Effect of closed negative pressure wound therapy after laparotomy for gastrointestinal perforation: A single-center observational study
Abstract
Aim
Laparotomy for gastrointestinal perforation is associated with a high risk of surgical site infection (SSI). The present study aimed to evaluate the effect of closed negative pressure wound therapy (NPWT) against SSI in patients undergoing laparotomy for gastrointestinal perforation.
Methods
This single-center, retrospective, observational study was carried out in a tertiary emergency medical center. We performed closed NPWT following emergency laparotomy for gastrointestinal perforation between May 2019 and May 2024, and compared it to standard dressings used between January 2016 and April 2019. Cases of open abdominal management were excluded. The primary outcome was the incidence of superficial and deep SSI between the two groups.
Results
The closed NPWT group comprised 65 patients, and the standard dressing group comprised 62 patients. There were no significant differences in patient characteristics, comorbidities, operative indications, and wound classification between the two groups. The rate of superficial and deep SSI was significantly lower with closed NPWT (26.2% with closed NPWT vs. 45.2% with standard dressing, p = 0.028).
Conclusion
The application of closed NPWT in patients undergoing laparotomy for gastrointestinal perforation was associated with a lower incidence of SSI compared with that of a standard surgical dressing.
INTRODUCTION
Surgical site infection (SSI) is a common complication in postoperative abdominal surgery that is important to prevent because it can prolong hospital stay and increase medical costs.1, 2 The incidence of SSI following emergency laparotomy is reported to be 25–40% higher than that for elective surgery, and it increases even more with contaminated surgery.3-5
Although prophylactic open negative pressure wound therapy (NPWT) for contaminated surgical incisions with a high risk of SSI and delayed suturing has been reported to be effective, delayed wound healing with open NPWT remains a problem.6, 7 The efficacy of closed NPWT for primary suture wounds in elective and emergency surgery with a higher risk of SSI has been reported for the prevention of SSI, but its usefulness in emergency contaminated abdominal surgery remains to be clarified.7-9 We hypothesized that closed NPWT would contribute to a reduction in the incidence of SSI and introduced closed NPWT to prevent SSI in primary sutured wounds following the performance of emergency laparotomy from May 2019.
This study aimed to evaluate the prophylactic effect of closed NPWT against SSI after emergency contaminated abdominal surgery for gastrointestinal perforation by comparing the incidence of SSI between closed NPWT and a conventional wound protection method.
METHODS
Study design, settings, and population
This single-center, retrospective, observational study was carried out in Kansai Medical University Hospital Critical Care and Emergency Center (Hirakata, Japan), a tertiary emergency medical center. The patients' electronic medical records were reviewed retrospectively. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Kansai Medical University (approval no.: 2024193).
The patients were divided into two groups, the closed NPWT group, which comprised patients whose primary wound closure was with closed NPWT managed using the PICO 7™ device (Smith and Nephew Healthcare, Hull, UK) between May 2019 and May 2024, and the standard dressing group, which comprised patients who underwent incisional wound management with a standard surgical dressing between January 2016 and April 2019. The wounds of both groups were washed with 200–300 mL of saline prior to wound closure. In the standard dressing group, wounds were stapled at a pitch of 1 cm, whereas in the closed NPWT group, they were loosely sutured with interrupted sutures or stapled at a pitch of 2–3 cm.
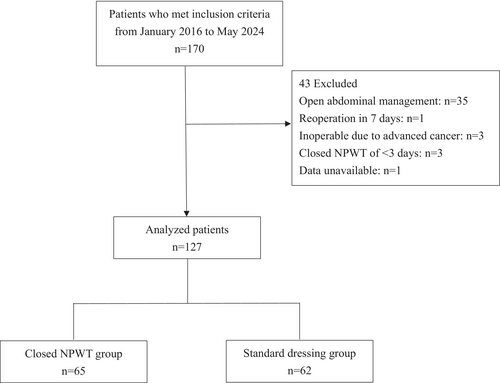
Patients who underwent emergency laparotomy for gastrointestinal perforation with Centers for Disease Control and Prevention (CDC) wound classifications of 3 (contaminated) and 4 (dirty) in midline laparotomy incisions or pararectal incision between January 2016 and May 2024 were included in this study. Patients who met at least one of the following criteria were excluded: open abdominal management, reoperation within 7 days, or inoperable due to advanced cancer. Cases with closed NPWT of less than 3 days were also excluded from the analysis in reference to previous studies (Figure 1).10, 11
Data collection and outcomes
Patients were analyzed for demographics, risk factors for wound complications, clinical characteristics, operative indications, and CDC wound classification. Patients were diagnosed as having disseminated intravascular coagulation (DIC) by Japanese Association for Acute Medicine DIC criteria.
