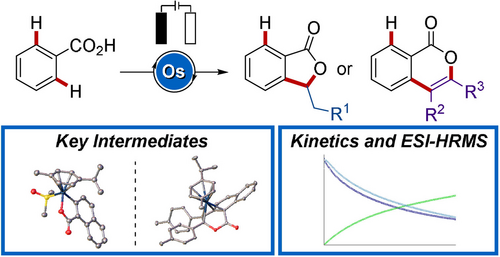
A Strategy for Site- and Chemoselective C−H Alkenylation through Osmaelectrooxidative Catalysis
Graphical Abstract
Abstract
Herein, we disclose osmaelectrocatalyzed C−H activations that set the stage for electrooxidative alkyne annulations by benzoic acids. The osmium electrocatalysis enables site- and chemoselective electrooxidative C−H activations with unique levels of selectivity. The isolation of unprecedented osmium(0) and osmium(II) intermediates, along with crystallographic characterization and analyses by spectrometric and spectroscopic in operando techniques delineate a synergistic osmium redox catalyst regime. Detailed mechanistic studies revealed a facile C−H cleavage, which allows for an ample substrate scope, providing provide robust and user-friendly access to annulated heterocycles.
Introduction
C−H activation has emerged as a powerful tool in molecular sciences, of which tremendous progress has been witnessed in recent years.1 Particularly, considerable advances in carboxylate-assisted2 oxidative C−H annulations3 by weak O-coordination4 have been realized by consecutive C−H and O−H bond scission of aromatic carboxylic acids (Figure 1 a). With pioneering contributions by Miura/Satoh,5 Ackermann,6 and Ison,7 among others,8 the modular assembly of five- or six-membered heterocycles has been established.9 Despite indisputable advances, these reactions generally require the additional use of stoichiometric chemical oxidants, such as copper(II) or silver(I) salts, to facilitate the catalyst reoxidation.
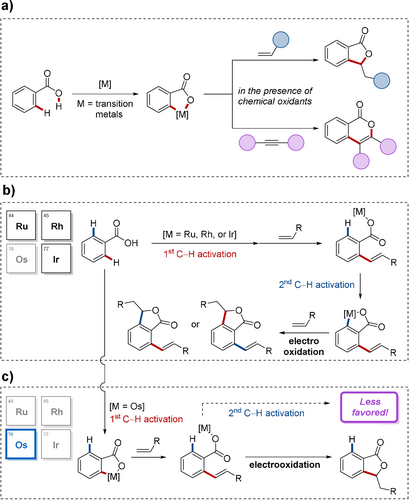
Transition-metal-catalyzed C−H annulations. a) General C−H annulations of benzoic acids. b) Mode of C−H activations by ruthenium, rhodium, and iridium catalysts. c) This work, presenting chemoselective osmaelectrocatalyzed C−H activation.
In sharp contrast, electrocatalysis has recently gained significant attention, providing chemists with a transformative platform for sustainable syntheses.10 Specifically, C−H activation has allowed for oxidative chemical transformations with electricity for the crucial catalyst reoxidation by an anodic event, thus avoiding expensive or toxic chemical oxidants.11 Hence, electrooxidative C−H annulations12 by ruthenium,13 rhodium,14 and iridium15 catalysis have thus far become viable (Figure 1 b). However, these catalytic processes largely suffer from a lack of product selectivity.
Osmium16 complexes feature prominently in stereoselective olefin difunctionalizations,17 but have been rarely used in organometallic catalysis18 compared to the homologues iron and ruthenium, arguably due to the inherently lower kinetic reactivity.19 Specifically, osmium-catalyzed electrooxidative C−H activations have thus far proven elusive to the best of our knowledge (Figure 1 c).
At the outset, we hypothesized that the metal center would enable selectivity control by repulsive steric interactions. Thus, we envisioned that unique regio- and chemoselectivity could arise from osmium complexes, which have distinct physicochemical features compared with other metals.20 As a result, we have established a novel osmium(II)-catalyzed electrooxidative C−H annulation by making use of a weak O-directing group. Notable features of our strategy include a) the first osmium-catalyzed C−H activation with electricity as a sustainable oxidant, b) a powerful regio- and chemoselective approach by osmaelectrocatalyzed C−H activation, c) ample substrate scope enabling the synthesis of five- and six-membered heterocycles, d) spectroscopic and spectrometric analysis, as well as e) detailed mechanistic and kinetic insights through the identification of key intermediates.
