Tailoring Electrochemical CO2 Reduction on Copper by Reactive Ionic Liquid and Native Hydrogen Bond Donors
Abstract
Electrochemical CO2 reduction (CO2RR) on copper (Cu) shows promise for higher-value products beyond CO. However, challenges such as the limited CO2 solubility, high overpotentials, and the competing hydrogen evolution reaction (HER) in aqueous electrolytes hinder the practical realization. We propose a functionalized ionic liquid (IL) which generates ion-CO2 adducts and a hydrogen bond donor (HBD) upon CO2 absorption to modulate CO2RR on Cu in a non-aqueous electrolyte. As revealed by transient voltammetry, electrochemical impedance spectroscopy (EIS), and in situ surface-enhanced Raman spectroscopy (SERS) complemented with image charge augmented quantum-mechanical/molecular mechanics (IC-QM/MM) computations, a unique microenvironment is constructed. In this microenvironment, the catalytic activity is primarily governed by the IL and HBD concentrations; former controlling the double layer thickness and the latter modulating the local proton availability. This translates to ample CO2 availability, reduced overpotential, and suppressed HER where C4 products are obtained. This study deepens the understanding of electrolyte effects in CO2RR and the role of IL ions towards electrocatalytic microenvironment design.
Introduction
Significant progress has been made in developing electrode materials,1 electrolyzer configurations,2 and various electrolytes3 to electrochemically convert CO2 to commodity chemicals. However, the initial electron transfer to activate CO2 that is otherwise linear and stable remains a critical challenge. In the 1970s, Bewick4 identified the initial electron transfer to CO2 as the most energy-demanding step. This continues to be one of the primary obstacles in commercializing technologies for the electrochemical CO2 reduction reaction (CO2RR), preventing CO2RR from being economically viable at a large scale, along with the related challenges of selectivity and catalyst stability. Therefore, developing novel electrolytes, such as ionic liquids (ILs), in parallel to heterogeneous electrocatalyst design is gaining momentum to modify the catalytic interface for CO2RR. ILs have desirable properties for these applications, such as wide electrochemical window, high conductivity, and high CO2 solubility.5 As a result, ILs have been used to modify electrode surfaces providing the catalytically active phase in the form of a thin film, which is also known as solid catalyst IL layer (SCILL).6-8 ILs have also been used as functional electrolytes, forming unique double-layer structures9, 10 that can interact with reaction intermediates of CO2RR through IL−CO2 adducts,11 hydrogen bonding12 or induced electric fields,13 thus catalyzing CO2RR. These interactions reduce overpotentials, and the presence of IL suppress hydrogen evolution reaction (HER),14 the competing side reaction in traditional aqueous electrolytes.
Prior literature focused primarily on the imidazolium (Im)-based ILs. Several investigations studied the behavior of n-alkyl substituted Im-based cations, such as 1-ethyl-3-methylimidazolium ([EMIM]+) and 1-butyl-3-methylimidazolium ([BMIM]+) and concluded that the formation of an Im-CO2 adduct,15 namely carboxylate, was the co-catalyzer in CO2RR over metal electrodes. This hypothesis was further supported by computational16 and in situ spectroscopy17 studies where the formation of [EMIM]+−COO− complex at C2-proton of Im was reported.
Another opinion on the mechanism of the observed co-catalytic effect of Im-based ILs considers the interactions between the hydrogen atoms of the Im cation and CO2⋅− radical anion, emphasizing the importance of the hydrogen bonding and the distinct electric field generated13 at the interface, thus promoting CO2RR. Lau et al.12 probed this alternative explanation by systematically substituting the Im ring to eliminate C2, C4 and C5 hydrogens individually. For instance, when the C2 position of the Im-ring was methyl-substituted, the catalytic activity of CO2RR stayed the same, whereas the substitutions at the C4 and C5 positions resulted in decreased currents. The authors suggested that the hydrogen bonding between C4/C5 protons and CO2⋅− is the effective mechanism in co-catalyzing CO2RR. Furthermore, Neyrizi et al.18 studied 1,3-dimethyl imidazolium bis(trifluoromethane)sulfonimide ([MM][TFSI]) on Au electrode with linear sweep voltammetry and computations where the C2 proton of the Im cation is discussed to interact with CO2⋅− absorbed on Au, contributing to the concerted coupled electron-proton transfer reaction. This study highlights the importance of available protons at the reaction interface in non-aqueous electrolytes for CO2RR. Similar to the cation-induced hydrogen bonding interaction, the addition of water- a hydrogen bond donor (HBD)- to non-aqueous electrolytes was found to positively affect the catalytic activity of CO2RR,19 where the hydrated double-layer facilitates the transfer of protons and electrons.