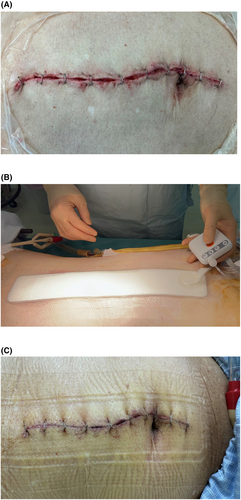
The primary outcome was the incidence of superficial and deep SSI at the last visit or 30 days postoperatively. The secondary outcomes were the length of intensive care unit (ICU) stay and hospital stay, in-hospital mortality, and incidence of complications as assessed by Clavien-Dindo grade. Wounds were managed with the PICO 7 device (Figure 2), which is designed to provide NPWT to wounds that have been sutured or stapled closed. In Japan, the PICO7 device can be used only at facilities that charge a specific hospitalization fee, such as a specific ICU management fee, for surgical wounds with a high risk of SSI, as covered by insurance. The PICO 7 dressing was placed at the time of wound closure, removed once on postoperative day 3, and if there were no findings of SSI after checking the wound, the PICO 7 dressing was re-applied and closed NPWT was continued until postoperative day 7 at the longest. The device was then removed, and the wound was left open to air or covered with dry gauze. If closed NPWT could not be maintained for 3 days for any reason, the closed NPWT device was replaced if the wound showed no complications. The PICO 7 dressing was changed once.
Evaluation
Wounds were classified as superficial or deep SSI if they met any of the following conditions: purulent drainage was present, localized fever and/or spreading erythema requiring treatment with antibiotics, the wound needed to be opened or debrided, or diagnosis by the surgeon or attending physician. Superficial and deep SSI were defined as follows: superficial SSI involves only the skin or subcutaneous tissue of the incision site, and deep SSI affects soft tissues (e.g., fascial and muscle layers) of the incision site. Infected wounds were then converted to open wound management with wet-to-dry dressings. Wound observation time was defined as that from skin closure to the patient's last visit or death.
Statistical analyses
Continuous variables are presented as medians and interquartile range (IQR) and were compared using Wilcoxon–Mann–Whitney tests. Categorical variables are presented as numbers and percentages and were compared using Fisher's exact test. Statistical significance was set at a value of p < 0.05. All statistical analyses were conducted using JMP Pro 17 software program (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographics
The closed NPWT group comprised 65 patients and the standard dressing group comprised 62 patients. The patient characteristics and prevalence of comorbidities that may contribute to SSI are shown in Table 1. The groups were demographically similar in terms of age, sex, body mass index, smoking history, and preoperative comorbidities. There were no statistically significant differences in the incidence of diabetes mellitus, coronary artery disease, heart failure, chronic obstructive pulmonary disease, chronic kidney disease, hemodialysis, liver cirrhosis, and immunosuppression medication between the closed NPWT group and the standard dressing group. In addition, no significant differences were found in the preoperative and postoperative use of vasopressors, presence of DIC, SOFA (Sequential Organ Failure Assessment) score, operation time, amount of blood transfusion, preoperative albumin level, duration of antibiotic administration, and American Society of Anesthesiologists-Physical Status (ASA-PS).
| Characteristic | Closed NPWT group (n = 65) | Standard dressing group (n = 62) | p-value |
|---|---|---|---|
| Age (years), median (IQR) | 73 (60–81) | 71 (60–80) | 0.875 |
| Men, n (%) | 29 (44.6) | 30 (48.4) | 0.724 |
| BMI (kg/m2), median (IQR) | 21.1 (18.7–23.2) | 21.9 (20–25.3) | 0.092 |
| Smoking history, n (%) | 25 (38.5) | 27 (43.5) | 0.592 |
| Diabetes mellitus, n (%) | 14 (21.5) | 15 (24.2) | 0.833 |
| Coronary artery disease, n (%) | 2 (3.1) | 6 (9.7) | 0.158 |
| Heart failure, n (%) | 4 (6.2) | 6 (9.7) | 0.524 |
| COPD, n (%) | 2 (3.1) | 3 (4.8) | 0.675 |
| CKD, n (%) | 9 (13.8) | 8 (12.9) | 0.999 |
| Hemodialysis, n (%) | 2 (3.1) | 4 (6.5) | 0.433 |
| Liver cirrhosis, n (%) | 2 (3.1) | 1 (1.6) | 0.999 |
| Immunosuppression medication, n (%) | 8 (12.3) | 7 (11.3) | 0.999 |
| Preoperative SOFA score, median (IQR) | 1 (1–4) | 1.5 (1–2) | 0.486 |
| Preoperative vasopressor, n (%) | 8 (12.3) | 3 (4.8) | 0.207 |
| Postoperative vasopressor, n (%) | 25 (38.5) | 22 (35.5) | 0.854 |
| Preoperative DIC, n (%) | 7 (10.8) | 6 (9.7) | 0.999 |
| Operation time (min), median (IQR) | 146 (116–185) | 149 (128–186) | 0.358 |
| Intraoperative blood transfusion, n (%) | 24 (36.9) | 22 (35.5) | 0.999 |
| Preoperative albumin level (g/dL), median (IQR) | 3.0 (2.5–3.6) | 3.1 (2.5–3.6) | 0.826 |
| Antibiotic administration (days), median (IQR) | 10.0 (7–15) | 9.5 (7–13.3) | 0.553 |
| ASA-PS | |||
| 1 | 7 (10.8) | 5 (8.1) | 0.332 |
| 2 | 9 (13.8) | 7 (11.3) | |
| 3 | 3 (4.6) | 2 (3.2) | |
| 4 | 46 (70.8) | 47 (75.8) | |
| 5 | 0 (0) | 1 (1.6) | |
- Abbreviations: ASA-PS, American Society of Anesthesiologists-Physical Status; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DIC, disseminated intravascular coagulation; IQR, interquartile range; NPWT, negative pressure wound therapy; SOFA, Sequential Organ Failure Assessment.
