Spatiotemporal Studies of Soluble Inorganic Nanostructures with X-rays and Neutrons
Abstract
This Review addresses the use of X-ray and neutron scattering as well as X-ray absorption to describe how inorganic nanostructured materials assemble, evolve, and function in solution. We first provide an overview of techniques and instrumentation (both large user facilities and benchtop). We review recent studies of soluble inorganic nanostructure assembly, covering the disciplines of materials synthesis, processes in nature, nuclear materials, and the widely applicable fundamental processes of hydrophobic interactions and ion pairing. Reviewed studies cover size regimes and length scales ranging from sub-Ångström (coordination chemistry and ion pairing) to several nanometers (molecular clusters, i.e. polyoxometalates, polyoxocations, and metal-organic polyhedra), to the mesoscale (supramolecular assembly processes). Reviewed studies predominantly exploit 1) SAXS/WAXS/SANS (small- and wide-angle X-ray or neutron scattering), 2) PDF (pair-distribution function analysis of X-ray total scattering), and 3) XANES and EXAFS (X-ray absorption near-edge structure and extended X-ray absorption fine structure, respectively). While the scattering techniques provide structural information, X-ray absorption yields the oxidation state in addition to the local coordination. Our goal for this Review is to provide information and inspiration for the inorganic/materials science communities that may benefit from elucidating the role of solution speciation in natural and synthetic processes.
1 Introduction
Soluble inorganic nanostructures (SoINs) are important states of matter to control synthesis and processing of nanomaterials.1 Probing solution-phase assembly processes enables (1) Understanding important processes in nature, synthesis and manufacturing; (2) Discovering and isolating novel material topologies and assemblies, and (3) Redirecting or optimizing reaction pathways in exploratory synthesis or manufacturing.2 Some functions of nanomaterials are also demonstrated in solution; e.g. catalysis,3 medicine,4 and sensing;5 and spatiotemporal characterization allows understanding and optimizing functions. Intriguingly, SoINs conceptually lie at the interface between inorganic molecules and solid-state inorganic materials, or infinite lattices. Inorganic molecules are monodispersed or demonstrate some supramolecular assembly. Various characterization techniques including nuclear magnetic resonance, mass spectroscopy and optical spectroscopy have been well-developed to probe molecular structures.6 Related periodic lattice inorganic materials possess long-range order that can be deciphered from X-ray diffraction methods.7 Between inorganic molecules and functional solid-state materials are SoINs whose composition, topology and supramolecular assembly are more challenging to characterize; which limits opportunity for indepth understanding of structure-property relationships and rational design of functional materials from SoIN building blocks.
In this review, SoINs include (but not limited to) 1) colloid nanoparticles (NPs),8 2) 2-dimensional nanomaterials,9 and 3) molecular clusters (MCs)10 that can be dissolved in diverse medium (e.g. traditional solvents and polymers). Solubility is a defining characteristic for SoINs, distinctive from other inorganic nanomaterials. Solubility of SoINs enables solution processing and large-scale manufacturing; i.e. for conformal coatings,11 nanolithography12 and ink-jet printing.13 Due to their well-defined structures, MCs are useful as model systems that represent surfaces and related bulk materials to understand formation mechanisms, reactivity, and structure-property relationships; and are therefore an important focus of this review.14 MCs are ensembles of bound atoms or molecules of intermediate dimensions between a molecule and a bulk solid, including noble metal clusters,6 metal oxide clusters (MOCs),15 metal-organic polyhedra (MOP),16 and polyhedral oligomeric silsesquioxane (POSS).17 Together, these families of MCs cover a vast compositional and topological space, featuring a huge range of nuclearities, sizes compositions and shapes. Abundant synthetic protocols have been developed and optimized to regulate atomic compositions, dimensionalities, topological structures, and surface functionalization for advanced applications including catalysis, photo-emission, and conductivity.18 Additionally, large surface areas and enriched surface-active sites of MCs can be functionalized with organic ligands and ultimately engineered into customized matrices.19, 20 The hybridization strategy enables access to organic-inorganic composite materials, granting the opportunity to marry functions from the individual structural units.21 Clear elucidation of the hierarchical structures is imperative to establish intrinsic structure-property relationships.
Single-crystal X-ray diffraction (SCXRD) of isolated MCs is an excellent starting point for X-ray/neutron scattering and absorption studies related with SoIN solutions, derivative SoINs that do not crystallize, and SoINs introduced into other matrices for function.22 Scattering measurements record statistically-averaged structural information, via a nondestructive optimized method. In principle, both scattering and diffraction originate from interactions between radiation and matter.23 Although scattering and diffraction methods share the similar basic principle, they yield different information. Diffraction relies on long range order in a periodic lattice, and Bragg's law is applicable. Diffraction yields structural parameters of the crystal lattice down to the sub-angstrom scale,24 while scattering provides spatial coverage at the meso-scale for different states of matter (solution, gel, and bulks), and crystallization/long-range order is not required.25 By combining data from ultra-small angle scattering, small angle scattering and wide-angle scattering, the accessible spatial length ranges from a micron to 1/10th of angstrom scales, covering 5 orders of magnitudes of spatial resolution (Figure 1). Complemented by absorption spectroscopy, the local environment of target atoms, such as the coordination number, bond length, and bond angle, can be precisely quantified; as well as oxidation state of the targeted atom.26 The exploitation of different radiation sources (e.g. X-ray, neutron, and laser light) can further provide contrasting complementary structural information, providing advantages to describe complex nanostructures.27 The isotope-sensitive nature of neutron scattering28 and anomalous X-ray scattering29 allows selective observation of the target components. Structural relaxation dynamics can be accessed from quasi-elastic/inelastic scattering measurements with broad spatiotemporal coverage by probing the energy transfer between X-rays or neutrons and matter.30 In addition to static structure characterization, in operando scattering experiments inform dynamics. State-of-the-art advanced light sources and task-specific end-station configurations enable time-resolved study of rapid evolution from basic structural units to higher order supramolecular assemblies under external fields.31 As an example, a hydrated metal cation is the basic structural unit which composes polynuclear clusters, and the polynuclear clusters undergo a multitude of interactions that vary with the solution media,32 driven by pH, electrochemistry, etc. This hierarchy can be probed by the techniques described in this review.
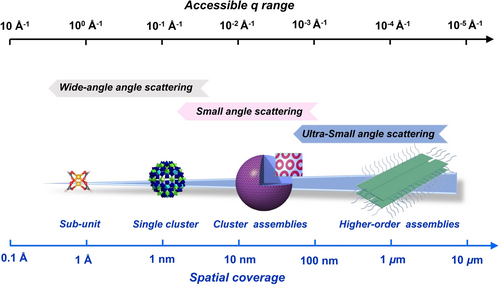
The accessible q ranges and corresponding spatial coverages of multiple X-ray and neutron techniques.
Herein, we mainly focus on SoINs and the recent advances on their spatiotemporal studies via scattering and absorption methods. Section 2 covers the basic principles of scattering/absorption techniques as well as brief information of currently available benchtop instruments and advanced light sources. In section 3, multi-modes of X-ray and neutron scattering are exploited for probing the static structures and providing quantitative spatiotemporal information of structural dynamics behavior at the meso-scale. Complementary total scattering and absorption methodologies is introduced in this section, which allows access to atomic-level information of SoINs. Finally, the challenges are discussed, and rational perspectives and outlooks are provided for the future advancement of this emerging field.
2 X-ray/neutron techniques
2.1 Fundamentals of X-ray and neutron scattering
Scattering can be simplified as a phenomenon that a wave alters its direction of propagation when meeting obstacles. The spatial distribution of the scattering wave is directly correlated with the structures of the illuminated sample.33 Common probes for scattering techniques are X-rays and neutrons. X-rays and neutrons share basic scattering principles, while their main differences originate from how they interact with atoms. X-rays are mainly scattered by electrons, whereas neutrons are scattered by nuclei.34 This means the obtained scattering length density (SLD) from X-rays and neutrons are different representatives of the same structure, providing complementary and comprehensive information.35 One must consider the composition of studied solutions; specifically absorption edges of the component elements. Scattering is most efficient away from absorption edges. Anomalous SAXS (ASAXS) exploits this by collecting series of scattering data across the edge energy to map out location of atoms within scattering species.
In general, scattering techniques can be categorized as ultra-small, small, and wide-angle modes based on the detectable scattering vector ranges. These scattering methods differ in their spatial coverages, leading to unique sensitivities of structure at different length scales. Due to cost and technology limitations, no single scattering configuration can cover the complete reciprocal space. Therefore, the selection of appropriate beamlines with required spatiotemporal coverages is governed by the information sought. With a reciprocal relationship, smaller q values translate to larger scale in real space. Pair distribution function (PDF) analysis, also called total scattering technique, which requires q-coverage as wide as possible. The PDF data is obtained by Fourier transformation of the total scattering signal and represents the distribution of all interatomic distances in the material.
Regarding instrumentation, high energy and high flux are provided by synchrotron radiation, while benchtop instruments are convenient. Another advantage of synchrotron instrumentation is the ability to tune the X-ray wavelength (energy) to the solution of study. Practical neutron facilities include reactor and spallation neutron sources, only at user facilities, and show poorer performance concerning flux and spatiotemporal resolution compared with X-ray sources. X-ray and neutron scattering both have advantages and disadvantages; and appropriate choice or complementary usage should be applied according to samples and structural information desired. To reduce possible artifacts in scattering measurements, it is important to select appropriate experimental setups, including containers, sample concentration and the homogeneity of the solutions. Meanwhile, the calibration of scattering measurements using standard samples followed by routine data reduction procedure is also key to ensure the quality of the data. More details concerning theories, practices, and data analysis protocols are summarized in the Supporting Information.
