or need drastic conditions.6a In contrast, hydroaminomethylation reactions work in a far more general manner. However until to date, catalysts for the latter transformation are based on noble metals, mainly rhodium complexes.7 Because of the scarcity of precious metals and increasing emphasis on sustainability, there is a strong interest to replace them by more available 3d-metals.8 In this context, especially cobalt-based catalytic systems come to mind as they are able to perform the essential hydroformylation reaction.1a, 9 Nevertheless, no such general process could be realized. Indeed, Bouwman, Bickelhaupt and co-worker stated very recently: “The use of cobalt-based catalysts in the HAM reaction (…) seems therefore not feasible”.10 Obviously, there are considerable challenges to realize the development of general cobalt-catalyzed hydroaminomethylations of alkenes (Figure 1 b). Both experiments and theoretical studies have shown that the kinetic barrier for the reductive amination step under hydroformylation conditions is high for representative cobalt catalysts.3b, 10, 11 In addition, competing alkene isomerizations mediated by Co-H species are typically faster than hydroformylation or reductive amination.6d, 1a, 11b Consequently, a mixture of isomeric olefins, aldehydes and alcohols is usually obtained. Finally, cobalt-based catalysts have limited functional group tolerance under the commonly applied reaction conditions, making any general methodology development difficult.9
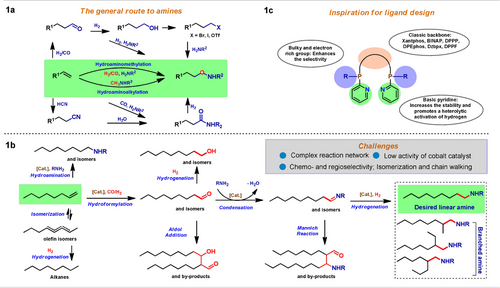
Catalytic hydroaminomethylation: Background and ligands development. a) Main known synthetic routes to aliphatic amines. b) Challenges and reaction network. c) Design of specific ligands for cobalt-catalyzed HAM.
To overcome all these limitations, we envisioned the development of a completely new cobalt catalyst with improved reactivity for reductive aminations under hydroformylation conditions. At the same time, the catalyst should suppress unwanted isomerization as well as other side reactions (Figure 1 c). Interestingly, several cobalt catalyst systems including BPE-phos, Duphos and Triphos have been used for hydrogenations recently;2b, 12 however, none of the reactions was performed in the presence of carbon monoxide. Notably, the presence of even small amounts of CO often completely shuts down the hydrogenation activity of homogeneous catalysts.13 Thus, for catalyst (ligand) design we speculated that multidentate phosphine ligands with both tert-butyl and 2-pyridyl groups in the phosphine fragment might overcome these limitations and synergistically promote the hydroaminomethylation (HAM) process. On the one hand, the electron-rich tert-butyl phosphine fragment increases the electron density of the cobalt center, which is beneficial for the oxidative addition of hydrogen to form the active Co-H species, while at the same time, the steric hindrance should be beneficial for selective reductive amination of the formed aldehydes. On the other hand, the 2-pyridyl group in the ligand scaffold can act as a proton shuttle for the formation of the metal hydride and the nitrogen atom is able to improve the durability of the catalyst via hemilabile coordination to the metal center in the catalytic cycle.14 Indeed, testing the combination of Co2(CO)8 (1 mol %) and more than 100 ligands, including commercial and own developed, at 100 °C, CO/H2=10/30 bar provided no successful catalyst system for the hydroaminomethylation benchmark reaction of 1-octene and N-methyl-aniline (Figure 2 and Scheme S1 in supporting information).

Model reaction and selected ligand effects.