Results and Discussion
Based on our hypothesis, we set out to explore viable reaction conditions for the electrooxidative C−H annulation of o-toluic acid I with olefin II in an undivided cell setup (Scheme 1).21 Orienting experimentation showed that [OsCl2(p-cymene)]2 was a viable catalyst in combination with a graphite felt (GF) anode and a platinum plate cathode, which provided 29 % of the C−H annulated product 1.
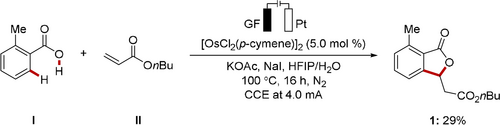
Initial observation of osmaelectrocatalyzed C−H annulation. Reaction conditions: I (0.2 mmol), II (0.6 mmol), [OsCl2(p-cymene)]2 (5.0 mol %), KOAc (2.0 equiv), NaI (2.0 equiv), HFIP (2.0 mL), H2O (2.0 mL). See the Supporting Information for the reaction setup and detailed optimization studies (Table S1 and Figure S1).
On the basis of these initial results, the effect of the additive was probed to improve conductivity and reaction performance (Scheme 2). Hence, a variety of additives, such as alkali metal based salts, organic salts, and iodine, were included in the osmaelectrocatalyzed C−H annulation. Interestingly, non-toxic and inexpensive potassium iodide provided the desired [4+1] annulated product 1 in 75 % yield by osmaelectrocatalysis. To highlight the advantages of the electrochemical approach, a set of experiments were carried out under air or with commonly employed chemical oxidants, such as AgOAc, Cu(OAc)2, Mn(OAc)3, PhI(OAc)2, or K2S2O8. All of the attempts met with unsatisfactory results, showing that electricity not only played a crucial role in the sustainable oxidation, but also gave optimal and unique efficiency for the osmium-catalyzed oxidative C−H annulation (Scheme 3).21
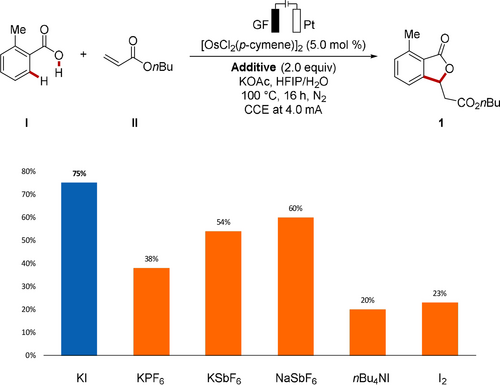
Examination of various additives.
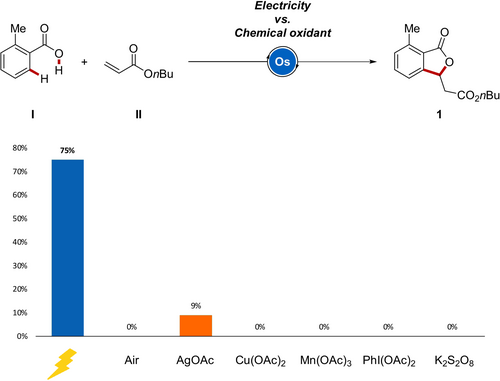
Reaction comparison between electricity and representative chemical oxidants.
