Configurationally Chiral SuFEx-Based Polymers
Abstract
Novel methods to make synthetic chiral polymers are highly desirable given their potential in a rapidly increasing number of bio-inspired applications. The enantiospecific sulfur–fluorine exchange (SuFEx) reaction of chiral di-sulfonimidoyl fluorides (di-SFs) with diphenols, was used to produce high-molecular-weight chiral polymers with configurational backbone chirality. The resulting new class of polymers, polysulfonimidates, can be efficiently produced via this step-growth mechanism for a wide range of di-SFs and diphenols, yielding MnPS up to 283 kDa with a typical dispersity Đ around 1.6. The optical activity of the resulting chiral polymers is largely due to the intrinsic asymmetry of the S atoms (configurational chirality). Finally, the enantiospecificity (ee>98 %) of the polymerization reaction was demonstrated by the degradation of a disulfide-containing polysulfonimidate. This novel route towards configurational main-chain chirality opens up new approaches towards tailor-made chiral polymers with precisely defined properties.
Introduction
The beauty of natural polymers as varied as DNA, proteins, and polysaccharides lies partially in their precisely controlled chirality, which is typically instrumental for their structure, function and specific reactivity. Inspired by this, the inclusion of chirality into synthetic polymers1 has turned into a highly fruitful motif, which has led to the formation of tailor-made polymeric materials that are useful as chiral catalysts or as chiral scaffolds for catalysts,2 as chiral stationary phases for high-performance liquid or gas chromatography,3 as crucial components for bio-interface modification,4 etc. In addition, polymeric materials are, increasingly investigated as a source to store information, and their chirality can play an important role in this.5 As a result, efficient approaches to access chiral synthetic polymers with tailor-made functionalities are in high demand.
The methods of introducing chirality into polymers can be sorted into three main categories by the type and position of the chiral source:6 Much of this work focuses on pendant chirality (as, for example, obtained in polyisocyanides that form stereodefined helices with chiral pendant groups)7 or conformational backbone chirality (as, for example, arising from helix sense-selective polymerization).1, 6, 8 The third, configurational main chain chirality (as, for example, obtained with isotactic polypropylene or chiral propylene oxide polymerizations), has received comparatively less attention9 although the concepts are, of course, recurrent in sequence-defined foldamer and DNA origami chemistry. Each of these classes of chirality has specific characteristics, and its own pros and cons. Configurational backbone chirality has as an advantage that the chain fully maintains its chirality even when any side groups are modified or even removed, and independent of any specific conformation of the polymer chain.5a To obtain configurational main chain chirality, various options are available, starting from enantiopure compounds that react in an asymmetric polymerization reaction to using non-optically pure chiral starting monomers, when either a chiral catalyst is used or a “majority rules”-selectivity is obtained.1, 4 In these polymerizations it is often a challenge to obtain and determine the degree of asymmetric induction of specific isotactic/syndiotactic polymers.5a, 10
A conceptually simple approach to obtain chiral polymers with a consistently high degree of enantioselectivity for each coupling reaction hinges on stereospecific reactions of enantiomerically pure starting materials without the need of chiral catalysts or auxiliaries, such as those involving chiral epoxide monomers.11 In principle, also polymer click chemistries have the potential to provide such configurationally chiral polymers and significantly increase the structural variations available for configurationally chiral polymers. Click chemistries do indeed display the required efficiency,12 but while defined as reactions that are “modular, wide in scope, give very high yields, generate only inoffensive byproducts that can be removed by non-chromatographic methods, and be stereospecific”,13 only recently the first intrinsically chiral click reaction was reported.14 This reaction is an example of a sulfur–fluoride exchange reaction (SuFEx)15 on a chiral sulfonimidoyl fluoride (SF, with −R−S*(=O)(−F)=NR′ as the reactive moiety). Since SuFEx has been proven to be an extremely powerful tool in the formation of polymers (Figure 1),16 we wondered whether this combination of efficiency and enantiospecificity would bring us closer to the bio-inspired goal of configurationally chiral synthetic polymers.