While the Im cations have been the main focus in mechanistic studies, it is the IL anions that were mainly discussed to control CO2 solubility in ILs through a Lewis acid-base interaction between CO2 and the anion based on the alkalinity. Few studies also discussed the role of anions in enhancing CO2RR activity. Sharifi et al.20 investigated the impact of the anion on Cu by varying anion size, hydrophilicity, and CO2 affinity. They reported the highest formate selectivity with [TFSI]−, which had the lowest hydrophilicity and highest affinity towards CO2 among the investigated anions. Furthermore, the high hydrophilicity of dicyanamide ([DCA]−) was found to increase HER. These results demonstrate that the anion can control the species at the interface and CO2RR outcomes. Moreover, 1,2,4-triazolide ([1,2,4-Triz]−), a basic anion that reacts with CO2, was examined with different cations, such as trihexyltetradecylphosphonium ([P66614]),21 tetra-propyl-phosphonium ([P3333]),22 and n-octyltrimethyl ammonium ([N1118]).23 It was found in all cases that CO2 forms complexes with the anion and CO2RR proceeds at lower overpotentials yielding dominantly formate as the product. Dongare et al.24 recently reported a bifunctional CO2-reactive IL, 1-ethyl-3-methylimidazolium pyrrole-2-carbonitrile ([EMIM][2-CNpyr]). This IL exhibited the formation of carboxylate ([EMIM]+−CO2−]) and carbamate ([2-CNpyr]−CO2−]) species in addition to the protonated anion specie (2-CNpyrH) (Figure 1), as previously observed by Lee et al.25 through chemisorption of CO2. The [EMIM][2-CNpyr] in acetonitrile demonstrated superior CO2RR activity over Ag electrode with >94 % selectivity for CO at lower overpotentials.
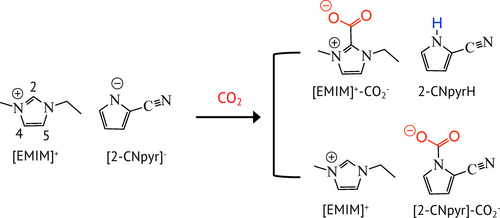
CO2 absorption by [EMIM][2-CNpyr] as determined by NMR analysis (Figure S1); consistent with the previous report by Lee et al.25 The formation of 2-CNpyrH and carboxylate ([EMIM]+−CO2−) through the CO2 complexation with carbene (deprotonated [EMIM]+ at C2 position) (top) and carbamate formation ([2-CNpyr]−CO2− (bottom)).
The thermodynamics and kinetics of CO2RR are highly dependent on the electrode-electrolyte interface which presents complexities in IL electrolytes. While the cations are attracted to the negatively polarized electrode surface, anions can influence the crowding of the interface26 and the cation adsorption on the surface through electrostatic interaction and hydrogen bonding.27 It was shown by sum frequency generation (SFG) technique on the Pt surface by tracking the change in intensities of the spectra that Im cations orient in a tilted orientation to the electrode with the C2 position facing the surface and that the ring becomes parallel to the surface with applied negative potentials.28 Such structural and compositional changes at the IL-electrode interfaces influence the reaction pathway and selectivity in CO2RR.29, 30 Therefore, there appears to be an interplay among the individual ions and the generated CO2 absorption species within the interfacial microenvironment that modulates the catalytic activity.