2.2 Fundamentals of X-ray absorption spectroscopy
X-ray absorption spectroscopy (XAS) is a very powerful structural technique, complementary to scattering techniques. XAS is element-specific, meaning it can selectively probe the local structure of specific elements within a material.26, 36 The XAS spectrum consists of the near edge and the extended region, i.e. X-ray Absorption Near-edge Structure (XANES) and Extended X-ray Absorption Fine Structure (EXAFS). EXAFS probes the local structure around the absorber through an interference effect. The excited photoelectron propagates as a spherical wave out of the absorber and is backscattered by neighboring atoms. The interference at the absorber site is detected as characteristic oscillations in the EXAFS region. The EXAFS process can be described analytically, resulting in the EXAFS formula, which is used to extract detailed information about bond lengths and coordination numbers. Because the intensity of the photoelectron wave is rapidly damped, EXAFS probes only few coordination shells.
The XANES region is also very sensitive to the local structure. In the absence of strong electron-electron correlation effects, the XANES is proportional to the partial density of states (DOS), whose shape is affected by the arrangement of several coordination shells around the absorber. Different from EXAFS, there is no XANES analytical formula which can be fitted to extract structural information. Instead, XANES spectra can be computed by first principles to validate underlying structural models. XANES is currently less exploited than EXAFS for structural studies due to the complexity of the analysis. The development of X-ray emission spectrometers to analyze fluorescence emitted by the sample following primary X-ray absorption initiated the acquisition of XANES in the High-Energy-Resolution Fluorescence Detected (HERFD) mode.37 HERFD XANES allows acquiring spectra with reduced core-hole lifetime broadening, resulting in sharper spectral features. This is particularly important for nanostructured materials, since the increased resolution allows to better appreciate small spectral differences. Supported by theoretical calculations, the use of HERFD XANES provides valuable structural information, as will be shown in the following sections. In Table S1, we have provided a summary of XAS beamlines in synchrotron radiation facilities, as well as some commercially available benchtop XAS instruments.
3 Study on soluble inorganic nanostructures with multi-modes of X-ray and neutron techniques
3.1 Static structural characterization
3.1.1 MOCs and their assemblies
Metal oxide clusters (MOCs) can be considered as soluble metal oxides and they are polynuclear species with multiple metal centers, linked and stabilized by H2O, OH−, and O2− ligands. These include classic early transition metal polyoxometalates (POMs), uranyl peroxide capsules, polyoxocations, and organically ligated metal-oxo clusters. Unlike metastable colloidal suspensions, ionic charge and discrete and small sizes (from sub-nm to several nms) lead to stabilizations.15, 38 Though MOCs have been studied for over a century, they are still fertile for research discovery. The heavy metals of MOCs yield SAXS patterns with well-resolved and characteristic features from which the size and shape can be derived.39 Moreover, the typical q range of SWAXS (small and wide angles combined) lies between 0.01 to 2.5 Å−1, corresponding to hundreds to sub-nm spatial coverage, which is appropriate to describe the structure of discrete MOCs40 and higher order assemblies.41
To determine MOC topologies, model fitting should be conducted, and the development of the scattering functions of classical geometrical models is critical.42 Generally available shapes are limited, including spheroids, discs, and cylinders. {Mo154}, ([Mo154(NO)14O448(H2O)70H28]14−), a donut/torus-shaped cluster has been documented in prior studies43 (Figure 2a) to undergo structural transformation to derivatives such as {Mo150} and {Mo128Eu4}, or binding with rare earth ions, if present. To better describe the structures of {Mo154} and its derivatives, Yin developed a custom carved ellipsoid model and the scattering function was systematically derived (Figure 2b).44 This work provided a new geometric model for the scattering community and facilitates the structural studies of other toroidal clusters including [P8W48O184]43−,45 MIV70 rings (M=U, Ce, Zr),46 Mn-oxo rings,47 and palladate rings.48
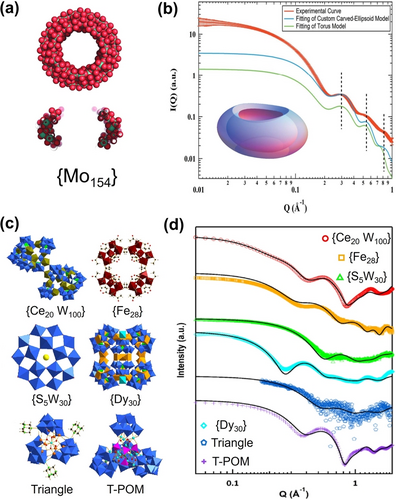
(a) Space filling model and cross-section of {Mo154}. (b) Carved-ellipsoid-shaped particle model to fit experimental SAXS of {Mo154}. (c) Polyhedral representation of POMs studied by SAXS. (d) Theoretical scattering curves compared to experimental SAXS data for POMs shown in part c. Adapted with the permission from Ref. [44]. Copyright 2020, International Union of Crystallography and Ref. [50]. Copyright 2020, Taylor & Francis.
In some cases, structural information of MOCs can be read directly from the scattering curves without complex model fitting. Tools such as SolX49 can very accurately simulate scattering data from crystal structures. For example, Yin et al. studied solution behavior of a series of POMs with diverse architectures (Figure 2c). The theoretical curves derived from crystal structure models match very well with the experimental SAXS, implying that their solid-state structures remain intact upon redissolution (Figure 2d).50 This analytical process is valuable when determining stability conditions, extracting information from polydisperse solutions and understanding assembly pathways. However, the necessary perquisite is a single crystal X-ray data for the cluster of interest. Oftentimes, supramolecular assembly or change in speciation can yield scattering data that is not identical to simulated data of the isolated cluster.51 Nonetheless, the structure is always a good starting point to understand complex and dynamic speciation.
Ion pairing is common for POMs and orchestrates assembly and function. However, studies of cations-POM assembly are usually based on indirect methods that are blind to structure. Antonio first used SAXS to directly observe the ion pairing of hexaniobate [Nb6O19]8− in aqueous solution.39b The value of radius of gyration (Rg) determined by Guinier analysis was used to detect ion-pairing. The Rg of [Nb6O19]8− associated with alkali metal ions is 0.3–0.8 Å larger than that of the bare anionic cluster and is consistent with the theoretical Rg calculated from SCXRD, revealing remarkable similarity between solution and solid association of alkalis and POMs. However, for large POMs, i.e. {Mo72Fe30}, the change of Rg due to ion association is negligible. ASAXS also accesses ion-pairing information by resolving element-specific contributions to the X-ray scattering intensity. By varying the incident X-ray energy around the Rb+ absorption edge, the contribution of Rb+ to the SAXS of {Mo72V30} varies and indicated replacement of K+ with Rb+ in solution (Figure 3a). Based on ASAXS, Liu et al. also determined the accurate distribution of Rb+ and Sr2+ around {Mo132}.52 The resonant term of ASAXS data is only related to the spatial distribution of counterions and is fitted with multi-layered spherical shell model (Figure 3b) to calculate the radial concentration profiles of Rb+ and Sr2+ (Figure 3c). It was found that most Rb+ ions were in the Stern layer of {Mo132} while more Sr2+ ions loosely associated with {Mo132} in the diffuse layer. This result explained the lower critical coagulation concentration of {Mo132} with Rb+ compared with Sr2+ (Figure 3d).53

(a) ASAXS curve for 0.052 mM {Mo72V30} in aqueous solution without Rb+ (upper plot) or with Rb+ (lower plot). (b) ASAXS profiles obtained from ASAXS data reduction and the corresponding fits with multi-layered spherical shell model. (c) Radial concentration profiles of Rb+ and Sr2+ in {Mo132}. (d) Graphical representation of the distribution for Rb+ (red) and Sr2+ (green) around {Mo132}. Adapted with the permission from Ref. [52]. Copyright 2010, American Chemical Society and Ref. [53]. Copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA.
For X-ray and neutron scattering, the structure factor describes the contribution of interactions among scatters and it can be used to characterize the interaction potential among POMs as well as the formation of supramolecular assembly (Figure 4a). SAXS is exploited by Antonio et al. to study the solution behaviors of Keggin type POMs and the structure factor is used to accurately identify the interaction potential.54 Two correlation peaks (P1 and P2) can be observed from the plot of P-HPA ([PW12O40]3−), while only P1 peak appears for Si-HPA ([SiW12O40]4−), and Al-HPA ([AlW12O40]5−) (Figure 4b). The P1 peak of Si-HPA and Al-HPA solutions corresponds to a correlation distance of 14.6 Å, which is larger than the sizes of Keggin type POMs. This suggests they remain as discrete clusters without aggregation. As for the P2 peak of P-HPA, a correlation distance (8.6 Å) close to the POM's diameter can be quantified, demonstrating the aggregation of P-HPAs via direct contact. Meanwhile, its P1 peak corresponding to 20.2 Å manifests the existence of long-range interactions among the P-HPA units. Further study shows that the temperature and concentration are important factors in governing the interplays among POMs in solutions (Figure 4c and 4d). These experimental observations are well consistent with simulation data. Also, the pH-regulated interactions of POMs are systematically investigated by Bera et al.55 The at pH=0 exhibits a shoulder peak near q=0 due to the strong attractive forces, whereas the repulsive interactions dominate and the peak at low q disappears. MSA model fitting on data provides comprehensive structure information, verifing that the POM charge changes from −3 to −7 at pH=4.7 (Figure 4e and 4f). Bauduin et al. applied the same protocol to study the effects of ionic strength in regulating the inter-POM interactions.56 Moreover, SAXS methodology offers deeper insights into the crystallization process for Keggin POMs.57 The structure factor data directly reflects that the crystallization is proceed via two-step processes. The first step involves the formation of liquid-like correlated phases, followed by the crystal growth inside the as-formed dense liquid phase in the next step.