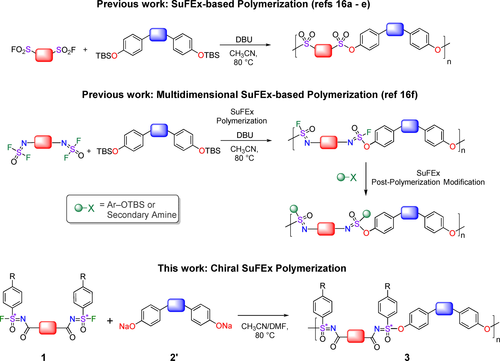
SuFEx-based polymerizations: Previous work focused on accomplishing SuFEx polymerizations under mild reaction conditions, and on introducing SuFEx-based post-polymerization modifications. The current work introduces chirality in SuFEx-based polymerizations.
Herein, we report the first SuFEx click reaction-based configurational main chain-chiral polymerization, without the need for chiral auxiliaries or chiral catalysts, for the reaction of chiral di-sulfonimidoyl fluoride compounds 1 with diphenol(ate)s 2/2′ (Figure 1). This polymerization results in a new class of polymers, namely chiral and high-molecular-weight polysulfonimidates (MnPS up to 283 kDa, ca. 750 repeating units) with a stable and intrinsic backbone-based chirality (>98 % ee per step).
Results and Discussion
We started our study by exploring the potential to form polysulfonimidates, an entirely new class of polymers, from disulfonimidoyl fluorides (in short: di-SFs) with (unprotected) diphenols. To first investigate the factors controlling the reaction and find optimal reaction conditions, non-chiral di-SFs were used. In the presence of DBU as a base, all di-SFs 1 a–f (Table 1) were reacted using equimolar amounts of di-SFs and diphenols (typically 0.10 mmol) in acetonitrile (CH3CN) at 80 °C for 16 h, and the resulting polymers were isolated and purified by precipitation. The reactivity is mainly governed by two molecular features.
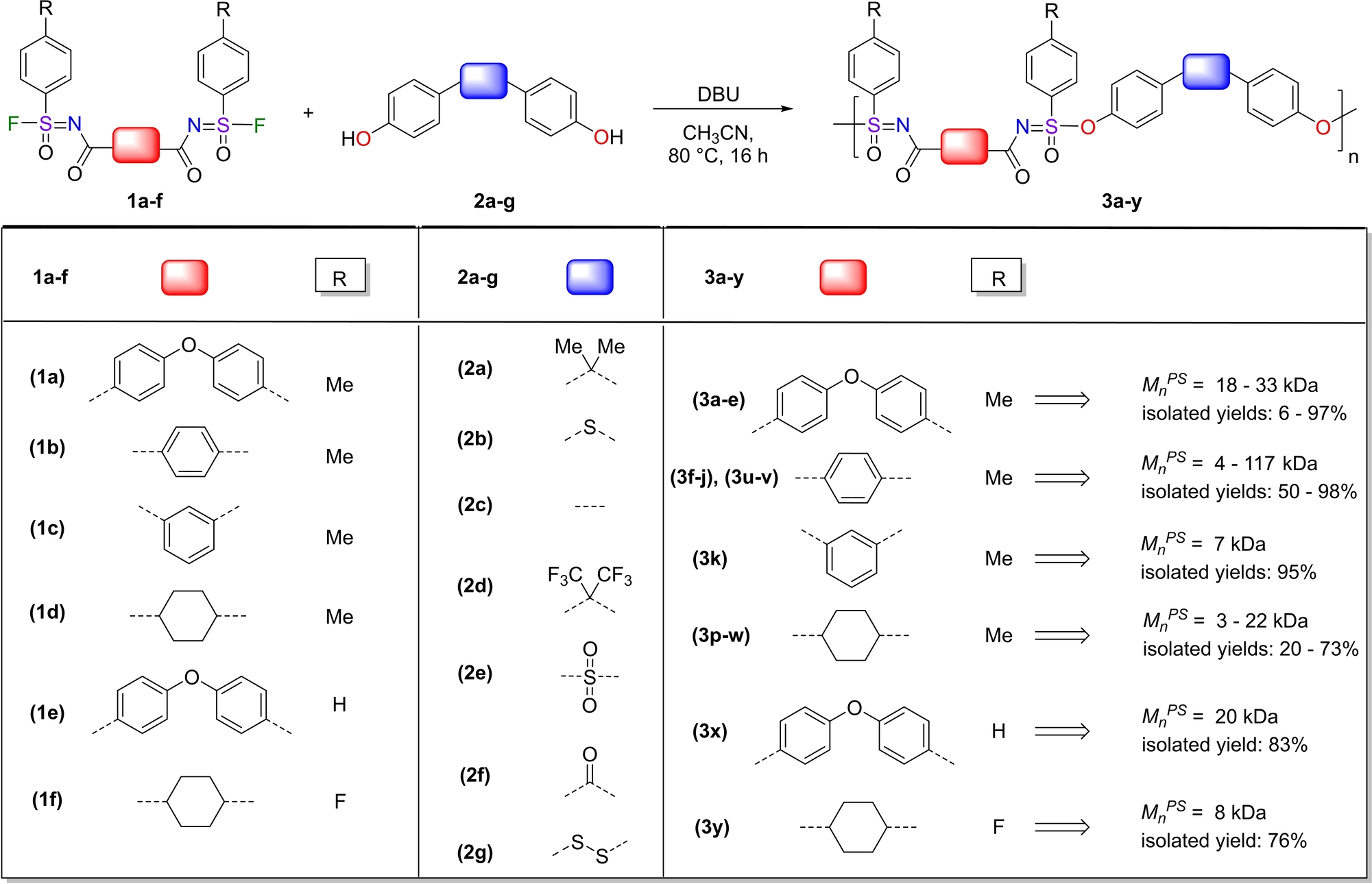
- [a] Note: Scale 0.10 mmol di-SF compound 1, 0.10 mmol diphenol 2 and 0.11 mmol of diazabicyclo[5.4.0]undec-7-ene (DBU) in 0.2–0.3 mL CH3CN. Number-average molecular weight (MnPS, using polystyrenes as standard) determined by gel permeation chromatography (GPC), using THF as the eluent.
The first is the solubility, the second (slightly less important) is the electronic structure of the reactant. The solubility of the thus formed polymers turned out to dominate the molecular weight reached and the overall isolated yield. For linkers that did not contribute significantly to the solubility, like the trans-1,4-cyclohexyl