Here, we selected the bifunctional IL, [EMIM][2-CNpyr], to further understand its role in modulating the CO2RR reaction pathways and energetics over the Cu electrode, focusing specifically on its voltage-driven behavior and modification of the interface. The reaction of this IL with CO2 in acetonitrile based supporting electrolyte follows the known reaction Scheme (NMR in Figure S2), consistent with the previous observation by Dongare et al.24 Cu was explicitly selected in this study as it is the only known metal catalyst that can produce high carbon products other than CO in aqueous electrolytes.31, 32 With in situ surface-enhanced Raman spectroscopy (SERS) and electrochemical impedance spectroscopy (EIS), both the surface species and the interfacial changes were determined. EIS is a powerful technique to probe the electrode-electrolyte interfaces including potential-dependent capacitance, surface adsorption, and charge transfer reactions.33 Specifically, careful analysis of the impedance data as a function of frequency through a circuit model fit can be used to determine capacitance due to excess charge accumulation.34 In complement, SERS35 provides chemical information in relation to the surface species and molecular orientations. We have shown the simultaneous use of EIS and SERS as an effective approach for examining the structuring of ILs, deep eutectic solvents, and similarly concentrated electrolytes at electrode surfaces.36-39
This study captures the orientational change of [EMIM]+ on the Cu surface upon negative polarization and links it to the observed decay in capacitance revealing double-layer thickening. These results motivated the hypothesis that interface enrichment of Im species with negative polarization traps the CO2 saturation products (Figure 1), including the protonated anion that functions as a native HBD, thus resulting in a unique microenvironment to drive CO2RR at lower overpotentials. Even though the concentration-dependent investigations of 0.1, 0.5, and 1.0 M IL in acetonitrile revealed that the diluted IL can form interfacial microenvironment similar to the neat IL, the surface coverage by [EMIM]+ at high concentrations ( 1.0 M) and thickening of the double layer decreases the catalytic activity. Furthermore, the HBD component that forms in situ by the absorption of CO2 contributes to CO2RR at high reaction rates with reduced overpotentials. Through gas chromatography (GC) and nuclear magnetic resonance (NMR) spectroscopy analysis, the formation of CO, CH4, C2H4, C2H6, H2, formate, succinate, formaldehyde and butane were identified with prolonged electrolysis experiments conducted at −2.1 V vs. Ag/Ag+.
Results and Discussion
Constructing of an interfacial microenvironment through CO2-reactive IL
Linear sweep voltammetry (LSV) was performed with neat [EMIM][2-CNpyr] to investigate the electrochemical behavior of the IL under N2 and CO2 on the Cu surface, as shown in Figure 2A. Under CO2 environment, a reduction potential at −1.8 V vs. Ag/Ag+ is observed and attributed to CO2RR since it was absent under N2. In addition, the open circuit potential (Eoc) shifts from −1.0 to −1.11 V. This is associated with the changes in the pH and composition of the electrolyte due to CO2 absorption. The surface species are [EMIM]+, 2-CNpyrH, carbamate, and carboxylate, as shown in Figure 1, formed upon CO2 chemisorption. Specifically, [EMIM]+ adsorption is evident as more clearly seen under the N2 environment where the current gradually increases in the potential range of −1.0 V to −2.1 V. Beyond −2.1 V, current increases sharply due to the reduction of [EMIM]+ to an adsorbed radical or carbene.40 Other general observations are the lower current under CO2 compared to N2 and the shallower slope in current beyond −1.8 V. This is due to the complexation with CO2 that results in increased viscosity and decreased conductivity; consequently, species diffuse slower to the electrode surface, slowing charge transport rate.
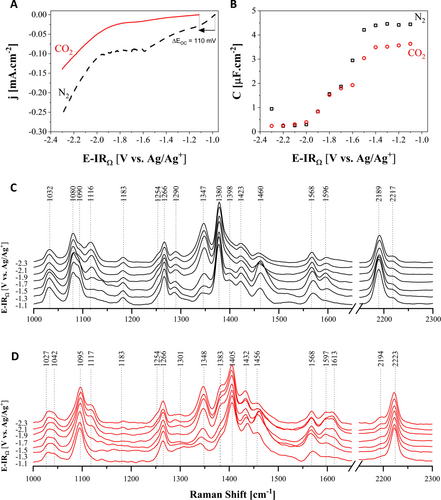
Electrochemical and spectroscopic analysis of [EMIM][2-CNpyr] on Cu electrode. LSV of the IL under N2 and CO2 (A); Differential capacitance curve of neat IL (B); Potential-dependent in situ SERS of neat IL under N2 (C) and CO2 (D). The spectra are normalized with respect to the −C≡N stretching peak located at 2189 cm−1 for N2 and 2223 cm−1 for CO2.
Differential capacitance (Cdiff) measurements were performed to probe the electrode-electrolyte interface, as shown in Figure 2B. The capacitance curve starts with a plateau at −1.1 V vs. Ag/Ag+ followed by a decay in capacitance with negative polarization up to −2.0 V and another plateau at potentials more negative than −2.0 V where a minimum of 0.25 μF.cm−2 is reached for the Cu|[EMIM][2-CNpyr] interface under both N2 and CO2. Initial capacitance of the interface is lower under CO2, suggesting a difference in the interface, consistent with the shift in Eoc. As increasing negative polarization causes more and more cations to accumulate on the electrode surface, [EMIM]+ cations crowd the interface, as interpreted from the Goodwin-Kornyshev model which treats the interface through the mean field theory.41 Accordingly, the charge density at the interface increases with increased applied potential until the ions feel their excluded volumes. Since the neat IL has no solvent molecules to displace, it can only admit voids upon increased polarization. However, when the cations admit all the voids, further increase in potential results in the increased double layer thickness, known as crowding, which leads to decrease in Cdiff as seen in Figure 2B.