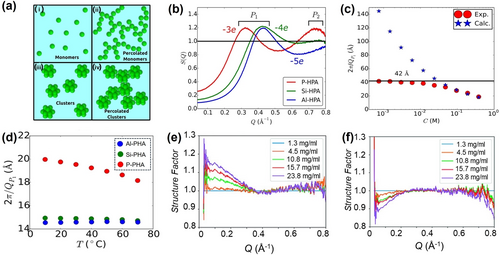
(a) Possible solution structures of NPs. (b) Normalized plots of P-HPA, Si-HPA and Al-HPA slolution. (c) The correlation distance determined from peak P1 as a function of concentration for P-HPA. (d) The correlation distance determined from peak P1 as a function of temperature for all POMs. (e) and (f) data for P-HPA solution at pH=0 and pH=4.7, respectively. Adapted with the permission from Ref. [54]. Copyright 2015, American Chemical Society and Ref. [55]. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KgaA.
Oberdisse et al. proposed a general approach based on the reverse Monte Carlo (RMC) computation to identify the percolation.58 The experimental SAXS profile is reproduced using RMC in a simulation box with length . The information of NP aggregations was extracted from the configurations by employing an aggregation recognition algorithm (Figure 5a and 5b).58 The slice images of the RMC simulation box at different volume fractions of NPs are consistent with the transmission electron microscopy (TEM) observations, which proves the validity of this protocol (Figure 5c). This method can be universally applied to diverse chemical systems.59 Moreover, the application of this method doesn't require any experience in analyzing SAXS data, which further promotes its versatility. The combination of scattering and RMC techniques provides a powerful toolbox for quantitative studies on the complicated solution behaviors of nanostructures, in addition to correlating non-intuitive SAXS with intuitive imaging.
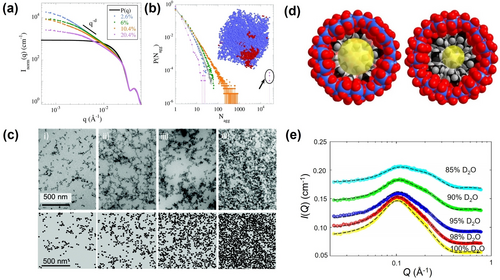
(a) SAXS profile normalized to NP's form factor for different NP volume fractions. (b) Distribution function of Nagg deduced from SAXS profile. (c) TEM pictures (top) and the respective slices of RMC-simulation box (bottom) for different NP volume fractions. (d) Schematic of the cross sections of {C2Mo132} (left) and {C4Mo132} (right). (e) CV-SANS profiles of {C3Mo132} solutions in D2O/H2O mixture. Adapted with the permission from Ref. [58]. Copyright 2018, The Royal Chemical Society Ref. [61]. Copyright 2020, American Chemical Society.
Capsule-like POMs are ideal models for studying the nanoconfined water.60 Yin used contrast variation small angle neutron scattering (CV-SANS) to investigate the distributions of water molecules inside capsule POMs (Figure 5d).61 The scattering contrasts are regulated by varying the ratios of H2O and D2O. A global-fit strategy assuming the water-filled POM as a core-shell structure is adopted to analyze the experimental data at various contrasts, and the number of water molecules inside the cavity was quantified (Figure 5e). Also, the density of confined water was quantified by excluding the volume of internally bound ligands (alkyl chain ligands with different lengths), and it was found that the water density decreases with the increasing length of ligands. Interestingly, the POM cavity exhibited complete dewetting with a core dimension of ≈11 Å, which is much larger than water molecules. This critical dewetting dimension is consistent with the length scale of a hydrogen bonded network of water, yet, a hydrogen bond network is geometrically impossible in this condition. The observed unique dewetting behavior confirms the existence of crossover of hydrophobicity at the nanometer scale.
3.1.2 Hybrid nanostructures and supramolecular assemblies
Through efforts of Cadot and Falaise,63 there has been considerable success in using cyclodextrin (CD) to create supra-structures of POMs and metal-metal bonded clusters. Hydrogen bonding between the CD and the anionic clusters is the driving force for assembly. In one example, they assembled a cluster inside two CD rings, inside a molybdate ring, formulated [Re6Te8(CN)6]@2(γ-CD))@[Mo152O457H14 (H2O)68] or Re6Te8@CD@Mo152 for short (Figure 6a).62 They used both SAXS and SCXRD to benchmark the assembly process. SAXS comparing {Mo152} alone and {Mo152} plus CD and Re6Te8 shows differences, which are readily explained by the pair distance distribution function (PDDF) analysis (Figure 6b). Specifically, while the maximum linear extent remains identical between the two solutions, commensurate with the diameter of {Mo152} (≈35 Å), there is a distinct difference in intensity of the maxima centered at ≈15 Å. The PDDF of {Mo152} alone has the characteristic shape of a core-shell profile with a lower shoulder on the left side, due to diminished scattering from the empty center. The maximum increases in intensity above that of the peak at ≈30 Å (from the {Mo152}shell) with introduction of the Re6Te8 cluster plus the CD, which is due to the higher electron density of the core when it contains this heavy element cluster. This SAXS study clearly shows pre-assembly of Re6Te8@CD@Mo152 prior to crystallization.
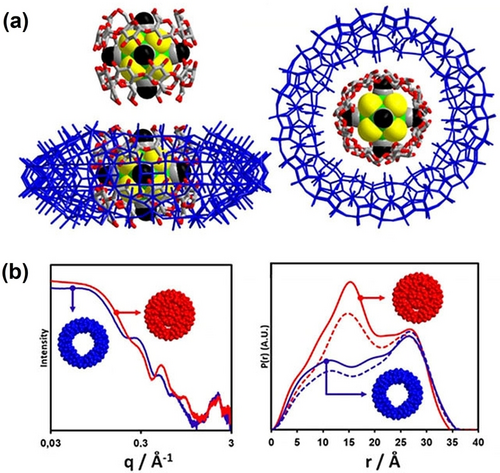
(a) Two views of Re6Te8@CD@Mo152.62 Blue wire framework is the {Mo152}, green, yellow, and black space-filling model is the Re6Te8 cluster, surrounded by the gray and red CD ring. (b) SAXS (left) of {Mo152} solution (blue) and {Mo152} solution plus CD and Re6Te8 (red), and corresponding PDDFs (right). For the PDDFs, the solid lines are experimental, the dotted lines are simulated. Adapted with the permission from Ref. [62]. Copyright 2021, Wiley-VCH Verlag GmbH & Co. KgaA.
Metal organic polyhedra (MOP) skeletons provide robust molecular templates for the fabrication of hybrid nanostructures.64 Enriched functional groups including alkyl chains,65 polymers,66 and clusters,67 can be appended to the surface of MOPs via coordination driven self-assembly. Scattering methods offer overwhelming advantages in characterizing this type of hybrid structures, due to the intrinsic paramagnetic features and complicated hierarchical structures.68 Taking PS-MOPs (polystyrene functionalized Cu-MOPs) as an example, scattering methods are used to characterize their structures.69 Regarding the organic-inorganic structural features of PS-MOP, SAXS and SANS cooperatively reveal their core-shell structures with a high electron density core and hydrogen-rich shell from the geometric model fitting, which agrees well with the molecular design (Figure 7a and 7b). The surface-grafted polymer layer significantly altered the solvation structure of PS-MOP, as determined by SANS. In the deuterated THF solution of PS-MOPs, the chemical composition of the solvent inside the porous MOP core, which is indexed by the scattering length density (SLD), remains that of the original solvent, rather than the deuterated solvent. This suggests the presence of the rigid and thick PS layer severely prohibits mass exchange between the MOP core and external environment.
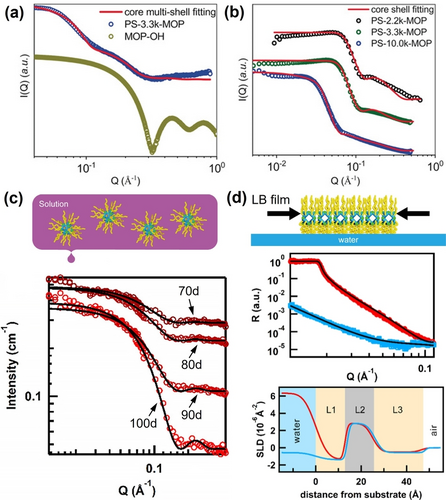
(a) SAXS and (b) SANS of different PS-MOPs in solution; scattering data are fit with a core-shell model. (c) Schematic of C18-MOP solution (inset); CV-SANS curves (red circles) and fitting curves (black lines) of the C18-MOP in THF at different deuteration ratios. (d) Neutron reflective data of C18-MOP LB films and the corresponding fitted scattering length density (SLD) profile. Adapted with the permission from Ref. [69]. Copyright 2020, Wiley-VCH Verlag GmbH & Co. KgaA. and Ref. [71]. Copyright 2019, American Chemical Society.