moiety in 1 d and 1 f, the polymerizations proceeded but were not very effective (MnPS=3–22 kDa; see Table 1 for selected data set, and Table S1 and full analytical data of all polymers under current study in the Supporting Information). For more soluble compounds 1 a and 1 e with oxygen-linked benzoyl moieties, and 1 b with the terephthaloyl linkage increased molecular weights were obtained (MnPS=18–33 kDa and MnPS=4–117 kDa, respectively), although heavily influenced by the nature of the diphenol. The electronic structure was indeed the second factor determining the reactivity. Control over the reaction outcome could be obtained via a broad variety of linking moieties between the two phenol groups (S, C(CH3)2, C(CF3)2, C=O, SO2; see Table 1), and via the para-substituent R of the phenyl group bound to the sulfur atom (here: CH3, H, F). Apart from the electronic, also the steric features of the resulting polymers played a role, as—in contrast with the terephthaloyl-linked polymers—the more sterically encumbered polymer that results from polymerization of the isophthaloyl-containing 1 c was not formed very efficiently, with MnPS=7 kDa. These molecular features of monomer solubility and electronic structure turned out to be more important than variations in the solvent and/or reaction temperature to determine the overall efficiency of the polymerization. For example, GPC analyses indicate that due to the high efficiency of the SuFEx click reaction,14 already after 1 h of reaction molecular-weight values up to 72 % of the eventually reached molecular-weight values could be obtained, but then a considerable fraction of low-molecular-weight polymers was then still present. As would be expected for a step-growth mechanism, such as seen for other SuFEx-based polymerizations,16e these smaller fragments could be slowly converted to more high-molecular-weight polymer chains by simply increasing the reaction time, although the precipitation of high-molecular-weight polymers during the reaction complicates the mechanistic picture and precludes sharp mechanistic distinctions in this case.
