Photocatalytic O- to S-Rearrangement of Tertiary Cyclopropanols
Abstract
Despite the importance of heteroatom-substituted cyclopropane derivatives in drug design and organic synthesis, cyclopropanethiols remain critically underexplored. Inspired by the wide use of the Newman-Kwart rearrangement to access valuable thiophenols from phenol feedstocks, we report the development of a photocatalytic approach for efficient ambient temperature aliphatic O- to S-rearrangement on tertiary cyclopropanol derivatives. After demonstrating that a range of cyclopropanethiols—that are difficult to access by other methods—can be obtained with this strategy, we show that these rearranged products can be easily hydrolyzed and further derivatized. We conclude this study with mechanistic findings that enabled an initial extension of this approach toward other classes of aliphatic alcohols.
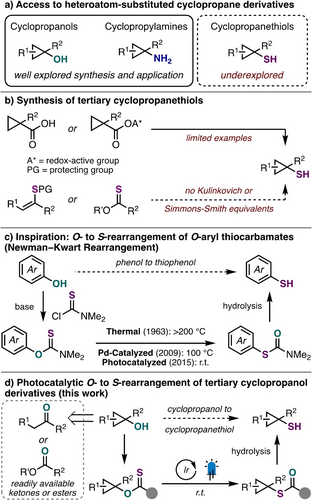
Heteroatom (nitrogen, oxygen, sulfur)-substituted cyclopropane derivatives are of great importance in modern drug design and as versatile building blocks in organic synthesis.1-3 While there exists a wealth of robust chemistry to prepare valuable cyclopropanols and cyclopropylamines via Kulinkovich- or carbenoid-type chemistry, related cyclopropanethiol derivatives have received considerably less attention (Scheme 1a).4 For instance, when considering methods for free cyclopropanethiol synthesis, particularly those that are tertiary or 1,2-disubstituted, there are only a handful of reports demonstrating single example access to these building blocks (Scheme 1b).5-8 This is despite a growing number of cyclopropanethiols found in valuable small molecules, albeit often as higher oxidation state derivatives (e.g., sulfonamides, sulfoximines).9, 10, 11-13 In addition to exploring biologically active chemical space with these alternative heteroatom-substituted cyclopropanes, derivatives of cyclopropanethiols have also found use as convenient precursors for the synthesis of other medicinally relevant cyclopropanes in recent years.7, 14, 15
Approaches toward O- to S-rearrangement.
When considering a new approach toward elusive cyclopropanethiols, we became inspired by the Newman-Kwart rearrangement (NKR) (Scheme 1c). The NKR is a powerful transformation to convert O-aryl thiocarbamates to S-aryl thiocarbamates, formally providing access to thiophenols from more accessible phenol starting materials.16, 17 While the NKR generally requires high reaction temperatures (>200 °C) for efficient rearrangement via intramolecular nucleophilic aromatic substitution, palladium and photoredox catalysis have enabled efficient O- to S-aryl rearrangements at lower temperatures as pioneered by the Lloyd-Jones18 and Nicewicz19, 20 groups, respectively.21
Given our experience working with redox-active O-thiocarbamate auxiliaries in the context of Ni-catalyzed deoxygenative cross-couplings,22, 23 we wondered if we could leverage related thiocarbonyl derivatives to facilitate an aliphatic O- to S-rearrangement of tertiary cyclopropanol derivatives. Cognizant that previously reported aliphatic NKR strategies relying on polar reactivity (e.g., SN1) would be unsuccessful on the cyclopropane scaffold due to competitive ring-opening, our reaction design focused on a single-electron approach toward aliphatic O- to S-rearrangement.22, 24 Motivation for this pursuit was additionally driven by the ease of access of cyclopropanols by a variety of both traditional25 and contemporary strategies,26 which make them ideal precursors for the synthesis of tertiary cyclopropanethiols. Herein, we report the development of an efficient photocatalyzed O- to S-rearrangement of tertiary cyclopropanols activated with a redox-active thiocarbonyl auxiliary (Scheme 1d). Furthermore, mechanistic insight obtained during this investigation led to a preliminary extension of our strategy toward other classes of aliphatic alcohols.