Alkyl chain-coated MOPs (Cn-MOPs, n denotes carbon atom number of the alkyl chain) have demonstrated potential applications as selective ion channels, yet the underlying molecular mechanisms remain unclear.70 The neutral ‘hairy’ MOPs remain as discrete particles in solution, which enables topology studies. Scattering data confirms the typical core-shell architectures of the Cn-MOPs (Figure 7c).71 CV-SANS experimental results demonstrate that the solvent molecules can penetrate the surface alkyl chains and reside inside the MOP cores, which means the mass exchange between the Cn-MOPs and external environment is possible. LB thin films of Cn-MOPs were fabricated at the water/air interface. Neutron reflectometry measurements were conducted to probe the monolayer structures normal to the surface of hairy MOPs under compression (Figure 7d). The neutron reflective data suggests the MOP cores are in the middle while alkyl chains are mainly distributed upward and downward. The bottom alkyl layer is thinner with higher packing density due to hydrophobic interactions.
SoINs can be chemically integrated into gel networks.72 Johnson et al. prepared a set of hybrid gels, in which the MOP cores are served as junctions for stabilizing the gel networks.73 SANS well describes the hierarchical structures of these gels (Figure 8a and 8b).73 A summed model fits the experimental scattering data well and the derivate structure parameters conform to the expected values, proving that the gel structures are as designed. Photo-reversible molecular units are further employed for the fabrication of smart hybrid gels.74 After UV irradiation, the SAXS curve exhibits a well-resolved peak at low q region and five new peaks arise in the high-q regime, corresponding to the form factor of Pd24L48 cage with a 2.9 nm radius (Figure 8c). SAXS verifies the structural transitions of MOP junctions within the gels (Figure 8d), which provides the molecular origin of the observable changes of their mechanical properties. Recently, Richtering et al. harnessed the superchaotropic effect of silicotungstic acid (HSiW) to crosslink the hydroxypropylcellulose (HPC), and thereby facilitating the gelation process.75 Notably, upon on the addition of 1 mM HSiW, the correlation peak at low q can be observed as the structure factor for the repulsive interaction among charged NPs. Cylinder model fitting at high q region affords a characteristic dimension, which shows monotonic increase with increasing HSiW loadings. This suggests that nanoscaled HSiW induces the aggregation of HPC chains (Figure 8f). Due to the electrostatic screening effects of NaCl, the repulsions among HPC chains are dramatically suppressed, supported by the disappearance of correlation peaks in the SANS plot (Figure 8e).
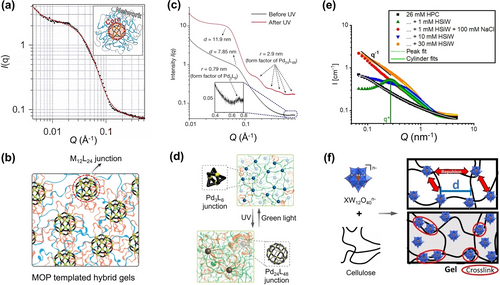
(a) SANS data (black) and model fitting curve (red) for supramolecular metallogel. (b) Schematic illustration of the gel with MOP junctions. (c) SAXS plots of the metallogel before and after UV irradiation. (d) Schematical illustration of the MOP reversible rearrangement from Pd3L6 to Pd24L48 upon irradiation. (e) SANS data of HPC solutions with the addition of different amounts of HSiW and NaCl. (f) Schematical illustration of the interaction mechanism for HPC-HSiW gels. Adapted with the permission from Ref. [73]. Copyright 2015, Nature Publishing Group, Ref. [74]. Copyright 2018, Nature Publishing Group, and Ref. [75]. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KgaA.
3.2 Spatiotemporal studies of complex nanostructures
3.2.1 Reaction kinetics and formation mechanisms of metal oxide clusters
Many metal oxide clusters are first discovered by crystallization and structural analysis, however dedicating efforts and time are required for crystallization. Solution scattering characterization provides new insight into kinetics and mechanisms of cluster formation, and then the SCXRD data becomes supporting information via simulated scattering data. The structure of ferrihydrite, poorly crystalline iron oxyhydroxide that comprises rust, soil, and the core of ferritin, has been the subject of debate for decades. Central to the debate is the presence of tetrahedral iron in the structure. Michel and Parise76 proposed a structural model that contains the Fe13 Keggin cluster as the core building unit, via PDF from X-ray total scattering. The Fe13 Keggin cluster contains tetrahedral iron surrounded by twelve octahedral irons, akin to POM Keggin ions discussed throughout this review. Sadeghi applied SAXS to study the formation and manipulation of Fe13 in solution (Figure 9),51a informed by crystallizing the compound with capping Bi3+-countercations. This highly reactive Fe13-core can be stabilized in water via capping with six Bi3+ plus ligands, Bi6[FeO4Fe12O12(OH)12(O2C(CCl3)12]+1 (Bi6Fe13, Figure 9a, blue box inset). Bi6Fe13 redissolved in acetone yields scattering consistent with the complete Bi6Fe13 cluster (Figure 9a, blue). The scattering of the synthesis solution (Figure 9a, green), on the other hand is consistent with the Bi6Fe13 cluster without the ligands, suggesting the Bi3+ is important in stabilizing the Fe13 core, but the ligands just assist crystallization. In contrast, SAXS of the synthesis solution without Bi3+ shows higher scattering intensity at low q, representing polydispersity and formation of larger aggregates (Figure 9a, red). A log-normal particle size distribution analysis informs a mode radius of 11.2 Å (Figure 9a, inset), approximately 8× larger in volume than the isolated Fe13. This highlights the significance of Bi3+ in stabilizing the small Fe13 cluster without aggregation. Removing the ligands and Bi3+ leads to supramolecular assembly of the cluster into chain-like nanostructures with a primary particle radius of 11 Å (Figure 9b), consistent with the linear arrangements of natural ferrihydrite NPs. TEM images provide intuitive evidence for the chosen form factors for data modeling, and selected area electron diffraction indicates incipient formation of ferrihydrite (Figure 9b). The SAXS analysis supports that Fe13 is a key pre-nucleation cluster for the Michel-Parise model for ferrihydrite formation.76 Shaw and colleagues later executed a chemically simpler experiment by exploiting pseudo in situ SAXS measurement (Figure 9c).77 They simply pH-adjusted a ≈7 mM solution of Fe3+ from 0.1 to 4 with NaOH. The reaction solution was continuously stirred and pumped through the sample chamber for analysis, and data was collected as a function of increasing pH. This scattering data similarly showed the formation of Fe13, prior to assembly of larger linear aggregates.
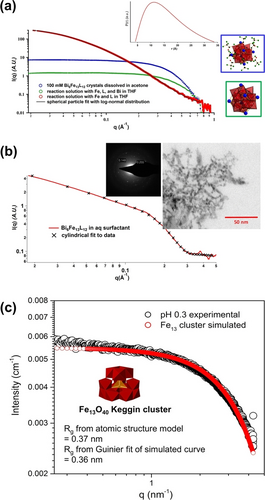
(a) SAXS of the synthesis solutions and redissolved crystals of Bi6Fe13. (b) SAXS of Bi6Fe13 in conditions that remove the Bi and the ligands and retain the iron-core. Inset is a TEM image (right) plus electron diffraction (left). (c) Scattering curve from in situ SAXS study of formation of Fe13, followed by aggregation as a function of pH, showing initial Fe13 formation at low pH. Adapted with the permission from Ref. [51a]. Copyright 2015, The American Association for the Advancement of Science and Ref. [77]. Copyright 2016, American Chemical Society.
Among the POM families, polyoxoniobates (PONbs) are unique with high negative charges that lead to strong interactions with alkali-countercations. Beyond the role in charge-balancing, Sures showed that alkalis alone can drive speciation changes in PONbs (Figure 10a).78 Specifically, addition of ACl (A=Li, Na, K, Rb, Cs) to aqueous decaniobate (Nb10O286−, Nb10), as observed by SAXS, leads to formation of much larger clusters. Moreover, the rate of the reaction and the resultant cluster size increase with alkali size. The increasing size can be seen with increasing scattering intensity and shift of the Guinier region to lower-q, Figure 10a. At the time of publication,78 Nyman was never able to grow crystals to prove what was present in solution. In a separate study, they employed the same strategy using alkali carbonates, and the increased basicity from the carbonate yielded [Nb24O72]24− (Nb24) in solution that are not linked by the alkalis.79 Again, this was determined primarily by SAXS analysis. PDDF analysis to obtain a real-space shape representation of these cluster solutions (Figure 10b) produced a profile representative of a core-shell type structure with a size similar to Nb24 (Figure 10c), reported prior.80 With this hypothesis as a starting point, they simulated alkali-linked Nb24 units and determined that increase size of species detected by SAXS with increased alkali size is due to increased alkali-linking of Nb24 (i.e. dimers for Li and Na, tetramers for Rb and Cs).78 They characterized both Nb10 and Nb24 by benchtop PDF in solution, strengthening the argument for Nb10 to Nb24 conversion.78 Moreover, they hypothesized the challenge to crystallization was the lability of the three H2O−Nb=O units (Figure 10c) that link the three Nb7 units (polyhedral representation, Figure 10c) into the ring structure. In follow-on attempts to crystallize Nb24, they added uranyl to the reaction, with a more stable O=U=O unit. Indeed two structures were obtained that feature the Nb24 unit with substitution of the uranyl on the H2O−Nb=O site,81 confirming assignment of structure from only the SAXS data was correct.
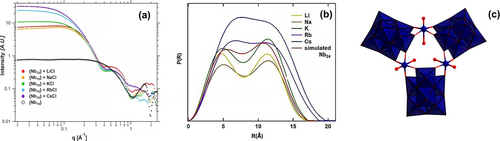
(a) SAXS of Nb10 plus added alkalis showing conversion to Nb24 linked by alkalis. (b) PDDF representation of Nb24 with alkali carbonates. (c) Structural representation of Nb24. Black spheres are carbonate, red spheres are oxygen. Adapted with the permission from Ref. [78]. Copyright 2018 American Chemical Society, Ref. [79]. Copyright 2021, Wiley-VCH Verlag GmbH & Co. KgaA, and Ref. [80]. Copyright 2006, Wiley-VCH Verlag GmbH & Co. KgaA.