Most of the used mono- and bisphosphines, e.g., triaryl-, trialkylphosphines, and privileged bidentate ligands (DPPP, Naphos, BINAP, Xantphos) showed basically no activity (<5 % of 3 aa). Surprisingly, in the presence of commercially available CataCXium, Sphos and Johnphos, more promising results for N-methyl-N-nonyl-aniline 3 aa were obtained (yield 4–17 %). Thus, several biaryl and aryl-heteroaryl phosphines were further investigated. Unfortunately, no superior catalyst system could be identified. Also testing of other commercially available Xantphos derivates did not improve the original results. Gratifyingly, introducing a Xantphos derivative with modified backbone (2,7-di-tert-butyl-9,9-dimethyl-4,5-bis(di-tert-butyl-phosphino)-xanthene, L9) enhanced the yield of the desired amine to 16 % (n/iso=93/7). Next, the effect of substituents on the phosphorous atom was explored. Recently, we have shown that tert-butyl-pyridylphosphino groups increase both activity and selectivity in palladium- and platinum-catalyzed alkoxy- and hydroxycarbonylations.15 In addition, it is known that the use of P,N-ligand improves the hydrogenation of imines in the presence of 3d metals.16 Thus, the ligand L10 (tert-BuPy-Xantphos) was prepared,17 and indeed gave an improved yield of 3 aa (24 %). While testing L11–12 with other substituents on the phosphorous center resulted both in less activity and lower regioselectivity. Even more interesting, this new catalyst system provided an excellent n/iso selectivity (99/1, Table S1) for the corresponding amine. To the best of our knowledge, this is the first highly regioselective hydroaminomethylation in the presence of a non-noble metal catalyst.
After suitable variation of important reaction parameters (temperature, solvent, partial pressure of H2/CO, and additives, Tables S2-S4), desired 3 aa was obtained in 70 % yield and 96/4 (n/iso) selectivity in the presence of 2.0 mol % Co-tert-BuPy-Xantphos complex (Co-1) at 120 °C and 40 bar CO/H2 (1:3) in MTBE (methyl tert-butyl ether) as solvent (Table S5).
To explain the origin of the high activity and regioselectivity utilizing the more hindered ligand L10 with amphoteric substituents on the P center several control experiments (Table S6,S7) and a kinetic profile of the model reaction (Figure S3) were completed. In conclusion, the observed performance can be explained as follows: (A) The initial Co-catalyzed hydroformylation step is positively influenced by the presence of N,N-dialkylaniline, which was used as a model for the amines present in the hydroaminomethylation protocol (Table S6,S7). (B) Notably, small amounts of water, which are generated from the condensation of the intermediate aldehyde with N-methyl-aniline improved the reaction still further. We speculate that water is beneficial for the formation of Co-H active species.18 Both these positive effects (amine and water) are reliable in the presence of the phosphine ligand. (C) Thus, the catalyst then hydrogenated the in situ-formed imine to give the final product. Notably, the catalyst mainly promotes the selective hydrogenation and not the condensation in this process. Furthermore, in the presence of the ligand, aldol side-reactions and unwanted hydrogenation of the starting olefin are avoided. (D) Comparing the reductive amination of the branched and linear aldehydes, the present catalyst shows a high selectivity for the latter one, which contributes to the high regioselectivity of the amine products observed.
Having an optimal cobalt catalyst in hand, we were interested in the generality and functional group tolerance of our system. As shown in Figure 3, diverse anilines gave the C1-homologated amines in good yields with high regioselectivities. Despite the low nucleophilicity, even N,N-diphenylamine provided the corresponding product 3 ad in 51 % yield. The parent compound aniline gave 3 ai in 53 % yield and 71/29 n/iso ratio with 13 % dialkylaniline byproduct 3 aj. Using other representative anilines with both electron donating group -OCH3 and electron withdrawing group -CF3 showed the functional group tolerance of this catalyst system. Furthermore, anilines with heterocyclic motifs, sterically hindered substituents as well as indoles underwent the desired transformation smoothly and the respective linear amines are obtained in moderate yield, e.g., 3 al-3 ap.