Compared with previous SuFEx-based polymerizations that used fluorosulfonates, yielding ∼R−S(=O)2−OR′∼ linking moieties in the polymers,16a, 16d, 16e the resulting polymers 3 a–y display sulfonimidate [∼N=SR(=O)−OR′∼] linking moieties. These bear significantly more functionality, but also display a concomitantly increased steric hindrance. This process thus yielded polymers with a moderate to reasonable molecular weight, up to MnPS=117 kDa, with Đ ranging from 1.3 to 2.3 (typically: 1.4–1.8) and isolated yields up to 98 %. When the polymerization was performed in a larger scale (0.4 g of 3 i, at r.t.), the molecular weight significantly enhanced from MnPS=6 kDa to MnPS=117 kDa and the isolated yield increased from 64 % to 98 %, due to a combination of reduced small scale-induced losses, improved stirring and more precisely determined equimolar of the reactions. However, two factors still needed to be improved to make high-quality polymers with additional features: the molecular weight needed to be increased also at a smaller scale, and chirality should be added to the main chain.
To obtain chiral polysulfonimidates, we first synthesized a series of chiral di-sulfonimidoyl fluorides 1 (Figure 2). Here we made use of the fact that, while some chiral sulfinamides 5 are commercially available, both enantiomers for a wide variety of compounds 5 can generically be easily prepared from the corresponding sodium aryl sulfinates 4 via chemical resolution using (R)-(+)-N-benzyl-1-phenylethylamine.17 Initially, di-sulfinamides 7 were prepared by coupling two chiral functionalized sulfinamides 5 with dimethyl esters 6 through amidation reactions. Afterward inspired by the work of Bull and co-workers,18 a synthetic strategy was adopted to prepare chiral di-SFs 1 by deprotonation and oxidative fluorination using Selectfluor.
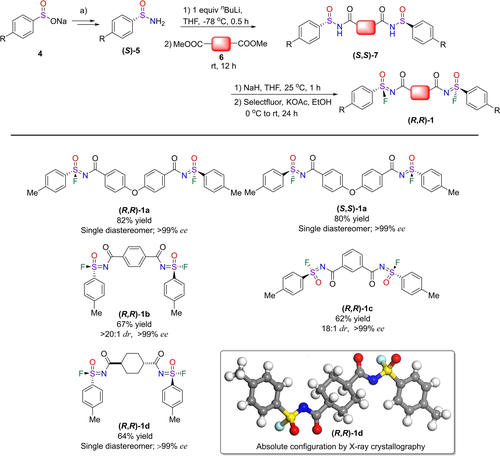
Synthesis of chiral disulfonimidoyl fluorides 1. a) Both enantiomers of 5 could be obtained by reacting arylsulfinates 4 with (R)-(+)-N-benzyl-1-phenylethylamine, to yield diastereomeric toluenesulfinamides that could be separated, and subsequently transformed into methyl arylsulfinates, and finally into enantiopure (R)- or(S)-arylsulfinamides 5. b) dr=ratio of diastereomers; ee=enantiomeric excess. Both dr and ee were determined by chiral HPLC with a UV detector, since the diastereomers could not be separated by flash column chromatography or distinguished by 1H NMR.
Four structurally different chiral di-SFs (Figure 2) were prepared, and of 1 a both enantiomers were made (R,R)-1 a and (S,S)-1 a). The optical purity and activity were assessed with chiral HPLC and circular dichroism (CD), respectively (Supporting Information, Section 2 and Figure S22, respectively). Additionally, the absolute configuration of (R,R)-1 d was confirmed by single-crystal X-ray diffraction analysis (Supporting Information, Section 2.3).