POMs are stable in solution and can be detected via SAXS in reaction solutions to optimize conditions for crystallization. Falaise82 demonstrated that the capsule-shaped uranyl peroxide polyoxometalate U28 ([(UO2) (O2)1.5]2828−), forms instantaneously upon dissolving studtite (UO2(O2)(H2O)2 ⋅ 2 H2O) in LiOH. The SAXS of these uranyl peroxide capsules (Figure 11a) are very distinct due to the high electron density contrast between core (containing water and alkali-cations) and shell (composed of uranyl peroxide), including the presence of strong oscillations. The solution was aged for 60 days, which led to a slight shift in both the oscillations and the Guinier elbow to higher q, indicating evolution to a slightly smaller capsule. Falaise presumed this indicated evolution to U24 ([UO2(O2) (OH)]2424−), with decomposition of the peroxides and replacement by dihydroxides. Indeed, U24 was crystallized from solution via vapor diffusion of methanol, proving this hypothesis correct.
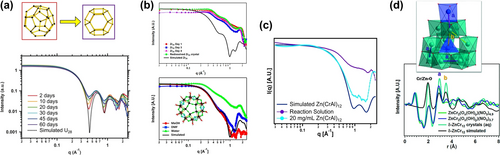
(a) SAXS of uranyl peroxide capsules showing evolution of U28 (top left) to U24 (top right) over 60 days. (b) SAXS of reaction solution (top) and redissolved Zr25 (bottom). (c) SAXS of reaction solution for Zn(Cr,Al)12, and redissolved crystals. (d) PDF of reaction solution and redissolved crystals of ZnCr12. Adapted with the permission from Ref. [82]. Copyright 2016, Wiley-VCH Verlag GmbH & Co. KgaA, Ref. [84]. Copyright 2019, American Chemical Society, Ref. [87]. Copyright 2016, Elsevier Ltd, and Ref. [88]. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KgaA.
On the other hand, in multiple instances, reaction solutions of polyoxocations reveal polydispersity and smaller cluster fragments via SAXS. This is due to: 1) the lability of these clusters that feature water and hydroxyl ligands instead of the multiply bound oxo-ligands that stabilize the surface of transition metal and uranyl polyoxometalates,83 or 2) extreme synthesis conditions that promote equilibrium between clusters and fragments. The latter phenomenon was observed in assembly and crystallization of Zr25-peroxide ([Zr25O10(OH)50(O2)5(H2O)40]20+, Figure 11b, bottom, inset).84 Zr25 was obtained simply by room temperature evaporation of a 1 : 1 peroxide:Zr solution. Over four days of evaporation prior to crystallization, the SAXS remained invariant, suggesting a mixture of cluster fragments much smaller than the fully-formed pentagonal shaped Zr25 (Figure 11b, top). Isolated Zr25, can be redissolved in methanol and DMF, in addition to water. Redissolved in water, the SAXS of Zr25 again indicates fragmentation. However, dissolution of Zr25 crystals in the organic solvents show intact Zr25; again, by comparison to simulated scattering (Figure 11b, bottom).
Aluminium Keggin ions, i.e. Al13 (AlO4Al12(OH)24(H2O)127+) are amongst the most studied polyoxocations for developing aqueous reactivity and speciation diagrams, building blocks for materials, and commercial products in water treatment and personal care.85 Given the similarities between aqueous Al3+ and Cr3+, there were early attempts to isolate a Cr13 analogue.86 Wang87 and Amiri88 accomplished this goal, and a central tetrahedral Zn2+ was one key to isolate both a mixed Zn(Cr,Al)12 Keggin ion and a ZnCr12 Keggin ion; the former confirms the similarity of Al3+ and Cr3+. However, this was not the only key, and X-ray scattering providing insight into unusual reaction pathways. SAXS and mass spectroscopy indicated that both Zn(Cr,Al)1287 and ZnCr1288 never fully assembled in the reaction solution, rather fragment formulae were detected (Figure 11c, Zn(Cr,Al)12). To complement SAXS and ESI-MS, the ZnCr12 assembly was studied by benchtop PDF (Figure 11d), and this also evidenced reaction solutions only contained cluster fragments. In this combined PDF/SAXS study, reaction time points differing by the amount of nitric acid resulted in growth of peaks a and b (Figure 11d), which represents Cr−Cr and Zn−Cr pair correlations indicating assembly of larger cluster fragments. On the other hand, SAXS and PDF of redissolved crystals indicated their stability in water, again by comparison to simulated scattering (Figure 11b and 11c). The solution conditions for both studies (high acidity and high nitrate) supress hydrolysis, which only permits formation of smaller clusters. Yet synthesis conditions that could support the full Keggin ion (lower ionic strength) only yielded amorphous precipitates.
The indepth Zn(Cr,Al)1287 study concluded that the fully-formed clusters must assemble at the air-liquid interface where nano-environments promote lower acidity and higher metal concentration, forcing hydrolysis and nucleation, without uncontrolled bulk precipitation. Bera et al.89 showed exactly this by in situ grazing incidence XAS, comparing the bulk (monomers) to the surface (clusters) of an erbium chloride solution. To summarize, controlled growth and especially crystallization of aqueous polyoxocations (examples reviewed here include Zr-peroxide, Al and Cr) require conditions that do not support persistence of the fully-formed clusters, and X-ray scattering was key to providing this information. However, much information is still missing. To access this information, advanced synchrotron instrumentation that allows spatiotemporal studies (i.e. distinguishing bulk from surface) such as that demonstrated by Bera is extremely important.
Recently, Sommers et al.90 used crystallization and SAXS to describe the atomic level details of the Zr−Hf industrial solvent extraction separation process, crucial to the nuclear industry since the 1950s.91 Hf is extracted selectively into an organic phase from crude Zr ore (containing a few % Hf) in an aqueous HCl-ammonium thiocyanate solution. SAXS informed every step of the extraction. First, oxo-centered tetrahedral clusters OM4 (M=Hf, Zr, [M4O(OH)6(NCS)12](NH4)4, Figure 12a inset) were crystallized from both aqueous Zr-ammonium thiocyanate and Hf-ammonium thiocyanate solutions, prepared separately to determine speciation differences between Zr and Hf. This result did not explain the difference between Zr and Hf, but SAXS of these aqueous phases began to provide an answer (Figure 12a). First, simulated SAXS of OHf4 matches very well with the aqueous Hf solution, while the aqueous Zr solution shows the presence of larger species (≈10 % by volume larger species, determined by size distribution analysis). The larger clusters were coaxed out of solution with addition of Cs-salt, and SCXRD revealed a cluster with a nuclearity of 48, Zr48. Simulation of Zr48 scattering (Figure 12a) confirmed the Zr48 was responsible for the shifted Guinier region and increased intensity, compared to the analogous Hf solution. SAXS of the organic phases, post Hf and Zr extraction, respectively show extraction of OHf4, and minimal extraction of any Zr-species (Figure 12b). While the simulated OHf4 matches well with the organic Hf-extractant, the organic Zr-extractant exhibits a shift to higher-q compared to the simulated scattering. Aside from comparison to the simulated scattering data, there are other indications of poor extraction. The intensity at the lowest observed q-value of the Hf scattering is an order of magnitude higher than that of the Zr scattering. Second, comparison of solvent scattering (q>1 Å−1) to molecule scattering (q<1 Å−1) shows that the Hf-organic phase contains considerably higher concentration of extracted species than the Zr-organic phase.
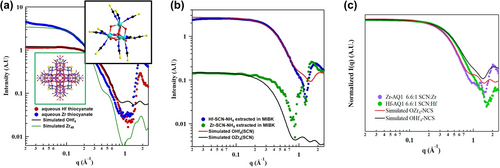
(a) SAXS of aqueous Hf-ammonium thiocyanate (red) and Zr-ammonium thiocyanate (blue) plus simulated scattering for OHf4 (black) and Zr48 (green). Inset in green box is Zr48, inset in black box is OM4 (M=Hf, Zr). (b) SAXS of organic phase, post extraction from Zr-ammonium thiocyanate solution (green) and Hf-ammonium thiocyanate solution (blue). (c) Scattering of aqueous solutions with doubled amount of ammonium thiocyanate, showing suppression of formation of Zr48. Adapted with the permission from Ref. [90]. Copyright 2022, American Chemical Society.
All of these SAXS and crystallization experiments were performed using a 3.3 : 1 NH4SCN : M (M=Hf, Zr) ratio, identical to that used in the industrial process, and only 10 % excess SCN compared to stoichiometry of OM4. By doubling the ratio to 6.6 : 1, formation of Zr48 is supressed and both the Hf and Zr solutions clearly show pure phase OM4 (Figure 12c). In this experiment, the power of X-ray scattering, specifically sensitivity to electron density, is revealed. Although isostructural OZr4 and OHf4 have the exact same size, OHf4 appears smaller in both the experimental and simulated scattering, corresponding with a shift to higher-q in the Guinier region, compared to OZr4. This is due to higher electron density contrast between core Hf and peripheral S, which decreases the magnitude of scattering vectors through the cluster.