[a] General conditions: Olefin substrate (1.0 mmol), amine substrate (1.0 mmol), Co2(CO)8 (1.0 mol %), ligand L10 (2.0 mol %), solvent (2.0 mL), CO (10 bar), H2 (30 bar), 120 °C, 24 h; [b] reaction conditions: 1-octene (10 mmol), N-methyl aniline (10 mmol), Co2(CO)8 (0.5 mol %, 0.05 mmol, 17.1 mg), ligand L10 (1.0 mol %, 0.1 mmol, 65.2 mg), mesitylene (as internal standard for GC analysis, 5 mmol, 600 mg), Methyl tert-butyl ether (MTBE) as solvent (30 mL), CO (10 bar), H2 (30 bar), 120 °C, 24 h; [c] cobalt active complex Co-1 as catalyst (2.0 mol %); [d] 125 °C, 24 h; [e] Displacement ellipsoid plot of Co-1 (30 % probability level, without C-bound H and co-crystallized solvent).
Next, the hydroaminomethylation of a selection of olefins 1 ba-1 bx with N-methyl aniline was investigated. Aliphatic terminal alkenes ranging from 1-pentene to 1-octene gave the desired products in good yields (62–71 %) and excellent regioselectivities (n/iso=94/6). Interestingly, sterically bulky alkyl substituents are tolerated in the substrate, and the corresponding amines 3 bd-3 bg are obtained in similar good yields and selectivities. The selective synthesis of versatile amino alcohols without using protecting group chemistry is a challenging task. In this respect, the chemo- and regioselective transformation of unsaturated alcohol to amino alcohol 3 bl in 44 % yield (n/iso=90/10) presents interesting synthetic possibility. Importantly, diverse olefins with (thio)ether 1 bi-1 bk, silyl 1 bo,1 bp, and other functional groups including esters 1 bn, amides 1 bq, as well as a sensitive carbohydrate building block 1 bw as an example for bio-derivatization, are well tolerated by our protocol which thereby enables the straightforward synthesis of many interesting amines directly from ubiquitous olefins. In all these reactions the presented cobalt catalyst showed excellent performance, even compared to a state-of-the-art rhodium phosphine carbonylation catalyst (Table S8).19 Apart from the shown aliphatic olefins, also styrene was reacted with N-methylaniline. However, in this case apart from the desired hydroaminomethylation product (14 %), ethylbenzene is mainly formed (78 %) because of the easier hydrogenation.
To evaluate the potential of the presented Co catalyst system for practical applications, upscaling experiment was completed from a bulk industrial feedstock 1 ca (Figure 4 A). Gratifyingly, using 0.05 mol % of catalyst, product 3 ca was obtained in 73 % yield and >99 % selectivity on >100 g-scale. In addition, inspired by the observation of olefin isomerization, we investigated the reaction of octene mixture (C8) from industry. To our delight, 3 aa was obtained with an 62 % yield and 91 % regioselectivity (Figure 4 B). Finally, the distinctive behaviour of the novel catalyst is showcased in the functionalization of a highly hindered olefin 1 cb, which are considered very demanding substrate for such methodology. Indeed, to the best of our knowledge this represents the first case of hydroaminomethylation of a tetra-substituted olefin.

Applications of the presented Co catalyst.
A general and selective synthesis of amines via hydroaminomethylation of olefins in the presence of the specific Co-tert-BuPy-Xantphos catalyst is presented. The optimal catalyst shows good to excellent linear-to-branched (n/iso) regioselectivity for hydroaminomethylation (HAM) reactions under mild conditions. This novel system is complementary to previously known noble metal catalysts and constitutes one of the most general catalysts for HAM. The utility is highlighted by the synthesis of specific amines on >100 g-scale.
Acknowledgements
We thank the analytical staff of the Leibniz-Institute for Catalysis, Rostock, for their excellent service. Funding: We gratefully acknowledge the support from the Federal Ministry of Education and Research (BMBF), the State of Mecklenburg-Vorpommern. Finally, we thank Prof. Zheng Huang (Shanghai Institute of Organic Chemistry, SIOC) and Prof. Jiawang Liu (Shanghai Jiaotong University) for helpful discussions and advice. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.