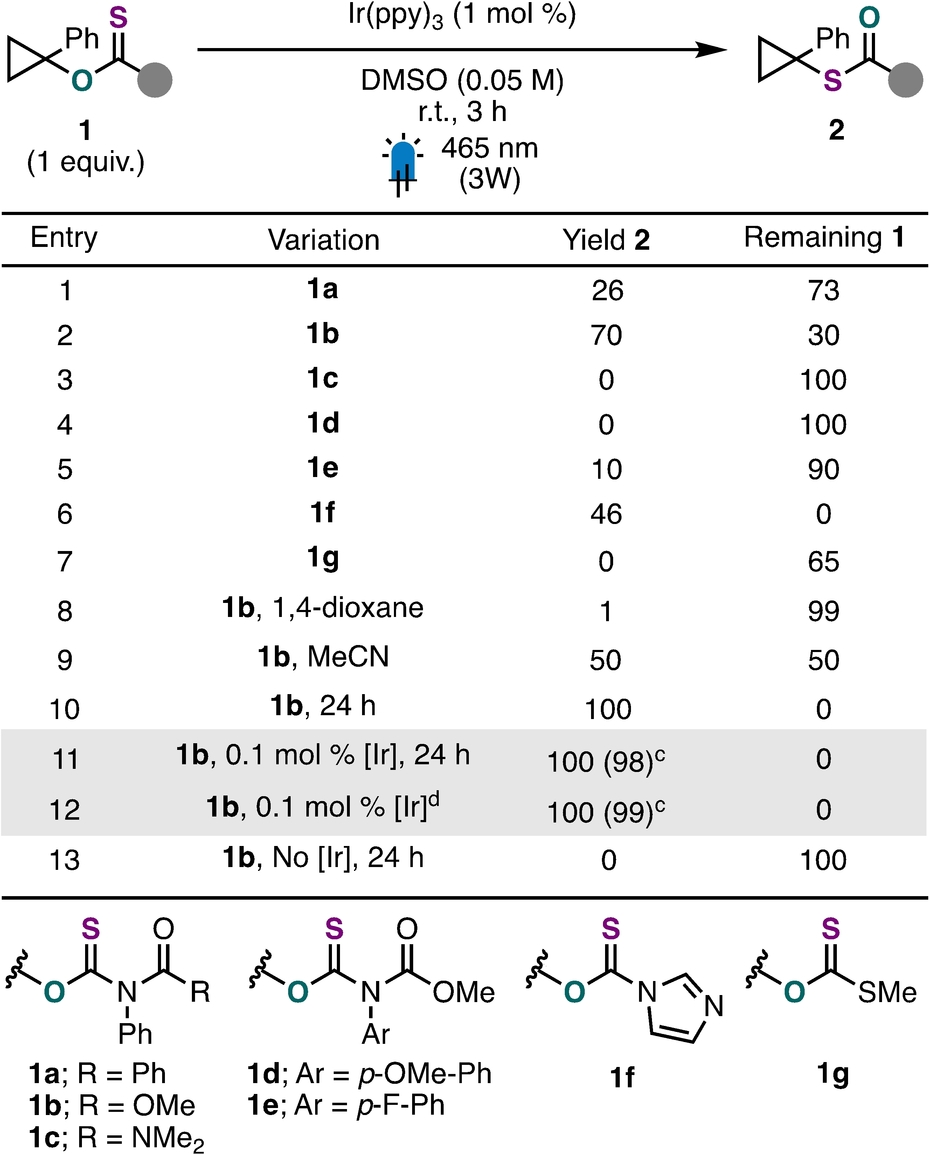
To accomplish our objective, we chose to explore a reductive photochemical approach given precedent for single-electron reduction of thiocarbonyl groups in the context of photoinduced electron/energy transfer reversible addition-fragmentation chain transfer (PET–RAFT) polymerization27, 28 and tin-free Barton-McCombie deoxygenation chemistry.29 We thus began our investigation by evaluating the reactivity of 1-phenylcyclopropanol activated with a variety of different thiocarbonyl-containing auxiliaries in the presence of Ir(ppy)3 as a photocatalyst (Table 1). Using our previously disclosed O-thiocarbamate group (1 a),22 rearranged product 2 was observed in modest yet promising yield (26 %) with the mass balance being remaining starting material (entry 1). Encouraged by this result, a variety of related analogues (1 b–1 e; entries 2–5) and other derivatives used in Barton-McCombie deoxygenations (1 f, 1 g; entries 6 and 7)30 were investigated. This evaluation revealed that the N-phenyl-N-(methyl formate) substituted O-thiocarbamate auxiliary (1 b), which can be easily synthesized in a one-pot, two-step reaction in good yield, afforded the highest yield of 2 (entry 2). These results highlight the importance of the specific thiocarbonyl group used with this photocatalytic approach for aliphatic O- to S-rearrangement. Other solvents, such as 1,4-dioxane or acetonitrile, yielded lower quantities of rearranged product (entries 8 and 9). Extending the reaction time for the rearrangement of 1 b to 24 h was found to give S-thiocarbamate product 2 in near quantitative yield (entry 10). Notably, lowering the loading of Ir(ppy)3 to 0.1 mol % did not impact reaction efficiency (entry 11). The addition of DIPEA (2.0 equiv.) provided access to 2 in quantitative yield in a shorter reaction time (3 h, entry 12). Finally, we confirmed that the photocatalyst was essential for reactivity (entry 13).