Inspired by this unprecedented reactivity, we were interested in interrogating its robustness (Scheme 4).21 To this end, we performed a series of metallaelectrocatalyzed reactions with benzoic acid III possessing two accessible ortho-C−H bonds (Scheme 4 a). Interestingly, osmaelectrocatalysis showed unique chemoselectivity (35.5:1), while rhodium, iridium, and ruthenium catalysis gave significantly lower selectivities (5.2:1, 7.4:1, and 6.3:1 respectively). This aspect was also displayed in H/D scrambling experiments in the presence of an isotopically labeled solvent mixture (Scheme 4 b). With the present osmium electrocatalysis regime, deuterations occurred at the C6 position in a highly selective fashion. In sharp contrast, comparable C2 and C6 deuterations were observed in the reaction with the other Group 8 metal, ruthenium. These findings hence represent a novel tool for selective hydrogen isotopic exchange (HIE).22
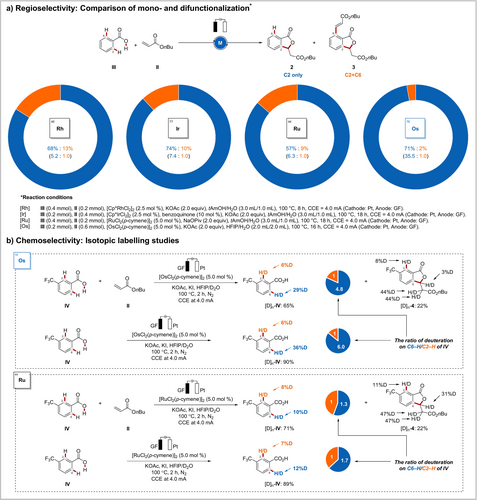
Selectivity of electrochemical osmium catalysis.
With these observations in mind, we set about to study the working mode of the electrooxidative osmium-catalyzed C−H annulation (Scheme 5).21 To this end, novel key intermediates, Os-I and Os-II, were selectively prepared, which were found to afford the desired products 5 and 6, respectively, in both stoichiometric and catalytic reactions (Scheme 5 a). Also, in operando NMR studies revealed the consumption of Os-I and alkyne VI with concomitant formation of the new osmium(0) sandwich complex Os-II, while an induction period was not detected (Scheme 5 b). Interestingly, the seven-membered intermediate formed by migratory insertion of alkyne IV was hardly observed, which presumably suggests the facile formation of the osmium(0) complex Os-II after the insertion step. Furthermore, the successive formation and consumption of key species were also clearly demonstrated by high-resolution electrospray ionization mass-spectrometric (HR-ESI-MS) analysis (Scheme 5 c). Additionally, cyclovoltammetric analysis of Os-II showed that the oxidation event occurred at E=0.58 V vs. Fc/Fc+, representing a slightly lower value than for the Ru0 to RuII oxidation potential (Scheme 5 d and Figures S11–S13).13f
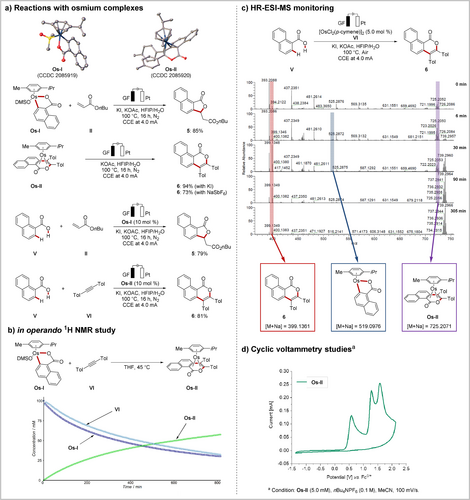
Mechanistic understanding of osmaelectrocatalysis.25
Next, we performed detailed kinetic studies (Scheme 6).21 First, intermolecular competition experiments with differently substituted benzoic acids were carried out (Scheme 6 a). The competition experiments showed that electron-donating groups in benzoic acid VII gave a higher reactivity while an alkyne VI with an electron-donating group afforded less product formation. Recent studies have proposed a base-assisted internal electrophilic substitution-type (BIES) mechanism to be operative in such a scenario.23 Additionally, a Hammett correlation was found for benzoic acids with different substituents in the meta-position to the C−H bond to be cleaved (Scheme 6 b). A linear correlation with a negative ρ value was found, indicating that an electrophilic mechanism is likely operative in the C−H bond cleavage step.23 Importantly, we observed a considerable dependence of the conversion upon an alteration of the current, being suggestive of a turnover-limiting electron transfer step (Scheme 6 c).14d Subsequently, an intermolecular competition reaction and a pair of parallel reactions were performed with o-toluic acid I and an isotopically labeled compound [D]7-I to determine the kinetic isotope effect (Scheme 6 d). In both cases, a negligible KIE of 1.1 and 1.2 was observed, respectively, suggesting a facile C−H scission. On the basis of our mechanistic findings, a plausible catalytic cycle for the osmaelectrocatalyzed C−H activation is depicted in Scheme 6 e. The mechanistic rationale commences with a facile C−H bond cleavage, which constructs osmacycle B. Thereafter, migratory insertion of the alkene or alkyne occurs, which enables the formation of intermediate D. Next, reductive elimination—in the case of a [4+1] annulation, a β-hydride elimination step is additionally involved—delivers osmium(0) sandwich complex E. Finally, the key anodic oxidation—via a proposed redox-mediator event when iodide is present—regenerates the catalytically active complex A, while liberating the desired product.
