Total Syntheses of (±)-Dracocephalone A and (±)-Dracocequinones A and B
Abstract
Described herein are the first total syntheses of (±)-dracocephalone A (1) and (±)-dracocequinones A (4) and B (5). The synthesis was initially envisioned as proceeding through an intramolecular isobenzofuran Diels–Alder reaction, a strategy that eventually evolved into a Lewis acid-promoted spirocyclization. This highly diastereoselective transformation set the stage for trans-decalin formation and a late-stage Suárez oxidation that produced a [3.2.1] oxabicycle suited for conversion to 1. Brønsted acid-mediated aromatization, followed by a series of carefully choreographed oxidations, allowed for rearrangement to a [2.2.2] oxabicycle poised for conversion to 4 and 5.
Chagas disease (CD) is transmitted by the protozoan parasite Trypanosoma cruzi and, despite its prevalence and lethality across Central and South America, is considered to be a neglected disease.1 Efforts to develop effective treatments for CD have proven problematic due to the necessity for prolonged drug regimens that result in associated adverse side-effects and the development of resistance.2 Thus, effective therapeutic agents remain scarce and the treatment of CD presents an important unmet medical need.3 In 2003, a 20-nor-abietane diterpenoid, (+)-dracocephalone A (1), along with (−)-cyclocoulterone (2) and (+)-komaroviquinone (3) (Figure 1), were isolated from the perennial semishrub Dracocephalum komarovi, a traditional medicine used for Inflammatory diseases and hypertony in Uzbekistan.4
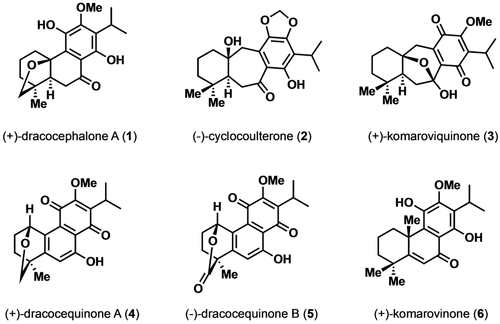
Representative diterpenoids from Dracocephalum komarovi.
In preliminary studies, extracts from D. komarovi showed strong in vitro trypanocidal activity against epimastigotes of T. cruzi. Inspired by these initial findings, further studies on the isolated components from the extracts revealed the minimum lethal concentrations (MLCs) of 1, 2, and 3 to be 200, 20, and 0.4 μM, respectively.4 Several years later, three new structurally related diterpenoids, (+)-dracocequinone A (4), (−)-dracocequinone B (5), and (+)-komarovinone (6), were also isolated from D. komarovi (Figure 1) and the former two were found to exhibit trypanocidal activity (MLC=12.5 and 25 μM, for 4 and 5, respectively).5 While the icetexane diterpenoids 2 and 3, have been prepared by total synthesis, their corresponding nor-abietane congeners (1, 4, and 5) have not yet succumbed to total synthesis.4-11 The scarcity of available methods for accessing nor-abietanoids, coupled with the interesting activity of 1, 4, and 5 against CD led us to develop the divergent synthetic strategy reported herein.
As illustrated retrosynthetically in Scheme 1, we envisioned 1, 4, and 5 as deriving from a common intermediate (7), which bears the requisite carbocyclic framework and oxidation patterns for diverging to each of the targets. We suspected the [3.2.1] oxabicycle embedded in 7 could be accessed via a late-stage Suárez oxidation, which would follow silyl ketal fragmentation and decarboalkoxylation of the [2.2.1] oxabicycle 8. The latter oxabicycle would be assembled from lactone 9 via tandem isobenzofuran generation/Diels–Alder cycloaddition. Lactone 9 would arise from aldehyde 10 and benzamide 11 via a directed Snieckus ortho-metalation/nucleophilic addition sequence and subsequent lactonization.12
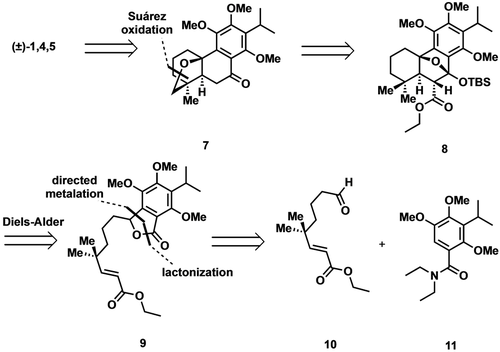
Initial retrosynthetic analysis of (±)-dracocephalone A (1) and (±)-dracocequinone A (4) and B (5).