We next investigated the scope of this photocatalyzed aliphatic O- to S-rearrangement. We found that 1-aryl cyclopropanol substrates bearing electron-rich or -deficient substituents at various positions on the aromatic ring underwent efficient O- to S-rearrangement (Scheme 2, 2 a–k). Aryl halide handles (2 d, 2 e, 2 j, 2 k) capable of engaging in subsequent transformations, as well as protected phenols (2 f, 2 g) and an aniline derivative (2 h), were well tolerated. In addition to 1-arylcyclopropanol derivatives, 1-heteroaryl substrates (2 l, 2 m, 2 n), as well as a 1-alkenyl (2 o) and a bicyclic cyclopropanol substrate (2 p), underwent O- to S-rearrangement with moderate to excellent efficiency. A cis-1,2-disubstituted cyclopropanol could also be rearranged to produce a mixture of products, favouring the trans-cyclopropanethiol (trans-2 q). Importantly, unreacted starting material could be recovered in cases where appreciable quantities remained (2 o, 2 p).31 Since 1,1-thiocarbonyldimidazole (TCDI) is commercially available, we further investigated our preliminary result using thiocarbonyl imidazolide starting material 1 f (see Table 1, entry 6).32 Although this auxiliary was not compatible with the addition of exogenous tertiary amine reductant, we found that the addition of 1,4-dioxane as a co-solvent afforded the rearranged product (2 r) in good yield (Scheme 2, see Table S2 for full details). A small representative scope of 1-aryl cyclopropanol derivatives containing electron-rich or -deficient substituents at the para- (2 s, 2 t) and ortho-position (2 u) of the aromatic ring were shown to be compatible with this alternative auxiliary. Finally, when the efficiency of 1-alkyl cyclopropanol rearrangement was examined with both auxiliaries, we were only able to observe low conversion to desired product using our designer thiocarbamate (2 v).33
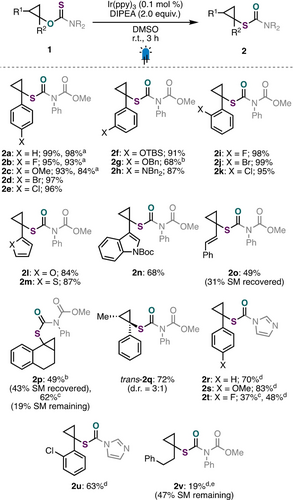
Scope of the reaction. Reactions performed on 0.20-mmol scale. Isolated yields are reported. [a] No DIPEA added, 24 h instead of 3 h. [b] 1.0 mol % Ir(ppy)3 instead of 0.1 mol %, 24 h instead of 3 h. [c] 72 h instead of 24 h [d] No DIPEA added, 1,4-dioxanes: DMSO (3 : 1) instead of DMSO, 24 h instead of 3 h. [e] 1H NMR yield versus 1,3,5-trimethoxybenezne as an internal standard. See Supporting Information for full details.