To further investigate the nature of Cdiff and the interfacial species, in situ SERS was performed with N2 and CO2-saturated electrolytes (Figures 2C and 2D). Table S1 summarizes the observed peaks and vibrational assignments. The prominent peaks are consistent with both N2 (Figure 2C) and CO2 (Figure 2D), and the spectra point to the co-existence of both the anion and the cation on the electrode surface. However, as discussed below, there are notable changes related to the [EMIM]+ orientation with increased polarization and compositional changes with CO2 absorption.
Spectral peaks related to [EMIM]+ ring located at 1347, 1380, and 1423 cm−1 under N2 (Figure 2C) intensify with applied polarization, indicating a stronger interaction with the electrode surface and further blueshift to 1348, 1383, and 1432 cm−1, respectively, under CO2 (Figure 2D), confirming the change in its environment due to CO2 absorption. In parallel, [2-CNpyr]− ring stretching at 1398 cm−1 (Figure 2C) that is weakly seen under N2 intensifies and shifts to 1405 cm−1 (Figure 2D). This is due to the closer approach of the neutral 2-CNpyrH specie that forms upon CO2 saturation to the surface. The presence of 2-CNpyrH upon CO2 exposure is also evidenced by the shift in the −C≡N stretching vibration at 2189 cm−1 under N2 to 2223 cm−1 under CO2. The peak at 1423 cm−1 under N2 and 1432 cm−1 under CO2 are attributed to the symmetric stretching of the CH3 group. Since it presents very low enhancement with negative polarization, CH3 groups are interpreted to be away from the surface, pointing towards the bulk electrolyte. On the contrary, the peaks at 1116, 1342, and 1380 cm−1 associated with C4 and C5 of [EMIM]+ and N−CH2 moiety of the ethyl group increase in intensity with negative polarization, as seen in Figure 3A, indicating a change in orientation of the cation. This behavior is persistent with N2 and CO2, although it is better seen under N2 without interference from complexation with CO2. Since the surface is already crowded by [EMIM]+, the intensification of these specific ring modes is interpreted to be due to the changes in the orientation of [EMIM]+ from tilted (C2 position facing the electrode surface) to parallel configuration with respect to the electrode surface.
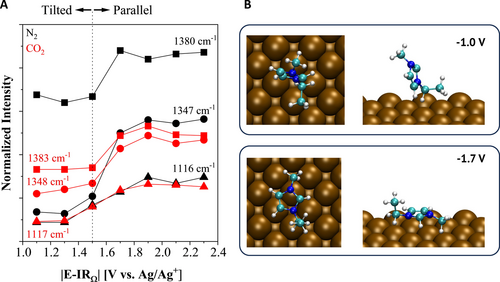
(A) SERS peak intensity as a function of absolute applied potential (|E-IRΩ|). Dotted line marks the potential of the change in the orientation of imidazolium species under N2 (black) and CO2 (red). Peaks: 1116 cm−1 (pentagons) for δ(C4C5−H); 1347 cm−1 for υ(Im ring)+υ(CH2(N)) (spheres); 1380 cm−1 for υ(Im ring)+υ(CH2(N))+υ(CH3) (triangles). (B) Lowest energy geometries calculated for [EMIM]+ at −1.0 and −1.7 V on Cu (100) (top and side views) indicating the preference of the parallel orientation at a more negative potential. Atom color code: blue=N; cyan=C; white=H.
The potential at which [EMIM]+ transitions from tilted to parallel can be easily seen from the recorded intensities of the associated peaks plotted in Figure 3A. Specifically, the intensities of the 1342 and 1380 cm−1 peaks associated with N-CH2 stretching increase sharply beyond −1.5 V vs. Ag/Ag+, which also coincides with the potential where the decrease in capacitance observed in Figure 2B during the negative potential sweep in EIS. When compared between N2 and CO2, the relative enhancement in the peak intensities used to identify the orientation change is lower under CO2 as the [EMIM]+−CO2− adduct forms. The change in the orientation of Im-species is further supported by IC-QM/MM calculations of the optimized geometries as shown in Figure 3B. At −1.0 V, the lowest energy structure shows a tilted orientation, while at −1.7 V the lowest energy structure changes to a parallel orientation. IC-QM/MM calculations at −1.5 V still show an energetic preference for the tilted orientation, however there is a clear transition to the parallel preference at more negative potentials, marking −1.5 V vs. Ag/Ag+ as the potential of transition. This trend continues into −2.0 V where the preferred orientation is parallel as shown in the relative energies given in Table S2.