Small clusters and counterions are usually considered as the transient molecular templates in the formation pathway of protein-sized MOCs, which refers to the well-accepted templated synthesis mechanism. Yin et al. used time-resolved SAXS to reveal the formation mechanism of [MoVI72MoV60O372(CH3COO)30(H2O)72]42− ({Mo132}).92 A theoretical scattering curve of {Mo132} provides a good match with experimental SAXS, confirming it's stability in solution. The original molybdenum precursors deliver featureless scattering plot. With time flies, characteristic oscillations appear at high q and become more distinct, indicative of {Mo132} assembling (Figure 13a). More importantly, the intensity of the first oscillation at q≈0.4 Å−1 can be used to quantitatively analyze the growth kinetics. The plot of peak intensity versus time gives a sigmoid curve, which is consistent with the kinetics data obtained by NMR. Combined with UV/Vis data, multi-step formation mechanisms, in which {MoV2(CH3COO)} is the transient template is proposed. In a separate study, SAXS was employed to uncover the formation processes for core-shell shaped Keg@{Mo72Fe30}.60b The operando SAXS data demonstrates formation of Keg@{Mo72Fe30} in solution with time (Figure 13b). Finally, a three-stage formation mechanism can be rationally deduced based on the in situ SAXS data with high spatiotemporal resolution.
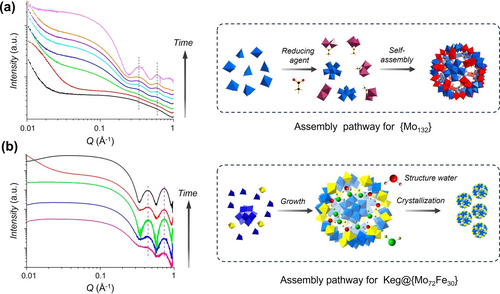
(a) Time-resolved SAXS of synthetic solution of {Mo132} from reaction time 0 to 19 h (left) and the proposed formation pathway for the giant clusters (right). (b) Time-resolved SAXS monitoring of the synthsis of Keg@{Mo72Fe30} with reaction time from 0 to 22 h (left) and the formation mechanism of Keg@{Mo72Fe30} (right). Adapted with the permission from Ref. [92]. Copyright 2016, American Chemical Society and Ref. [60b]. Copyright 2016, American Chemical Society.
3.2.2 Structural Dynamics and assembly pathways of hybrid nanostructures
Hybrid surfactant can self-organize into micelles, which further coagulates into nanobelts in water (Figure 14a). By applying time-resolved SAXS, the stepwise assembly processes were revealed by Liu et al.93 SAXS of the freshly prepared hybrid surfactants solution shows typical scattering of core-shell structures. The total radius, core radius, and shell thickness can all be quantified from model fitting. With aging, the characteristic core-shell oscillations became weaker and completely disappeared after 680 min, while a sharp peak centered at q=0.202 Å−1 rises and grows simultaneously (Figure 14b). This scattering feature corresponds to an interlayer distance of 3.11 nm, originating from the lamellar packing of the hybrids. Sigmoidal shaped feature was observed for the peak intensity plot as a function of time, suggesting the slow coalescence rate of the micelles at the initial time. More interestingly, Weinstock et al94 realized the successful polymerization of a bifunctional Keggin cluster to afford a linear polymer with entirely inorganic backbones (Figure 14c). The flexible μ-oxo-linked backbone permits the as-formed polymer to coil, analogous to typical linear polymers (inset picture in Figure 14d). By fitting the SAXS data, it is confirmed that compact sphere with average radius of ca. 15 nm (green curve) and individual Keggin ions (red curve) both exist (Figure 14d), which is supported by dynamic light scattering and cryo-TEM. This work demonstrates the exciting potentials of inorganic nanostructures as buliding blocks for the fabrication of functional materials with higher ordered structures.
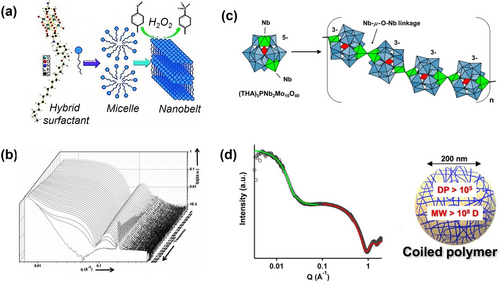
(a) Graphical representation of the self-assembly process of the hybrid surfactant. (b) Time-resolved SAXS data of the hybrid surfactant solution. (c) Formation of a polyether-like inorganic polymer from [PNb2Mo10O40]5−. (d) SAXS data for a concentrated sample of the solution (gray circles) and model fitted curves (green and red), inset picture represents that the flexible polymer chains coil into spherical nanoassemblies. Adapted with the permission from Ref. [93]. Copyright 2014, Wiley-VCH Verlag GmbH & Co. KgaA and Ref. [94]. Copyright 2020, American Chemical Society.
The study on the dynamics of coordination bonds is still in it's fancy. Yin et al. explored the dynamic behaviors of MOPs, that is, the coordination bonds can be dynamically broken and reformed at characteristic time scales in solutions.95 The high sensitivity towards light elements of SANS techniques brings overwhelming advantages for uncovering the hierarchical inorganic-organic nanostructures as well as their dynamics.96 The ligand exchange processes can be tracked by time-resolved SANS. The addition of excess PS ligand to the solution of alkyl chain-coated MOPs triggers the ligand exchange process. The free PS ligands adapted with Gaussian coil conformations are bonded together to form core-shell shaped nanostructures, supported by the SANS data (Figure 15a). In addition, ligand exchange among different MOP species is monitored during the reaction process. Initially, the SANS pattern implies a wider size distribution of mixed MOPs in the solution. Up to 48 h, the exchange reaction has reached equilibrium. The final product presents a narrower size distribution and relatively lower scattering contrast, which demonstrates the two cages are chemically fused yielding a stable hybrid MOP, resulting from dynamic ligand exchange (Figure 15b). This unique dynamic behavior can be further harnessed for the construction of MOP cross-linked vitrimer for gas separation.97
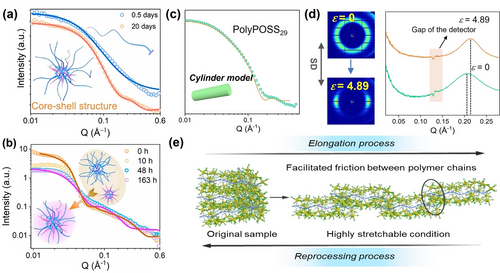
(a) SANS is used to monitor the ligand exchange of MOP and PS ligand at room temperature. (b) SANS tracks the ligand exchange between two MOPs at 120 °C. (c) SANS of PolyPOSS29 and fitting curve based on the cylinder model. (d) 2D SAXS (left) and corresponding 1D SAXS (right) for PolyPOSS29, before and after stretching. (e) Proposed structural models to interpret the strain-hardening behaviors. Adapted with the permission from Ref. [95]. Copyright 2021, American Chemical Society and Ref. [99]. Copyright 2021, Wiley-VCH Verlag GmbH & Co. KgaA.
POSSs are a family of cage like molecular entities comprised of alternative connected Si−O bonds.98 Recently, norbornene was chemically-appended to the POSS surface.99 The polymerization of this precursor leads to the generation of a millipede-shaped hybrid polymer. Unexpectedly, this polymer has excellent mechanical properties without cross-linking and strong supramolecular interactions. SAXS and contrast complemented SANS provided comprehensive structural information from the solution structure to the packing structure at multi-scales (Figure 15c). Operando SAXS (solid-state) enabled in situ monitoring of structural transition in response to an external force (Figure 15d). Typical orientation of polymer chains are clearly detected, evidenced by the in situ SAXS. Additionally, the polymer backbone to backbone distance decreases, characteristic of more condensed interpenetrated microstructures resulting from stretching. The directional packing of polymer backbones greatly enhances the friction between polymer chains, accounting for the observable strain-hardening behaviors (Figure 15e). Overall, clear physical images of complex structures and dynamics were obtained.
3.3 Combined applications of PDF, XAS, and SAXS to metal-oxo clusters and small NPs
3.3.1 Coordination of metal ions in solution and cluster formation, M38 (M=CeIV, BiIII, PuIV)
Here we review related studies on a family of M38 (M=CeIV, BiIII, PuIV) oxocluster compounds, in which SAXS, PDF and XAS were employed in four separate studies. Notably, the Np38100 and U38101 analogues have also been synthesized and characterized by SCXRD. Briefly, the MIV38 (MIV=Pu, Ce, Np, U) all have isostructural cores that are similar to the CaF2 structure, and BiIII38 is quite different, likely a direct consequence of the lone-pair effect that drives distortion. Each M38 is centered around a M6O8 hexamer that is the inorganic node of the UiO-66 MOFs,102 but the Bi38 has an addition μ6-oxo in the center.
PDF from X-ray total scattering has been used by Soderholm, Skanthakumar and collaborators to characterize the coordination environment of metal ions in solution103 and the oligomerization and hydrolysis processes leading to cluster formation.104 Studying the hydrolysis and oligomerization of PuIV, they found monodisperse, nanometer size, surface stabilized (Cl−) clusters of plutonium dioxide. Crystals obtained from the solution revealed well-defined Pu38 clusters ([Pu38O56Cl54(H2O)8]14−)104b (Figure 16a), and the match between the measured PDF and the model built from the crystallized Pu38 supports proposed reaction pathways (Figure 16b). The absence of hydroxo-bridged Pu in the Pu38 cluster suggests that the condensation reaction of PuIV may proceed by oxolation rather than olation, contrary to what previously reported,105 and that is similar to the hydrolysis chemistry seen for POMs.