Having demonstrated access to a range of protected cyclopropanethiol products, we next explored methods for effective auxiliary removal (Scheme 3). Treating products 2 a or 2 r to an excess of ethanolamine at room temperature effectively cleaved the auxiliary (Scheme 3a).34, 35 While 1-phenyl cyclopropanethiol 3 could be isolated in good yield, it was often difficult to purify from disulphide 4, which is presumably generated via oxidation by atmospheric oxygen.36 However, we found that S-thiocarbamate 2 a/2 r could be converted to disulphide 4, a non-odorous, stable solid, in near quantitative yield by prolonging the hydrolysis reaction time (48 h). Importantly, both cyclopropanethiol 3 and disulphide 4 were amenable to derivatization (see Supporting Information Section D for full details). For example, cyclopropanethiol 3 was converted to products 5 and 6 following telescoped SNAr and acylation reactions, respectively (Scheme 3b, 3 c). Starting from disulphide 4, sulfonamide 7 and sulfonyl fluoride 8 were prepared in good yields (Scheme 3d, 3 e). These compounds are valuable building blocks for various applications in areas such as medicinal chemistry, organic synthesis, and chemical biology.37
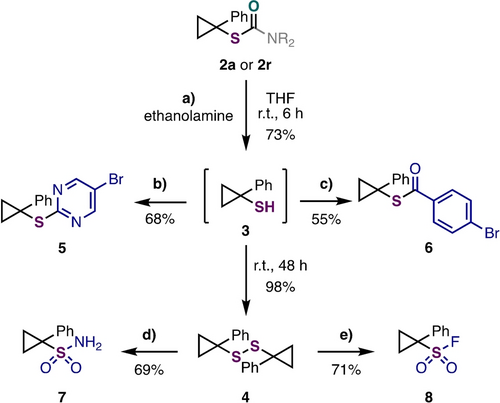
Hydrolysis and derivatization. See Supporting Information for full details. b) ArCl, NaH, DMF, r.t., 3 h; c) ArCOCl, DMAP (cat.), DIPEA, DCM, r.t., 16 h; d) PIDA, (NH4)CO3, MeOH, r.t., 16 h; e) SelectfluorTM, MeCN/H2O, reflux, 6 h.