These chiral di-SFs were then reacted with sodium phenolates (indicated with a ′ to distinguish them from the corresponding phenols) 2 a′–2 e′ to yield chiral polysulfonimidates (Table 2), as previous research has shown that the silicon-free SuFEx between sulfonimidoyl fluorides and sodium phenolates occurs in an enantiospecific manner.14 Crucial in this is not only the higher reactivity of the phenolates versus the phenols, but also that the sodium ions can capture any fluoride atoms formed, and thus preclude the fluoride-induced racemization of starting materials analogous to Bull's use of lithium salts to this effect.18
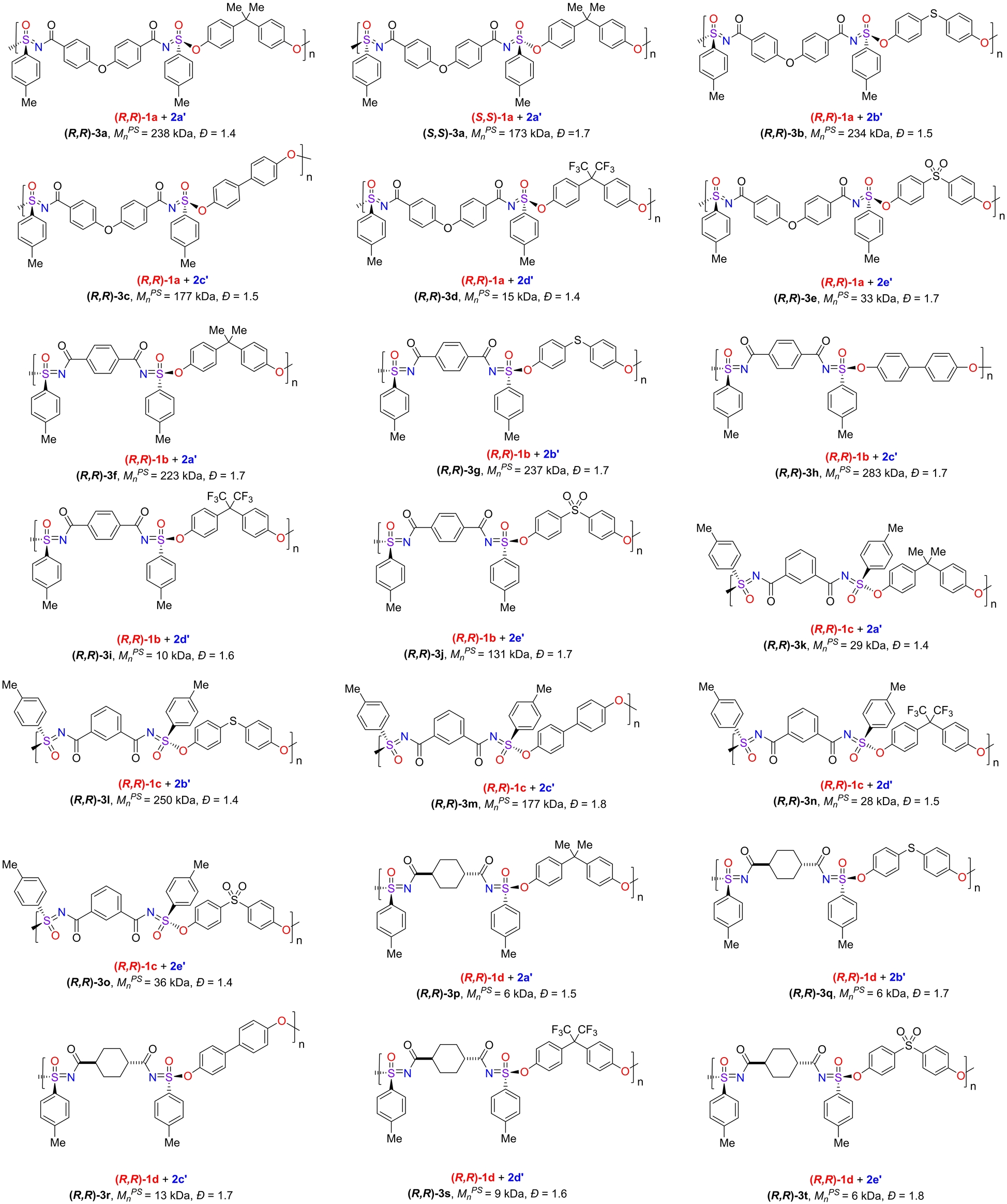
- [a] Typical polymerization conditions: 1 (0.05 mmol) and 2′ (0.05 mmol) were polymerized in dry N,N-dimethylformamide (DMF)/CH3CN (1.25 mL/1.25 mL) at 80 °C for 64 h. The MnPS and Đ were determined by GPC by using DMF:LiBr (0.1 % w/w) as the eluent.