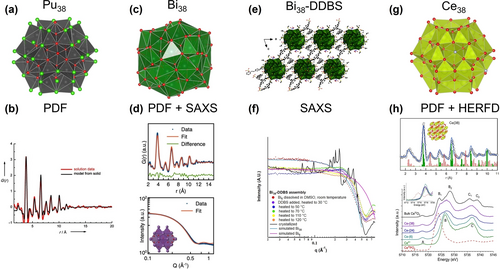
(a) Structure of the Pu38 cluster. (b) PDF data of clusters in solutions compared with calculated PDF from the XRD structure of Pu38. (c) Structure of the Bi38 cluster. (d) Experimental PDF (top) and SAXS (bottom) of the final product compared with calculated PDF and SAXS of Bi38 cluster. (e) Structure of the Bi38-DDBS framework. (f) SAXS showing the formation of Bi38 in DMSO, followed by addition of DDBS linker, organization into a framework, and finally crystallization. (g) Structure of the Ce38 cluster. (h) Experimental and calculated PDF on Ce38 (top) and Ce L3-edge HERFD on clusters of increasing nuclearity. Adapted with the permission from Ref. [104b]. Copyright 2008, Wiley-VCH Verlag GmbH & Co. KgaA, Ref. [106]. Copyright 2021, Wiley-VCH Verlag GmbH & Co. KgaA, and Ref. [109]. Copyright 2023, American Chemical Society.
Anker et al. recently combined in situ SAXS and PDF from total X-ray scattering to investigate the formation of the Bi38 oxo-nanoclusters (Figure 16c), and identify intermediate cluster nuclearities.106 In their study, they started with dissolution of [Bi6O5(OH)3(NO3)5] in DMSO, and followed the reaction, leading from the [Bi6O8] units to the [Bi38O45] final product. SAXS also provided information about the size, shape, and polydispersity of the intermediate products, while PDF from total scattering was used to determine the short-range atomic arrangement (Figure 16d). Contrary to the expectation, they identified [Bi22Oy] as the main metastable intermediate. The results are solid based on the parallel refinement of PDF and SAXS data with the same structural models.107
Amiri et al.108 took this study a step further and used SAXS to benchmark the formation of coordination polymer networks that feature the Bi38 node linked by DDBS (2,2′-[biphenyl-4,4′-diylchethane-2,1-diyl] dibenzenesulphonate) (Figure 16e). Using a benchtop instrument and in situ heating, not surprisingly, they also observed Bi38 rather than Bi6. With heating and addition of the DDBS linker, they were also able to track pre-organization in solution by modeling the structure factor, followed by crystallization of the framework, with the observation of diffraction peaks (Figure 16f). With the benefit of the Bi38-DDBS framework crystal structure, they were able to correlate cluster-to-cluster distances in solution and in the solid state, as well as confirm identification of the compound that crystallized in the in situ study.
Understanding the evolution of atomic structure during cluster assembly with increasing nuclearity can be complemented by parallel investigation of the evolution of the electronic structure. In a recent study, Estevenon et al.109 investigated the atomic and electronic structure of Ce oxo-hydroxo clusters with PDF and Ce L3 edge HERFD XAS. Ce oxo-hydroxo clusters represent the fundamental building blocks in CeO2 nanoscale architectures, where molecular clusters can exhibit reactivity similar to CeO2 NPs.110 To explore assembly processes, a series of Ce-oxo clusters with nuclearity (Ce2 to Ce38) were synthesized and compared with small CeO2 NPs and bulk CeO2.109 The employed methods consistently revealed trends as the system size progressed from molecular Cen clusters (n=2, 6, 24, 38, and 40) to bulk CeO2. From PDF, the increased nuclearity can be readily seen and good agreement is found between the data and the PDF calculated from available structures of Cen clusters (Figure 16g). The shortest correlation in PDF, corresponding to Ce−O bonds, showed a decreasing disorder when going towards larger nuclearity to match the final single Ce−O distance in CeO2. HERFD XAS at the Ce L3 edge concomitantly probed the evolution of the electronic structure upon size increase.
Analysis of the Ce L3 pre-edge region, denoted as peak A (Figure 16h) and arising from transitions between the 2p and 4f valence orbitals, revealed that Ce clusters are only made of CeIV,111 and no CeIII species were found (inset in Figure 16h). Inspection of the post edge region of Ce L3 edge HERFD XAS additionally showed that the shape of Ce 5d states gradually changed from that of CeIV in aqueous solution, characterized by two primary peaks, labeled B and C in Figure 16h, to that of bulk CeO2, where B and C are further split in B1–B2 and C1–C2 by the Oh crystal field of CeO2 fluorite structure. This behavior reflects the effect of the building-up of the cubic crystal field, which splits the Ce 5d density of states into eg and t2g bands, corresponding to B1 and B2.112 With increased nuclearity, the density of states is progressively more ordered until it matches that of crystalline CeO2.
The role of 5d orbitals in the bonding, and electronic and magnetic structure of Ce complexes have been discussed by Moreau and co-authors.113 They conducted a study involving the synthesis and characterization of two novel alkali metal-capped Ce oxo compounds. Through the use of HERFD XAS measurements at the Ce L3 edge, it was determined that both the imido and oxo materials possess an intermediate valence ground state for Ce. The authors′ calculations further indicated a significant 4f/5d hybridization and a variation in the distribution of d-state DOS, depending on the specific bonding configuration of Ce.
3.3.2 Oxide nanoparticles and colloids
Plakhova and colleagues conducted a study on the structural modifications of small CeO2 NPs ranging from 2 to 8 nm.114 By employing ab-initio calculations using FEFF, they demonstrated that the Ce L3 HERFD XAS spectral shape changes due to the presence of OH groups on the NP surfaces, indicating the significant influence of surface composition on the reactivity and performance of CeO2 NPs. Particularly notable changes in the spectral profile were observed when the oxygen surface groups of the 2 nm CeO2 NPs were hydroxylated. As the particles increased in size, the effects of the OH groups on the spectra diminished, becoming less visible for 5 nm CeO2 NPs and disappearing completely for particles as large as 8 nm.
The structure of PuO2 NPs has been extensively studied by a combination of techniques including SAXS, SANS, EXAFS and HERFD XAS.115 In a recent review, Virot and collaborators provide a comprehensive summary of the collective efforts made to study the size, morphology, and crystallinity of PuO2 NPs.116 The center of the discussion was the oxidation state and the bonding of Pu at the surface. Conradson and co-workers were the first to fit EXAFS with multiple Pu−O bond lengths and claimed the presence of Pu(V).117 In the investigation of intrinsic colloid formation, Rothe et al. found only Pu(IV) and explained the splitting of the Pu−O bonds in EXAFS by condensation of Pu(IV) phases with Pu-OH groups at the surface.118 Bonato et al. also reported the splitting of the Pu−O shell in PuO2 NPs and explained it with disorder of the local structure at the surface.119 The same group found further evidence of surface disorder by combining SAXS and XAS on PuO2 NPs obtained by different synthetic methods.120 SAXS was used to measure the outer size of NPs and reveal the different morphologies obtained by different methods. XAS showed a PuO2-like core, smaller than the size estimated by SAXS. Gerber et al. combined EXAFS, Pu L3 and M4 HERFD XAS and PDF on PuO2 synthesized from different Pu precursor and they found only Pu(IV) in the end products.121 The EXAFS analyzed by simple fitting and by sophisticated Monte-Carlo simulations was well-reproduced by a single Pu−O bond length. HERFD XAS was also employed by Kvashnina et al. to determine the structure of an unknown intermediate phase, which was stabilized during PuO2 NPs synthesis.114b Pu M4 edge HERFD XAS identified the unknown phase as a Pu(V) phase. The structure was then determined to be NH4PuO2CO3 by comparing the Pu L3 edge HERFD data with XAS calculations from different possible Pu(V) inorganic structures. The same authors investigated the formation of PuO2 NPs at low pH121b and determined that the predominant oxidation state for the particles was Pu(IV). However, when the PuO2 NPs were formed at pH=1, the presence of multiple oxidation states was observed, including Pu(III), Pu(IV), and Pu(VI). It was concluded that the contribution of Pu(III) and Pu(VI) observed in the HERFD XAS spectrum of PuO2 NPs at pH=1 originated from the solution rather than from the NPs themselves. This detection was possible due to the HERFD method‘s high sensitivity to the concentration of Pu under these conditions.
The sensitivity of the HERFD XAS method in detecting oxidation states has also been demonstrated in another study on the formation of UO2 NPs.123 The authors revealed that although U(IV) was the dominant oxidation state of the freshly prepared UO2 NPs, they oxidized to U4O9 over time and under the influence of the X-ray beam. Additionally, it was observed that the oxidation process of the NPs was accompanied by an increase in their size to 6 nm. The importance of small polynuclear metal-clusters in the assembly path of actinide dioxide NPs was recently revealed for ThO2 and PuO2. Amidani et al. combined HERFD XAS and PDF to ThO2 synthesized by chemical precipitation.124 In a first study, they found that for freshly precipitated ThO2 NPs (2.5 nm size), a feature in the post-edge of Th L3 edge HERFD XAS disappears. They performed electronic structure calculations, considering non-relaxed structural models, which successfully reproduced the trends observed in the experimental data. Through examination of the simulations involving Th atoms located in the core and on the surface of the NP, it was revealed that the presence of the first post-edge feature is highly sensitive to the reduction in the number of coordinating atoms, as shown in Figure 17a. The signature of very weak Th−Th coordination at the surface was confirmed by an independent study on ThO2 and UO2 NPs.125
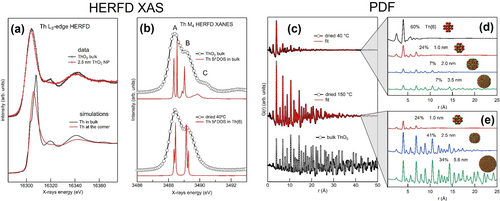
(a) Experimental (top) Th L3 edge HERFD XAS of 2.5 nm NPs (red) and bulk (black) ThO2 compared with calculations (bottom) for a Th atom in bulk (black) and at the corner of a NP (red). (b) Experimental (black dots) Th M4 edge HERFD XAS of bulk ThO2 (top) and as precipitated NPs (bottom) compared with calculations (red lines) of Th bulk and Th6.122 (c) PDF data (black dots) on freshly prepared (top) and 150 °C dried (middle) ThO2 NPs compared with bulk (bottom). The fits of PDF data on NPs are shown in red and were obtained from an ensemble of NPs. (d) and (f) show the PDF contributions from different sizes of NPs used in the fit model for freshly prepared and 150 °C dried NPs.