In accord with our synthetic plan, aldehyde 12 was first accessed by a known protocol reported by Frontier and co-workers (Scheme 2A) and advanced to the homologated aldehyde 10 via a 3-step sequence involving Horner–Wadsworth–Emmons olefination, desilylation with tetra-n-butylammonium fluoride, and Dess–Martin oxidation.13 Having prepared the requisite electrophile 10, we turned our attention to the preparation of benzamide 11 (Scheme 2B). The latter was accessed in short order by subjecting known bromide 13 to metal-halogen exchange (n-BuLi), followed by addition of the resultant organolithium to N,N-diethylcarbamoyl chloride.14 With the coupling fragments in hand, the directed ortho-metalation was effected under conditions developed by Snieckus and the derived anion exposed to aldehyde 10 (Scheme 3).12 Following work-up, exposure of the intermediate alcohol (not shown) to p-toluenesulfonic acid then afforded lactone 9 in modest yield. We were pleased to find that upon treatment with lithium hexamethyldisilazide (LiHMDS) and tert-butyldimethylsilyl chloride (TBSCl), 9 was converted to the corresponding siloxyisobenzofuran 14 which, upon warming to room temperature, smoothly underwent the desired Diels–Alder cycloaddition to deliver the sensitive silyl acetal 8 as the only isolable product.15 With 8 in hand, we screened a number of conditions to effect opening of the silyl acetal to the requisite decalin. To our dismay, no products corresponding to the desired C−O cleavage were ever detected. Instead, under all conditions tested, cleavage of the C−C bond outpaced that of the C−O bond thereby giving rise to a spirolactone, the structure of which was unambiguously defined as illustrated (15) by single crystal X-ray analysis.
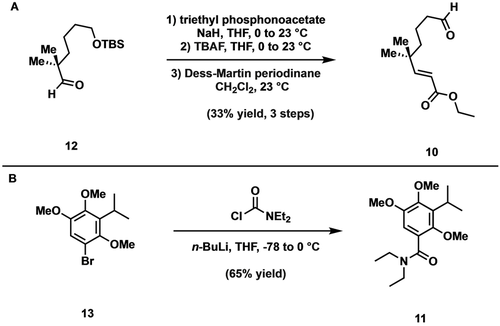
A) Synthesis of aldehyde 10 and B) synthesis of benzamide 11.
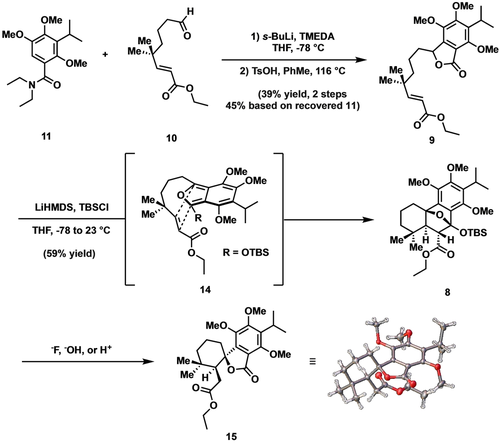
Syntheses of Diels–Alder cycloadduct 8 and undesired lactone 15.
Given the sensitivity of the silyl acetal moiety of 8 coupled with its propensity to undergo an undesired retro-Dieckmann reaction, it was clear that an alternative route was needed. However, given its accessibility, 15 remained an attractive intermediate for constructing the decalin. In considering potential deviations from our initial synthetic strategy, we envisioned that decarboxylative formation of an alkyl anion from the ester moiety present in 15 would set the stage for reforming the pivotal C−C bond and deliver the required decalin. To this end, preliminary studies were conducted to convert 15 into its corresponding carboxylic acid. Unfortunately, all attempts to affect selective hydrolysis of the ethyl ester moiety failed. Accordingly, we sought access to a more malleable allyl ester 18.