In the light of SERS analysis, the interfacial liquid structure can be considered a cation-rich layer infiltrated with anions under N2. Under CO2, the surface is dominated by [EMIM]+, [EMIM]+−CO2−, 2-CNpyrH, and fewer [2-CNpyr]−. The crowding and thickening of the interface with [EMIM]+ upon negative polarization accompanied by reorientation are believed to impair the ability of the anion to escape from the interface, especially when the neutral form of the anion (2-CNpyrH) gets reduced, the effect of which is discussed later. Therefore, we observe features of the anion in SERS at 2189 cm−1 under N2 and 2194 cm−1 under CO2. The existence of the pyrrole species contributes to the formation of a unique microenvironment for CO2RR since the anion also complexes with CO2 ([2-CNpyr]−CO2−) and can serve as a CO2 carrier to the electrode surface. In addition, the protonated anion, 2-CNpyrH, can act as HBD.
CO2RR catalytic behavior modulated by the microenvironment
Neat [EMIM][2-CNpyr] has a viscosity of 68 cP at 25 °C, which increases to 247 cP upon saturation with CO2;25 these high viscosities is a limiting factor for CO2RR. Therefore, acetonitrile was used as a diluent to minimize the mass transfer limitation and increase the ionic conductivity in CO2RR. The concentration-dependent ionic conductivity and the viscosity of diluted [EMIM][2-CNpyr] (0.0 to 3.0 M) are provided in Figure S3. A maximum ionic conductivity at 1.5 M and a consistent increase in viscosity with increasing IL concentration was observed. Because the measured ionic conductivity of [EMIM][2-CNpyr]/acetonitrile decreased after CO2 saturation, 0.1 M tetraethylammonium perchlorate (TEAP) was also added as the supporting electrolyte to maintain a stable ionic conductivity. Therefore, further analysis of the interface and CO2RR with IL/acetonitrile electrolytes was performed with 0.1, 0.5, and 1.0 M concentrations of IL and 0.1 M TEAP supporting salt.
Interestingly, the general behavior of the Cdiff curves is nearly identical between the IL electrolytes in acetonitrile (Figure 4A) and with the neat IL (Figure 2B), which suggests that the ionic microhabitat and the structure of the double-layer formed on the electrode surface are similar. With 0.1 and 0.5 M IL electrolytes, the decay in capacitance with a negative potential sweep gradually starts at about −1.5 V vs. Ag/Ag+. The decline in capacitance occurs at a notably faster rate with 1.0 M IL. This decay further confirms the higher [EMIM]+ concentration covering the electrode surface, thickening the double-layer faster than the relatively diluted electrolytes (0.1 and 0.5 M IL), and impeding the diffusion of new IL−CO2 adducts towards the electrode surface, i.e., increased mass transfer overpotential. In addition to the similarities in Cdiff, the comparison of the SERS spectra obtained from the neat (Figure 2C and 2D) and diluted IL electrolytes (Figure S4) further confirm the similar microenvironment for CO2RR where Im-species (i.e., [EMIM]+ and [EMIM]+−CO2−) dominate the interface with entrapped [2-CNpyr]− and HBDs (2-CNpyrH). This microenvironment is unique compared to prior literature involving Im-based ILs since more active species exist in the interface along with native HBD (in situ generated due to CO2 absorption).