Highly effective chiral polymerization was achieved using a 50 : 50 (v/v) CH3CN−DMF mixed solvent system at 80 °C, yielding polymers with a number average molecular weights (MnPS) up to 283 kDa (see Figure 3 and Table 2) and Đ between 1.4–1.8. Both the higher molecular weights and lower Đ range can be explained by the overall more reactive sodium diphenolates, and these low Đ values are, fortunately, on the low end of what can be expected for this type of polymerization.16a, 16f Since only low-molecular-weight fragments are soluble in THF, precipitation in THF turned out to be an efficient manner to isolate these chiral polymers. Similar to achiral polymerization, many factors affect the degree of polymerization; several trends can be seen in the data in Table 2, namely solubility, steric and electronic character. Right-skewed band broadening was observed for high molecular weight polymers (see Figure 3), but not for similar polymers with smaller MnPS values, pointing to the limits in our current GPC-based analysis. To avoid this broadening, more specific, high-efficiency GPC columns may be required.19
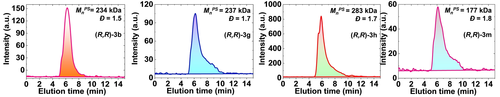
GPC elution profiles: From left to right: (R,R)-3 b; (R,R)-3 g, (R,R)-3 h, and (R,R)-3 m. Note: GPC results (UV absorption at 270 nm) display molecular weights calibrated relative to linear polystyrene standards in the elution solvent (DMF+LiBr (0.1 % w/w)).
The first factor is the solubility of the polymers. In general, the polymers resulting from di-SFs (R,R)-1 a (-p-C6H4-O-p-C6H4-linked) and (R,R)-1 b (-p-C6H4- linked) are more soluble in CH3CN/DMF and yield higher molecular weights. Typically, polymers derived from (R,R)-1 d were less soluble (Supporting Information Table S3), and lower molecular weight polymers resulted. Second, the structure of the polymer is crucial. As already observed for the non-chiral polymers, isophthaloyl (-m-C6H4- linked) polymers derived from (R,R)-1 c would be expected to display roughly the same electronics as those derived from (R,R)-1 b, but the molecular weights were about one order of magnitude less. We attribute this to the increased steric hindrance, which hampers the reactivity of individual S−F moieties during the reaction, a factor that is likely becoming more important during the later stages of this “step-growth-like” polymerization. As a point in case, when (R,R)-1 b and (R,R)-1 c were reacted with diphenolate 2 a′ this yielded chiral polymers (R,R)-3 f (MnPS=223 kDa, Đ=1.7) and (R,R)-3 k (MnPS=29 kDa, Đ=1.4), respectively. Third, when reacted with a specific fluoride, different phenolates also exhibited different reactivities, as can be seen in the polymer series (R,R)-3 a→e, (R,R)-3 f→j, (R,R)-3 k→o, and (R,R)-3 p→t. This is primarily due to differences in the electronic factors: Compared with electron-rich diphenolates 2 b′ and 2 c′, the -SO2-linked diphenolate 2 e′ is expected to be less reactive due to strongly electron-withdrawing linkage, and a concomitantly lower degree of polymerization was always obtained when reacting with identical chiral di-SF's. This lower reactivity does not imply that the degree of polymerization is necessarily poor: when reacting with (R,R)-1 b the resulting polymer (R,R)-3 j yielded MnPS=131 kDa; however, when (R,R)-1 b reacted with electron-rich diphenolates, the molecular weight was even higher. Such poor electronics dominate over high solubility, as is seen for fluorinated phenolate 2 d′. The resulting fluorinated polymers turned out to be comparatively soluble in CH3CN/DMF mixtures, yet when compared with 2 a′, a lower degree of polymerization was always obtained. It must be said, however, that—apart from electronics—the introduction of the protruding C(CF3)2 moiety in the diphenolate linkers might also affect the overall conformation of the polymer and thus affect the progress of this polyaddition polymerization. For example, when compared to the less nucleophilic -SO2-linked diphenolate (NPA charge on the phenolate oxygen atoms=−0.84; wB97XD/6-311+G(d,p) calculations using the SMD solvent model to mimic CH3CN), the -C(CF3)2- linkage (NPA charge on the phenolate O atoms: −0.88) still yields significantly lower molecular weight.
In addition, the molecular weight for polymers 3 b and 3 i was also determined using Ubbelohde capillary viscometry (see for details Supporting Information Section 9). From the viscometry analysis in DMF, the molecular weights Mn obtained for polymers 3 b and 3 i are 239 kDa and 86 kDa, respectively, which are in close agreement with the MnPS results obtained from GPC, namely MnPS=232 kDa and MnPS=80 kDa, respectively.