By utilizing a similar sequence, illustrated in Scheme 4, efforts to prepare 18 were accompanied with optimization studies and commenced with subjection of aldehyde 12 to Masamune-Roush modified Horner–Wadsworth–Emmons olefination conditions. Subsequent aqueous HCl-mediated deprotection and Dess–Martin oxidation furnished 16 in a greatly improved yield relative to that observed for 10. Turning next to the coupling event, we found that the ortho-metalation/lactonization sequence could be significantly improved by the addition of lanthanum(III) chloride bis-lithium chloride complex followed by direct exposure to p-toluenesulfonic acid. These conditions efficiently delivered lactone 17. In further optimization efforts designed to bypass the intermediate siloxybenzofuran, we discovered that exposure of 18 to LiHMDS and catalytic quantities of copper(II) trifluoromethanesulfonate resulted in excellent yields of 18.16 Clearly, the exclusion of TBSCl circumvented formation of an isolable Diels–Alder adduct, thereby resulting in net spirocyclization to 18.
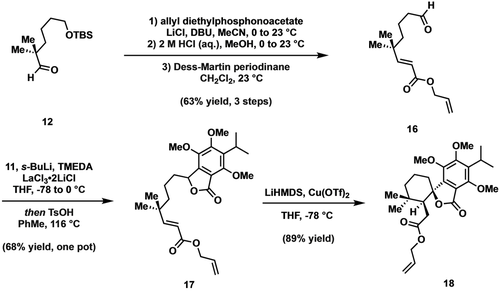
Modified synthetic route to allyl ester 18.
It is worth noting that the highly diastereoselective spirocyclization could arise via two distinct mechanistic pathways: either an anionic Diels–Alder/retro-Dieckmann cascade or an intramolecular Michael cyclization.17 Given that the sense and extent of diastereoselectivity very closely matched the outcome of the endo selective siloxyisobenzofuran Diels–Alder (see above, cf. Scheme 3), we hypothesize that the former is at play. Further, examples of anion-assisted furanone Diels–Alder reactions in the literature support the feasibility of such a process.18, 19
Having optimized the route, we set the stage for the end-game by preparing decagram quantities of 18 and then began advancing by subjecting the ester to palladium-mediated deallylation to furnish the corresponding carboxylic acid 19 (Scheme 5). Subsequent exposure of 19 to modified Hunsdiecker conditions smoothly induced decarboxylative iodination to furnish alkyl iodide 20.20, 21 Metal-halogen exchange using t-BuLi then initiated a rapid intramolecular nucleophilic acyl substitution reaction that produced trans-decalin 21 which,22 upon treatment with phenyl iodide diacetate (PIDA) and I2 under Suárez conditions, undergoes a diastereotopic group selective transannular oxidation of the syn-methyl group to furnish ether 7, thus completing construction of the [3.2.1] oxabicyclic ring system.23 At this point, all that remained was regioselective double demethylation to the hydroquinone, a transformation that in practice proved to be a considerable challenge due to the lability of 7 under acidic conditions. After considerable experimentation, it was eventually determined that cerium (IV) ammonium nitrate (CAN) can be employed to convert 7 to a transient quinone intermediate which, upon reduction with sodium dithionite, gives rise to dracocephalone A (1).6, 24 Notably, this efficient synthetic approach provided quantities sufficient for crystallization of 1 and further structural confirmation via single crystal X-Ray analysis.
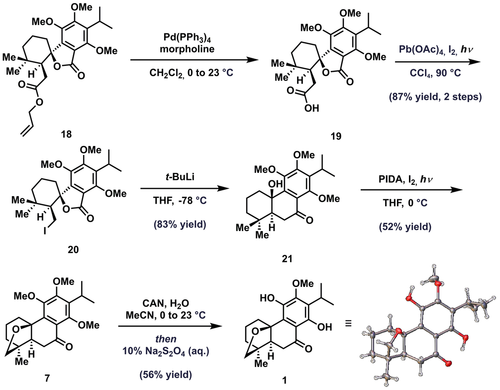
Completed synthesis of (±)-dracocephalone A (1).