We next conducted a series of experiments to elucidate the mechanism of this aliphatic O- to S-thiocarbamate rearrangement (Scheme 4). To probe the intermediacy of a cyclopropyl radical intermediate, we subjected cyclopropanol diastereomers trans-1 h and cis-1 h to the optimized reaction conditions and obtained a mixture of isomerized products, favoring the same trans-2 w product in both cases (Scheme 4a). Interestingly, the diastereomeric ratio of 2 w varied based on the diastereomer of starting material 1 h subjected to the reaction conditions. While trans-1 h yielded trans-2 w in 16 : 1 d.r., cis-1 h yielded trans-2 w in a more modest 4 : 1 d.r. This observation can be attributed to the barrier of inversion of cyclopropyl radical intermediates.22, 38 The intermediacy of an alkyl radical intermediate was further supported when the standard reaction was conducted in the presence of TEMPO. In this case, TEMPO adduct 9 was detected, while the desired product 2 a was not. We also observed 96 % of starting material 1 b remaining (Scheme 4b). After confirming that both starting materials 1 b and 1 f quench the photoexcited Ir(ppy)3 catalyst in Stern–Volmer studies (Figure S1 and S2), cyclic voltammograms of both substrates were collected (Figure S3 and S4), which revealed that single electron reduction with Ir(ppy)3 is feasible (Scheme 4c, *Eox [IrIV/IrIII*]= -2.39 V vs. Fc/Fc+),39, 40 see Supporting Information Section H for discussion). Quantum yield measurements for reactions involving substrates 1 b or 1 f, which both exceeded unity (φ >1), suggested that product formation is the result of a radical chain process (Scheme 4d). To confirm a radical chain mechanism, a cross-over experiment using an equimolar amount of O-thiocarbamates 1 b and 1 i was performed (Scheme 4e). As expected for a mechanism involving propagation via a radical chain, a near-equimolar amount of the four possible rearranged products (2 a, 2 b, 2 x and 2 y) was observed (Scheme S6). Taken together, these data suggest that this unique O- to S-alkyl rearrangement reaction could begin with a reduction of O-alkyl thiocarbamate starting material (1) to the corresponding radical anion by photoexcited Ir(ppy)3 ([*IrIII], Scheme 4f).29 Fragmentation (β-scission) of the radical anion 10 would generate O-thiocarbamate anion 11 and cyclopropyl radical 12. Cyclopropyl radical intermediate 12 could then react with another equivalent of O-alkyl thiocarbamate starting material (1) to generate S-alkyl thiocarbamate product 2 and another equivalent of cyclopropyl radical intermediate (12), propagating the chain. Finally, the ground state Ir(III) photocatalyst could be regenerated via single electron transfer from the tertiary amine reductant, DIPEA. Alternatively, given the efficiency of this reaction in the absence of external reductant and the relatively short-lived radical chain observed with ON/OFF light experiments (Figure S6 and S7), a redox neutral mechanism could also be operational. In this scenario, O-thiocarbamate anion 11 could be oxidized by the Ir(IV) intermediate to produce the corresponding radical 13, which could terminate radical chain propagation via coupling with cyclopropyl radical 12. While this oxidative quenching cycle is likely operating under the optimized reaction conditions, alternative mechanistic scenarios, such as those involving energy transfer (EnT), direct excitation, and α-amino radical initiation, can also be accessed depending on the specific reaction conditions employed (see Supporting Information Section H for details).
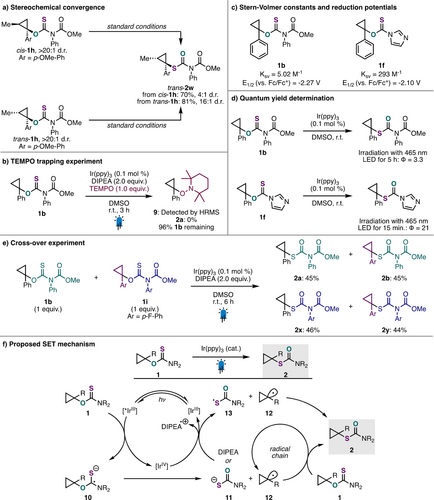
Mechanistic studies.