Interestingly, using the two enantiomers ((S,S)-1 a and (R,R)-1 a)), we were able to prepare, for the first time, a set of artificial “enantiomeric” polymer pairs via a click reaction, namely (S,S)-3 a (MnPS=173 kDa, Đ=1.7) and (R,R)-3 a (MnPS=238 kDa, Đ=1.4). As expected, the chirality of the monomer has no significant effect on the outcome of the polymerization reactions, and both polymers could be isolated easily on a multi-mg scale. To evaluate the chirality of these configurational main chain-chiral polymers, circular dichroism (CD) spectroscopy was used. Figure 4a displays the characteristic features of enantiomeric monomers (S,S)-1 a and (R,R)-1 a, and Figure 4b those of chiral polymers (S,S)-3 a and (R,R)-3 a). Evidently, these CD spectra display mirror-image features for the enantiomers of both the monomer and polymer. Interestingly, from the structure of the monomers and polymers, and the concentration of these molecules in solution, one can derive the approximate ratio of chiral S atoms in both the monomer and polymer solutions. For the polymer solutions, this number of asymmetric S atoms in this case turns out to be ca 72 % of those present in the monomer solution. Yet, the observed size of the CD effects (e.g., the maxima found for monomers and corresponding polymers, normalized for their optical absorption) is typically slightly higher than 72 %: for the data in Figure 4a,b these CD effects are roughly equal. As a result, we conclude that, while the configuration around the chiral S atoms dominates the CD effects, other factors likely also come into play (see below).
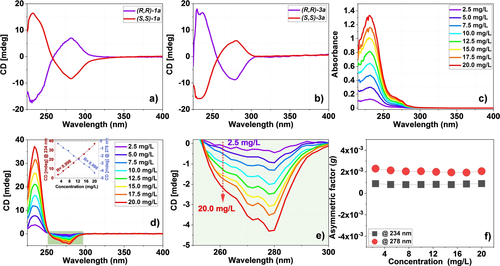
UV/Vis absorption and CD spectra of chiral polymer and monomers: a) CD spectra for chiral monomers (R,R)-1 a and (S,S)-1 a, measured (for structure see Figure 2). b) CD spectra for chiral polymers (R,R)-3 a (formed from (R,R)-1 a) and (S,S)-3 a (formed from (S,S)-1 a). c) UV/Vis absorption spectra of the polymer (R,R)-3 p. d) CD spectra for (R,R)-3 p; the insert shows linear correlation between the CD signal versus concentration (R2=0.998 at 234 nm, and R2=0.996 at 278 nm). e) Zoomed-in section of Figure 4d between 250–300 nm versus the concentration of (R,R)-3 p. f) CD and UV absorption asymmetry factor (g=0.1×CD [mdeg]/(3298.2×UV [abs])) of the obtained for chiral polymer (R,R)-3 p at 234 nm and 278 nm for different concentrations.
According to previous findings from our lab, the reaction between sodium phenolates and SF compounds is enantiospecific.14 First, we confirmed the enantiospecificity of the reaction between chiral di-SF's and phenolates (Supporting Information, Section 4). Building on this result, the UV/Vis spectra and CD spectra of both chiral monomers (S,S)-1 a and (R,R)-1 a and chiral polymers (S,S)-3 a and (R,R)-3 a were obtained. The UV/Vis absorption spectra display maxima at 232 and 272 nm for the monomers and 234 and 278 nm for the polymers, close to the observed maxima of the CD spectra (232 and 278 nm for the monomers and 232 and 278 nm for the polymers, respectively). (For all-optical data, see Supporting Information, Section 8.) These CD spectra also display a near-perfect mirror symmetry, which suggests that the chirality of the central chiral sulfur atom in the chiral monomer has been successfully transferred to the target polymer. Similarly, more asymmetric polymers were successfully prepared from the other chiral monomers (R,R)-1 a, (R,R)-1 c, and (R,R)-1 d, all with analogous mirror-image CD spectra between monomers and polymers (Supporting Information, Figure S22). This illustrates the wide scope of making highly functionalized and intrinsically chiral polymers in this manner.