Having accessed 1, we next sought to extend this strategy to the aromatized and further oxidized congeners 4 and 5. In accord with our retrosynthetic plan, our intent was to employ 7 (trivially O,O-dimethyldracocephalone A) as the point of divergence. Toward this end, aromatization of the central ring was most efficiently accomplished by treating 7 with catalytic p-toluenesulfonic acid in hot benzene to give naphthol 22 (Scheme 6A). A number of oxidants were screened in hope of effecting conversion of 22 to an intermediate quinone methide (not shown) from which transannular etherification would deliver 23. Although these efforts revealed 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)-mediated oxidations to be the most promising, they were initially found to be poorly reproducible.25 In anhydrous CH2Cl2, trapping of the aryl oxidation intermediate to deliver 25 was observed as the predominant event, with only traces of 23 being detected (Scheme 6B). Switching solvents to 1,4-dioxane gave improved but variable yields of 23 along with 25. Eventually, we discovered the course of this reaction to be reliant on water content of the solvent. Interestingly, the addition of superstoichiometric water was found to completely suppress formation of 25, thereby enabling isolation of 23 in good yield. At this stage, to complete the syntheses of 4 and 5, we hoped to apply the oxidative demethylation conditions that proved effective in the completion of 1. However, recognizing the potential for complications arising from the free phenol we opted to mask 23 as its corresponding acetate. To this end, acylation of 23 gave the crystalline naphthol acetate 24; the structure of which was confirmed via single crystal X-Ray analysis. Finally, oxidation of the trimethoxyarene to its corresponding para-quinone using CAN was followed by in situ removal of the naphthol acetate to furnish dracocequinone A (4).
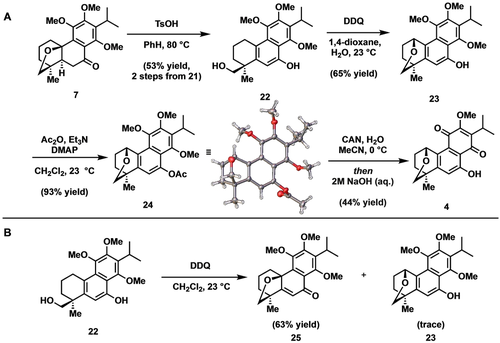
A) Total synthesis of (±)-dracocequinone A (4) and B) solvent/water-induced reversal of oxidation selectivity.
Turning to the completion of 5, a slightly modified course of events was required to access the requisite lactone 28 (Scheme 7). Specifically, selective protection of the naphthol 22 as the corresponding acetate (NaH/AcCl) was required since the free hydroxyl moiety was found to preclude selective oxidation of the primary alcohol. Subsequent Dess–Martin oxidation of the intermediate acetate proceeded to give aldehyde 26 in excellent yield over the two steps. Oxidation of 26 under standard Pinnick conditions resulted in smooth conversion to carboxylic acid 27. Much to our chagrin, efforts to advance 27 via the previously developed DDQ/water-mediated protocol failed and returned only starting material. Fortunately, we found that the desired lactone 28 could be prepared under Wohl-Ziegler bromination conditions, presumably via an SN1-type displacement of the transient benzylic bromide. Finally, CAN oxidation and removal of the naphthol acetate in the same pot secured access to dracocequinone B (5).
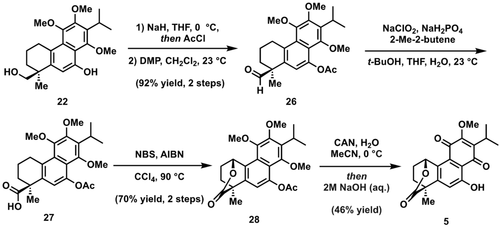
Total synthesis of (±)-dracocequinone B (5).
In summary, we have completed the first total synthesis of (±)-dracocephalone A (1) in 10-steps and 8 % overall yield from known materials. The developed synthetic strategy was successfully extended to the first total syntheses of (±)-dracocequinones A (4) and B (5), in 13 and 15 steps, respectively, from known materials. To the best of our knowledge, these represent the first total syntheses of C-20 nor-abietane natural products. The efficiency of the approach and ability to prepare intermediates on gram scale has greatly facilitated ongoing investigations into the trypanocidal activity of late-stage intermediates and simple variants,27 as well as the syntheses of other nor-abietanoids and related trans-decalin natural products.
Acknowledgements
The authors gratefully acknowledge Dr. X. Xu for assistance with NMR analysis, Prof. K. Klausmeyer for assistance with X-Ray crystallography, and Dr. A. Ramirez for mass spectrometry assistance. The authors gratefully acknowledge Kirk French and Prof. Kevin Shuford for their assistance with computational studies. The authors acknowledge financial support from Baylor University, the Welch Foundation (Chair, AA-006), the Cancer Prevention and Research Institute of Texas (CPRIT, R1309), NIGMS-NIH (R01GM136759), and NSF (CHE-1764240).
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