Having successfully demonstrated this aliphatic rearrangement approach to access cyclopropanethiols from cyclopropanols, we were eager to evaluate the generality of this transformation with non-cyclopropyl scaffolds. Unfortunately, initial attempts to extend this methodology using thiocarbamate activating groups toward other classes of alcohols were met with little desired reactivity (Scheme 5a, see Supporting Information Section F for details).41 Despite this, we were encouraged by the production of deoxygenated alkane and homo-coupled starting material during preliminary optimization efforts. Considering our proposed mechanism for product formation (Scheme 4f), we speculated that radical chain propagation was being outcompeted by deleterious hydrogen-atom abstraction and/or homo-dimer formation via radical-radical coupling. Given precedent for efficient radical chain propagation of O-alkyl thionoesters in the context of Barton- McCombie deoxygenation,42 we were interested in exploring this class of activated alcohol derivatives as precursors for a more general aliphatic O- to S-rearrangement. These redox-active precursors can notably be synthesized via an operationally simple and efficient Ni-catalyzed cross-coupling reaction.43 After brief optimization (Table S3), we found that this approach was successful in achieving rearrangement of activated O-alkyl thionoesters (14) (Scheme 5b). Products resulting from the rearrangement of secondary (15 a, 15 b) and primary (15 c–15 e) benzylic alcohols were obtained in synthetically useful yields. We also found that an α-hydroxy ester derivative could undergo O- to S-rearrangement, affording 15 f in 43 % yield. Although these products were obtained in moderate yields, this approach provides an alternative route in scenarios where steric effects (15 b, 15 d) or chemoselectivity (e.g. SNAr (15 e)) may make traditional substitution chemistry to thiols less appealing. Unfortunately, similar O-alkyl thionoesters derived from other unactivated alcohols were not amenable with this strategy and remain a target for future reaction development (see Supporting Information Section F for details). Finally, to confirm the feasibility of this approach for thiol synthesis, we found that treatment of rearranged product 15 c with excess ethanolamine at slightly elevated temperatures yielded disulfide 16 (Scheme 5c).
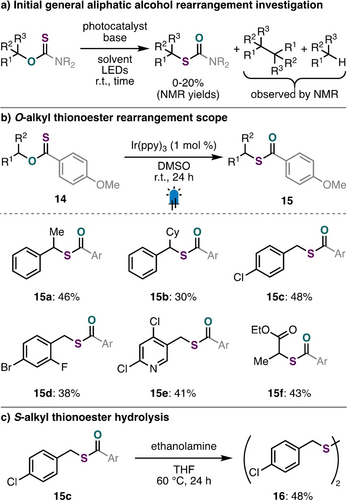
Extended reaction scope using O-alkyl thionoesters. Reactions performed on 0.20-mmol scale. Isolated yields are reported. See Supporting Information for full details.
In conclusion, we have investigated a photocatalytic approach to access valuable cyclopropanethiols via an aliphatic O- to S-rearrangement of readily accessible tertiary cyclopropanols. The products of these reactions can be readily derivatized to give access to a variety of S-functionalized cyclopropane derivatives. Through mechanistic insight, we have revealed the many nuances associated with this approach, where the choice of thiocarbonyl auxiliary and reaction conditions can dramatically impact the mode of starting material initiation. We anticipate that our O- to S-rearrangement strategy will inspire other single-electron approaches to synthesize valuable C(sp3)−S products from aliphatic alcohols, particularly in scenarios where traditional substitution chemistry is unfeasible. Work is ongoing in our group to develop a generally applicable aliphatic Newman-Kwart rearrangement.
Acknowledgments
We thank NSERC (Discovery Grants and Canada Research Chair programs), the Canada Foundation for Innovation (Project No. 35261), the Ontario Research Fund, the Alfred P. Sloan Foundation, and the University of Toronto for generous financial support of this work. We also acknowledge the Canada Foundation for Innovation (Project No. 19119) and the Ontario Research Fund for funding the Centre for Spectroscopic Investigation of Complex Organic Molecules and Polymers. J.J.M. thanks NSERC for graduate scholarships (CGS-M, CGS D) and the University of Toronto for a doctoral scholarship (FAST). J.W.P thanks NSERC for a graduate scholarship (PGS D). J.J.M. also would like to thank Kasumi Hayashi and Bryton Varju for assistance with Fluorescence Spectrophotometer machine operation and Santiago Tijaro Bulla for assistance with ElectraSyn 2.0 operation. We would all like to thank Tyler McDonald and Anthony Palermo for a critical reading of this manuscript.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.