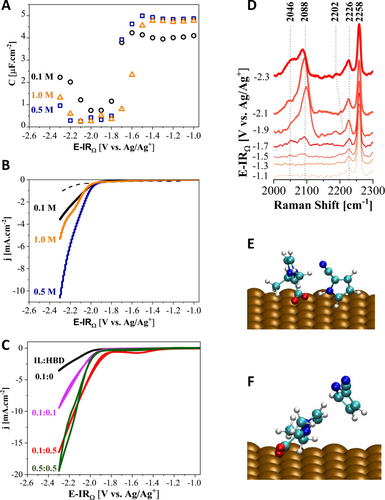
IL concentration and HBD effects on catalytic activity. Differential capacitance curves (A) and LSV measurements (B) for three different IL concentrations (0.1, 0.5, and 1.0 M) in acetonitrile with 0.1 M TEAP as the supporting salt (dashed line: N2 and solid line: CO2). The cyclic voltammograms (C) illustrate the effects of IL:HBD concentrations on CO2RR. SERS spectra (D) of the 0.1 M IL and 0.5 M HBD containing electrolyte showing COad peak (2088 cm−1). The spectra are normalized with respect to the −C≡N stretching peak of acetonitrile at 2258 cm−1. Optimized geometries of [EMIM]+−CO2− adduct with 2-CNpyrH demonstrating hydrogen bonding between −COO− and the HBD (E) and [EMIM]+−CO2− adduct with [2-CNpyr]− (F) at −1.7 V. Atom color code: blue=N; cyan=C; white=H; red=O.
The LSV measurements under CO2 suggest the onset potential for CO2RR is at about −1.9 V vs. Ag/Ag+ for all the IL electrolytes studied (Figure 4B). Consistent onset potential implies the activation energy for the charge transfer does not depend on IL concentration. However, the IL concentration significantly impacts CO2RR current densities, similar to our previous report with the Ag electrode.24 Increasing the IL concentration from 0.1 to 0.5 M results in a more than two-fold increase in CO2RR current due to the increased availability of CO2 at the electrode surface. However, the catalytic current decreases with further increase in IL concentration to 1.0 M. [EMIM]+ was reported by Urushihara et al.42 to cover the entire surface even at low concentrations. Therefore, the decrease in CO2RR activity is attributed to the surface layer formation by the cation and thickening of the double layer which prevents easy access of reactants to the electrode surface, thus increased mass transfer overpotential and lowered catalytic activity.
With respect to the role of the IL in lowering the overpotential needed for CO2RR, one widely acknowledged explanation involves the formation of the IL−CO2 adducts, i.e., carboxylate formation at the interface with CO2 binding to the C2 position of the Im ring. The facilitation of CO2RR by the carboxylate results in a decreased overpotential compared to its absence;43 however, after a particular concentration, the reaction becomes impaired, and the rate slows down, as seen with 1.0 M IL in Figure 4C, due to the double layer dynamics discussed above. Another explanation of the role of the IL focuses on the hydrogen bonding that also modulates the electrical field and interaction with the intermediates at the interface, especially with CO2⋅−. To probe specifically the impact of the hydrogen bonding network on CO2RR at the interface, we performed CV measurements with additional 2-CNpyrH (0.1 and 0.5 M) to vary HBD concentration independently in the IL electrolyte (Figure 4C).
We hypothesized that incorporating HBDs could enhance the CO2 reduction activity by increasing the -COOH intermediate formation. As seen in Figure 4C, additional 2-CNpyrH at 0.1 M does not influence the CO2RR onset potential whereas it increases the catalytic current, compared to 0.1 M IL electrolyte without the HBD addition. When the 2-CNpyrH concentration was increased to 0.5 M, the catalytic activity also increased along with a positive shift in the onset potential by 120 mV. These results suggest a substantial modification to the energetics of the first proton-coupled electron transfer step in CO2RR (i.e., formation of -COOH) as a function of HBD concentration.
The enhanced catalysis by HBD is also evident from the SERS spectra shown in Figure 4D, where the surface adsorbed CO peak (*COads in 2046–2088 cm−1 range) gains intensity at potentials more negative than −1.5 V vs. Ag/Ag+ compared to that without HBD (full spectra is given in Figure S5). The observed broad peaks indicate the presence of intramolecular C≡O stretching due to the binding of CO to the Cu electrode surface, consistent with multiple binding configurations.44 Furthermore, the results suggest that an optimal ratio of [EMIM]+ (hence carboxylate) and HBD may exist, highlighting the significance of the ionic microenvironment on the electrode surface for CO2RR and demonstrating a new approach to tune the microenvironment in non-aqueous electrolytes.
In complement, computational analysis of the simplified interface shown in Figures 4E and 4F using IC-QM/MM optimized geometries of [EMIM]+−CO2− with 2-CNpyrH and [EMIM]+−CO2− with 2-CNpyr, respectively, at −1.7 V demonstrate the hydrogen bonding in the presence of the native HBD. The HBD (CNpyrH) prefers to orient itself towards the carboxylate moiety interacting through hydrogen bonding and stays close to the surface while [EMIM]+−CO2− prefers a tilted orientation, consistent with the lower increase in intensities under CO2 (Figure 3A). Upon deprotonation and the formation of the anion, the [CNpyr]− prefers to move away from the negative Cu surface, however, gets trapped in the microenvironment due to the double layer thickening as seen from 2194 cm−1 feature in Figure 2D. From the computed anion-cation configurations, it is also seen that there is still hydrogen bonding between [CNpyr]− and [EMIM]+−CO2− through the C4, C5 protons of Im. This causes the cation to move more towards a parallel orientation as in Figure 4F.