As indicated by the data in Figure 4a,b, while the enantiospecific inversion of chiral S atoms would indeed be expected to yield configurationally chiral polymers, conformational chirality could also be a source of optical activity, for example, when these polysulfonimidates would form helical structures like those observed for e.g. the SOF4-based SuFEx-derived polymers,16e or higher-order intermolecular interactions forming chiral supramolecular aggregates. If the latter would be the case, then one would expect concentration effects. To test this, CD spectra of polymer (R,R)-3 p were obtained at different concentrations in the range of 2.5×10−3 to 2×10−2 g L−1. Over this concentration range, the asymmetry factor (or g factor)—i.e. the ratio of the intensity of the UV/Vis absorption (Figure 4c) to the CD signal at the wavelengths of 234 nm and 278 nm—was found to be constant (Figure 4f). This indicates that in this concentration range there is no evidence for higher-order aggregates that might affect the optical activity. Of course, highly stable higher-order chiral conformations, such as helices, cannot be excluded, but the polymer structures do, at these concentrations in THF, not give rise to expectations that would support chiral aggregates to contribute significantly to the observed CD effects.
Finally, our hypotheses on the structure of the configurational main-chain chiral polymers as discussed above critically hinge on the enantiospecific nature of the coupling click reaction. Therefore, we were interested in developing a method to demonstrate this enantiospecificity. To this aim, we used disulfide-containing diphenol 2 g (4,4′-disulfanediyldiphenol). The idea behind this strategy was that, following the SuFEx-based polymerization, such disulfide groups could be reduced under mild conditions with retention of the asymmetry of the S atoms. First, we investigated the product (R,R)-S2 resulting from the coupling of diphenol 2 g with two equivalents of a chiral mono-SF compound [(R)-N-benzoyl-4-methylbenzenesulfonimidoyl fluoride, S1], to find out how to mildly reduce the disulfide moiety without affecting the chirality of the S atoms (See Supporting Information, Section 6). Such mild reduction was shown to work with retention of the stereochemistry on S with PPh3 in tetrahydrofuran-water (1:1) at room temperature. When we subsequently applied this procedure to the polymer resulting from (R,R)-1 b (94:6 dr)+2 g′ (Figure 5), this yielded a −SH functionalized compound, which was difficult to analyze. However, mild alkylation with methyl iodide yielded the stable compound (R,R)-8. This compound was analyzed by chiral HPLC, and displayed, in repeated experiments, >92:8 dr, corresponding to >98 % ee of each SuFEx reaction in the polymerization. This result thus demonstrates the enantiospecificity of each step in the polymerization reaction, and the power of this asymmetric SuFEx reaction to construct highly functional polymers with main-chain configurational chirality.
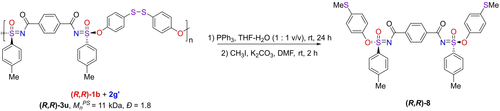
Disulfide reduction of polymer (R,R)-3 u to demonstrate the enantiospecificity of the SuFEx-based polymerization reaction.
Conclusion
For the first time, an intrinsically chiral SuFEx click reaction was used to produce a series of configurationally chiral polymers with MnPS up to 283 kDa. Variation of the constituting diphenol and di-sulfonimidoyl fluoride components allows for a wide range of polysulfonimidates, a novel class of highly functionalized polymers. The configurational enantiospecificity of the polymer-forming click reaction was shown by polymer degradation experiments. This configurational chirality gives rise to main chain-intrinsic optical activity, which might be enhanced by higher-order conformational effects, that are currently under study in our labs. Such easy-to-obtain, chiral polymers are expected to play key roles in a wide range of applications, ranging from solution-based polymer science via chiral catalyst-embedding and chiral polymer brushes to bio-interfaces, especially since the SuFEx reaction is highly compatible with the presence of a wide range of different substituents elsewhere in the molecule.
Acknowledgements
The authors thank Wageningen University, Tianjin University (postdoctoral fellowship to M.S.), and the National Science Foundation of China (grants 21871208 and 22011530163 to H.Z.) This research was carried out under project number C16030b in the framework of the Partnership Program of the Materials innovation institute M2i (www.m2i.nl) and the NWO Domain Science, which is part of the Netherlands Organization for Scientific Research (www.nwo.nl). We thank Tobias Stürzer (Bruker) for single-crystal X-ray diffraction studies,20 and Hans Beijleveld, Barend van Lagen, Fedor Miloserdov, Maarten Smulders, Andriy Kuzmyn and Frans Leermakers for helpful discussions.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