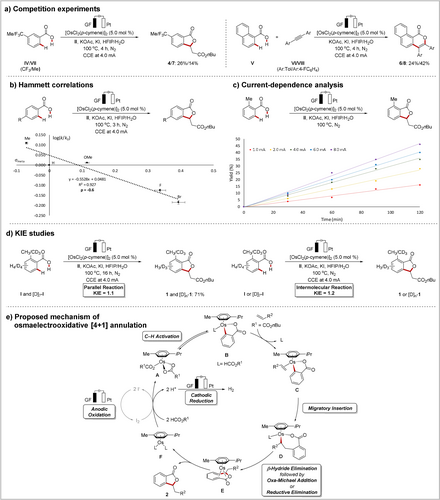
Detailed kinetic studies and proposed mechanism.
With detailed mechanistic insights in hand, we finally explored the viable substrate scope of the electrochemical osmium-catalyzed [4+1] and [4+2] C−H/O−H annulation of benzoic acids with alkenes 2 and alkynes 4 (Scheme 7). A wide range of benzoic acids gave the desired heterocycles, and valuable functional groups, such as halides (10, 11, 12, 17, 18, 19, and 20) or amines (13 and 21), were fully tolerated. Particularly, the reaction with a substrate having two competing olefins selectively afforded a single annulated product (31). The annulation manifold was not limited to the construction of five-membered rings, but six-membered heterocycle assembly with differently-substituted alkynes was also found to be viable, thus providing the extension of conjugated π-system. Notably, the synthetic utility of our electrooxidative osmium catalysis was mirrored by the development of a one-pot strategy of C−H/O−H activation (35) followed by enantioselective dihydroxylations (36).24
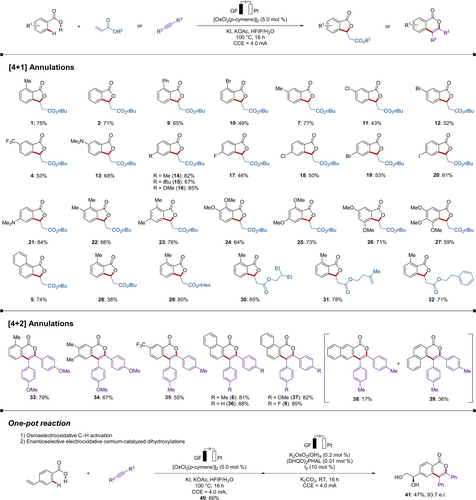
Versatility of the electrochemical osmium-catalyzed C−H annulation.
Conclusion
In summary, we have reported on the first electrochemical osmium(II)-catalyzed C−H activation involving benzoic acids by weak O-coordination. Detailed reaction analyses with analogous transition-metal catalytic systems highlighted advantageous characteristics of the osmium catalysis. Key osmium intermediates were isolated and characterized by X-ray crystallography. Spectrometric, spectroscopic, and kinetic mechanistic studies revealed the electrocatalytic mode of action. Mechanistic investigations provided strong support for a facile organometallic C−H osmation by a BIES mechanism. The ample substrate scopes of the [4+1] and [4+2] annulations reflected the robust and versatile features of the electrooxidative osmium catalysis.
Acknowledgements
Generous support from the Kwanjeong Educational Foundation (fellowship to I.C.), the European Union's H-2020 Marie Skłodowska-Curie Fellowship Grant No. 895404 (fellowship to A.M.M.), the CSC (fellowship to X.H.), an ERC Advanced Grant, and the DFG Gottfried-Wilhelm-Leibniz-Preis (conferred on L.A.) is gratefully acknowledged. We thank Dr. Christopher Golz (Georg-August-Universität Göttingen) for assistance with the X-ray diffraction analysis. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.