To distinguish between the microenvironments formed by different electrolyte concentrations, constant potential electrolysis experiments were conducted at −2.1 V vs. Ag/Ag+ for 3 hours in a two-compartment cell (Figure S6) coupled with in-line GC analysis. The gaseous products were identified and quantified by in-line GC (example in Figure S7) while the liquidus products were analyzed after electrolysis by NMR (example in Figures S8). Table S3 summarizes the quantified products from electrolysis. The catholyte was the IL electrolyte of interest and the anolyte was aq. 0.1 M H2SO4 serving as the proton source. The calculated Faradaic efficiencies (FE) for H2 and C1 products, such as CO, formate, and CH4, are given in Figure 5A, and that of the C2+ products, such as succinate, C2H4, and C2H6, are shown in Figure 5B. Selectivity for CO (C1, 2e−) increases from 23 % to 43 % as the concentration of IL increases from 0.1 M to 0.5 M, as seen in Figure 5A. Further increase in the concentration slightly decreases the selectivity for CO to 37 %. High local CO2 concentrations cause premature CO desorption from the Cu surface and lead to high CO selectivity. Increasing IL concentration increases CO2 concentration on the electrode surface, leading to CO desorption.45 Furthermore, [EMIM]+ adsorption may also replace the COads on the electrode surface, causing high CO selectivity.
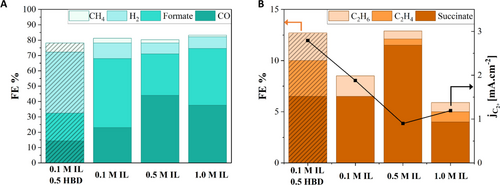
Faradaic efficiencies (FE) for C1 products and H2 (A), and partial currents (B) for C2+ products identified through GC and NMR after electrolysis for 3 hours at −2.1 V vs. Ag/Ag+.
The second major product is formate (C1, 2e−), with 45 % FE for 0.1 M IL, 26 % FE for 0.5 M IL, and 36 % FE for 1.0 M IL. The spectral evidence to -COOH in complement to significant formate presence in products strengthen the argument for the role of the proton dynamics between the Im species and HBD in controlling the mechanism leading up to CO formation and beyond in these non-aqueous electrolytes. As seen in Figure 5A, the selectivity of formate decreases from 45 % to 26 % when the electrolyte concentration is increased from 0.1 M to 0.5 M IL. This decrease is accompanied by an increase in the selectivity towards succinate (C4, 14e−), as seen in Figure 5B. Succinate has a relatively low selectivity with 6 %, 11 %, and 4 % FE for 0.1 M, 0.5 M, and 1.0 M concentrations, respectively. The initial increase in selectivity towards succinate may be due to the −COOH coupling that proceeds it; however, further increase in the concentration of −COOH does not result in increased FE for succinate, likely due to the over saturation of the electrode with [EMIM]+ as discussed earlier, thus preventing the C2−C2 coupling. FE for H2 is small, less than 10 %, since HER is suppressed in IL containing electrolytes, as generally reported in the literature46 and most of the observed HER is from water cross-over from the anolyte. However, HER enhances when HBD is introduced at 0.5 M (39 % FE) suggesting a high surface coverage by H+, which further explains the positive potential shift observed in Figure 4C. This finding underlines the role of HBDs in CO2RR selectivity and further suggests that selectivity can be tuned by IL : HBD composition and by replacing the aqueous anolyte with a non-aqueous system as the proton source. Increased H+ availability due to HBD also promotes the formation of CH4, C2H4, and C2H6, as seen in Figure 5B. Faradaic efficiencies in Figure 5 add up to 90 % of the total efficiency. Apart from the reported products in Figure 5, butane and formaldehyde were also observed in GC (Figure S9) and NMR (Figure S10), respectively, in negligible quantities. Table 1 compares the obtained Faradaic efficiencies with previous reports utilizing IL electrolytes in CO2RR on Cu-based electrodes. Uniquely, C4 products are obtained in this study owing to the constructed microenvironment presented.