In a following investigation, the same authors found the first evidence of the presence of Th-hexamer clusters in the first stages of ThO2 NPs synthesis by using PDF and HERFS XAS at the Th M4 edge. PDF data from X-ray total scattering were collected for freshly precipitated, 150 °C dried ThO2 NPs and bulk ThO2. The size increase with annealing can be readily seen in PDF data. The authors then fitted the data using a structural model of an ensemble of NPs of different sizes, cut-out from the structure of ThO2 bulk. The good agreement (Figure 17c) revealed that the freshly prepared sample contained up to 60 % of [Th6O32] hexameric units, very similar to Th6 cluster reported in literature (Figure 17d and 17e).122, 126 The HERFD XAS at the M4 edge of Th shows also relevant differences. In particularly, compared to the spectrum of bulk ThO2, the peak C is missed for freshly precipitated NPs (Figure 17b). Knowing from PDF that 60 % of the sample is made of Th hexameric units, the authors used the structure of published Th6 clusters to calculate the change in 5 f electronic structure and found agreement with the experimental data (Figure 17b). Insight into the calculation result elucidated that the absence of peak C was due to the 45° rotation of Th−O bonds at the surface of [Th6O32] clusters, showing the power of HERFD XAS to elucidate the structure at the surface for samples of few nm size.
The presence of Pu-hexamer clusters was also found by Cot-Auriol et al. for PuO2 NPs by SAXS and XAS.115a Moreau et al. investigated ultra-small ThO2 and UO2 NPs by combining SAXS, EXAFS and XANES.125 They found that a bulk-like core is accompanied by strong disorder of the Th−O and U−O bonds at the surface. Romanchuk et al. recently discussed EXAFS analysis as a tool to estimate NP size and disorder.127 They proposed an effective coordination number derived from a core-shell NPs model that perfectly fits the variations in a metal-metal coordination observed in EXAFS with decreasing NP size. Understanding the chemistry of actinides in the environment is fundamental to predict their mobility and small NPs are believed to be an important cause of environmental contamination due to their high mobility.128 Several studies are therefore utilizing structural characterization to understand the fate of actinides during environmentally relevant chemical processes, like their interaction with minerals. EXAFS was used to investigate the form assumed by Pu interacting with ferrihydrite. It was found that Pu sorption on ferrihydrite followed by transformation into goethite produces PuO2 and Pu is incorporated into the Fe mineral. Differently, co-precipitation of ferrihydrite and Pu only results in Pu incorporation into the structure of the Fe minerals.129 In another study, the same group investigated the interaction of Pu with calcite with EXAFS. They found that Pu(VI) is incorporated as plutonyl into the calcite structure and undergoes partial reduction to Pu(V).130
In a recent work, Neill and collaborators investigated the formation, composition, and structure of U(IV)-silicate colloids under the alkaline conditions relevant to spent nuclear fuel storage and disposal. By exploiting the complementarity of SAXS, PDF and EXAFS, they were able to determine the complex structures of the colloids formed.131 SAXS analysis provided an average particle size of 4.6–6.3 nm, in net contrast with the absence of PDF signal above 1.5 nm. The interatomic distances from PDF indicated the presence of U(IV)-silicate and UO2 interatomic distances at short range, with only UO2 interatomic distances surviving in the long-range up to 1.5 nm. For the EXAFS fit, on the other hand, good agreement was reached by considering only the local environment of U(IV)-silicate and no evidence of UO2 was found. The apparent conflicting results were interpreted as the evidence of core-shell structure colloids, i.e. a small 1.5 nm crystalline UO2 core surrounded by a thicker amorphous U(IV)-silicate shell, up to 4.6–6.3 nm. SAXS measured the outer size of the colloids, independently from the crystallinity. PDF detected only short distances of the amorphous layer and all distances involved in the crystalline core. EXAFS on the other hand was blind to the small UO2 core, constituting only 2.6 % of the colloids, and measured local structure of the dominant U(IV)-silicate phase.
4 Conclusion and Outlook
Structural characterization over multiple spatiotemporal scales, especially in solution, is important to understand and ultimately control formation processes and function of SoINs. X-ray/neutron scattering and X-ray absorption are a very powerful and flexible set of tools to obtain this information from sub-nm to μm scale. Our collective prospective is that there are opportunities for the interested scientific communities to use these tools more extensively, and opportunities for the instrument manufacturers and beamline scientists to develop next generation tools. These are briefly summarized below.
-
Higher beam flux is required to track the instantaneous structural evolutions and metastable intermediate products, with or without applied external fields. Yet potential beam damage can alter the sample's structure, chemical state, and other properties, thereby compromising the reliability of X-ray and neutron methodologies. It is crucial to understand and address these beam damage effects to obtain reliable and meaningful data. This requires careful optimization of experimental parameters, implementation of fast data collection mode, sample handling procedures, and data analysis techniques. On the other hand, researchers need access to flow cell configurations that can partially mitigate this challenge.
-
Widespread availability of other alternative sample environments will increase the value of in operando measurements, including atmosphere-controlled cells (specialty gases, applied pressure), flow and mixing configurations, and setups with applied photo-, electro-, or magnetic fields.
-
Integration of multiple X-ray and neutron techniques has been shown here to provide a more comprehensive understanding of nanostructured materials. Unlike real space imaging, the afforded spectra are not intuitive, and there is rarely one unique solution to data modeling. The development of advanced data analysis tools, including electronic structure calculations, molecular dynamics, machine learning, and artificial intelligence can aid in extracting structural information from these sophisticated spectra at broad spatiotemporal ranges. This includes indirect signatures in spectra; i.e. solvent scattering, which is often ignored. Moreover, the creation of databases containing reference spectra and fingerprint characters can accelerate the rapid identification towards the target structures.
-
Access to synchrotron facilities, crucial for conducting XAS experiments, presents another challenge. Limited availability of beamtime often restricts the number of SoIN samples that can be studied with X-rays and neutrons. Publication rates of such data is slowed by the intractable problem of never anticipating all the data required for a comprehensive study (i.e. control samples). To address this issue, the development of tabletop and laboratory-scale setups is underway,132 aiming to broaden the accessibility of X-ray and neutron experiments.
Acknowledgments
M. N. acknowledges support of the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Material Sciences and Engineering, under award DE SC0010802. P. Y. and J. Y. acknowledge the financial support from the National Natural Science Foundation of China (Nos. 22241501, 92261117, and 52203087), the Fundamental Research Funds for the Central Universities (No. 2022ZYGXZR055), TCL science and technology innovation fund (No. 20222056), and Postdoctoral Innovation Talent Support Program (No. BX20220114).
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Biographical Information
Panchao Yin received his B.Sc. degree from Tsinghua University and his Ph.D. degree from Lehigh University. In 2013, he moved to the University of Akron for postdoctoral research and later became a Clifford G. Shull fellow at Oak Ridge National Laboratory. In 2017, he joined South China University of Technology as a full professor. His group studies synthesis-structure-property relations of polymer nanocomposites. The photograph shows the laboratory members who have contributed to this Review. From left to right: Yuyan Lai, Binghui Xue, Jia-Fu Yin, Panchao Yin, Mu Li, and Jiadong Chen.
Biographical Information
Lucia Amidani is a scientist at the ROBL beamline at the ESRF, operated by the Helmholtz-Zentrum Dresden-Rossendorf. She received her Ph.D. at the University of Bologna. Her research focuses on the use of X-ray spectroscopies to investigate the electronic structure of materials and understand structure-property relationships. She has studied materials for LEDs, photocatalysis, rare-earth-based phosphors, and actinides. Her main interest is the fundamental understanding of f-electron systems using experimental X-ray spectroscopies and theoretical calculations.
Biographical Information
Kristina Kvashnina is the Head of Department and full Professor (W3) at the Institute of Resource Ecology, Helmholtz-Zentrum Dresden-Rossendorf (Germany). She is also responsible for the Rossendorf Beamline at ESRF in Grenoble and she holds a Professorship at the University Grenoble Alpes in France. She obtained her Ph.D. in physics from Uppsala University (Sweden) in 2006. She then moved to ESRF where she conducted research on actinide systems using advanced X-ray spectroscopy methods.
Biographical Information
May Nyman is a Professor of Chemistry at Oregon State University. She received her Ph.D. at the University of New Mexico with Prof. Mark Hampden-Smith. Her scientific passions include discovery of and solution X-ray characterization of new cluster topologies from across the periodic table. In addition to understanding fundamental aqueous speciation of metal cations, May′s research has spanned applications in nuclear waste management, inorganic lithography, environmental remediation; and more recently metal-metal separations and carbon capture.