Specifications |
Electrolyte |
|
Faradaic Efficiency |
Ref. |
||
|---|---|---|---|---|---|---|
H2 |
C1 |
C2-3 |
C4 |
|||
Cu foil 2-C H-cell CA at −2.1 V vs. Ag/Ag+ |
[EMIM][2-CNpyr] in ACN * |
7.6 % |
70 % CO+COOH−+CH4 |
1.5 % C2H4+C2H6 |
7.3 % C4H6O4 |
This work |
0.1 M [EMIm][2-CNpyr] +0.5 M 2-CNpyrH |
39 % |
33 % CO+COOH+CH4 |
≈6 % C2H4+C2H6 |
6.5 % C4H6O4 |
||
Cu foil 2-C H-cell CA at −2.5 V vs. Ag/Ag+ |
0.3 M [BMIM][BF4] +0.2 M [C12mim][BF4] in ACN |
N/A |
99.5 % CO |
N/A |
N/A |
[47] |
Poly(IL)- Cu 3-C Flow Cell CA at −0.85 V vs. RHE |
1 M KOH (aq.) |
8.1 % |
18.3 % CO+CH4+HCOOH+CH3OH
|
76.1 % C2H4+C2H5OH+ CH3COOH+C3H7OH |
N/A |
[48] |
Cu2O/IL-graphite 2-C H-Cell CA at −1 V vs. RHE |
0.1 M KHCO3 (aq.) |
20 % |
25 % CH4+CO+COOH− |
52.4 % C2H4+C2H5OH |
N/A |
[49] |
Cu foil 2-C H-Cell CA at −1.12 V vs. RHE |
0.1 M KHCO3 +10 mM [BMIM][TFSI] (aq.) |
17 % |
52 % CO+COOH−+CH4 |
20 % C2H4+C2H5OH |
N/A |
[50] |
BaO/Cu 3-C Flow Cell CP at 500 mA.cm−2 |
1 M KOH |
≈10 % H2 |
N/A |
≈81 % C3H7OH+CH3COO+ C2H5OH+C3H7OH |
N/A |
[51] |
Conclusion
The dynamics of reactive IL at the electrode-electrolyte interface was demonstrated by identifying the orientational change of [EMIM]+ through spectro-electrochemical studies complemented by IC-QM/MM calculations. The potential at which the orientation changes correspond to the start of the decline of double-layer capacitance, indicating the increase in double-layer thickness. The dynamics and the concentration of the IL were shown to influence the electrocatalytic behavior of CO2RR through transient voltammetry. By examining the in situ generated HBDs by SERS and simultaneously probing the independent HBD additions in electrolysis, the kinetics, the onset potential, and the reaction products of CO2RR are found to be dependent on the HBD composition in the electrolyte. The concentration of IL and HBD can switch the reaction pathways by preventing C−C coupling and increasing the hydrogenation of carbon products. Constant potential electrolysis revealed the formation of a broad spectrum of products (C2+ up to C4) enabled by the constructed microenvironment. The examination of the reaction mechanisms leading to C2+ products in these non-aqueous electrolytes by ultrafast in situ spectroscopy complemented by energetic calculations of the possible reaction steps by theory can guide synergistic electrode-electrolyte design for further tuning of product selectivity in CO2RR.
Author Contributions
O.K.C. performed the spectroscopy and electrochemical measurements and analyses. S.D. synthesized ionic liquids and analyzed gaseous products by gas chromatography. B.D. performed IC-QM/MM simulations and analysis. A.K. carried out the NMR analysis for identifying the liquid products. M.T. oversaw the computational methods and analysis. B.G. conceptualized the study, contributed to the discussions of the results, and managed the project. All authors contributed to the writing of the manuscript.
Acknowledgments
This study was funded by an NSF CAREER award (no. 2045111) from the Division of Chemical, Bioengineering, Environmental and Transport Systems (CBET), Interfacial Engineering, and Electrochemical Systems. The authors would like to thank the NMR Instrumentation Facility at the Department of Chemistry and the Raman Spectroscopy Facility at the Department of Chemical Engineering at Case Western Reserve University for access to instrumentation. Partial support for computational analysis of the electrolyte structure at the interface was from Breakthrough Electrolytes for Energy Storage (BEES)—an Energy Frontier Research Center (EFRC) of the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award # DE-SC0019409. S.D. was supported by the Center for Closing the Carbon Cycle (4C) EFRC, under Award # DE-SC0023427, for synthesis and chromatography analysis during electrolysis.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