The operative indications and CDC wound classifications are shown in Table 2. The indication for surgery was divided into gastroduodenal, small bowel, appendix, and colorectal and was not statistically different between the groups. Pararectal incisions were performed in two cases of appendicular perforation, whereas all other surgeries were performed through midline laparotomy incisions. There were no statistically significant differences in wounds of CDC class 3 or 4 (p = 0.573). In the closed NPWT group, four wounds were sutured with 2-0 nylon, whereas all other wounds were closed with staples.
| Indication/Classification | Closed NPWT group (n = 65) | Standard dressing group (n = 62) | p-value |
|---|---|---|---|
| Perforation site | |||
| Upper gastrointestinal, n (%) | 23 (35.4) | 16 (25.8) | 0.256 |
| Small bowel, n (%) | 7 (10.8) | 4 (6.5) | 0.531 |
| Appendix, n (%) | 5 (7.7) | 9 (14.5) | 0.265 |
| Colorectal, n (%) | 30 (46.2) | 33 (53.2) | 0.480 |
| CDC wound classification | |||
| Wound class 3 (Contaminated), n (%) | 22 (33.8) | 18 (29.0) | 0.573 |
| Wound class 4 (Dirty), n (%) | 43 (66.2) | 44 (71.0) | |
- Abbreviations: CDC, Centers for Disease Control and Prevention; NPWT, negative pressure wound therapy.
Outcomes
The outcomes are shown in Table 3. The combined incidence of superficial and deep SSI was significantly lower in the closed NPWT group compared to the standard dressing group (26.2% vs. 45.2%, p = 0.028). Although the incidence of superficial SSI (21.5% vs. 38.7%, p = 0.051) and deep SSI (4.6% vs. 6.5%, p = 0.713) tended to be lower in the closed NPWT group, the differences were not statistically significant when each was analyzed separately. The lengths of ICU stay and hospital stay and the in-hospital mortality rate were not statistically different. In the closed NPWT group versus the standard dressing group, the hospital stay was 20 [IQR 13–29] days versus 21 [IQR 15–33] days (p = 0.435), and the mortality rate was 1.5% versus 6.5% (p = 0.201).
| Outcome | Closed NPWT group (n = 65) | Standard dressing group (n = 62) | p-value |
|---|---|---|---|
| Superficial and deep SSI, n (%) | 17 (26.2) | 28 (45.2) | 0.028 |
| Superficial SSI, n (%) | 14 (21.5) | 24 (38.7) | 0.051 |
| Deep SSI, n (%) | 3 (4.6) | 4 (6.5) | 0.713 |
| Clavien-Dindo grade, n (%) | |||
| I | 40 (61.5) | 20 (32.3) | 0.005 |
| II | 10 (15.4) | 23 (37.1) | |
| IIIa | 12 (18.5) | 10 (16.1) | |
| IIIb | 0 (0) | 4 (6.5) | |
| IVa | 2 (3.1) | 1 (1.6) | |
| IVb | 0 (0) | 0 (0) | |
| V | 1 (1.5) | 4 (6.5) | |
| ICU stay (days), median (IQR) | 3 (2–5) | 3 (2–5) | 0.723 |
| Hospital stay (days), median (IQR) | 20 (13–29) | 21 (15–33) | 0.435 |
| In-hospital mortality, n (%) | 1 (1.5) | 4 (6.5) | 0.201 |
- Abbreviations: ICU, intensive care unit; IQR, interquartile range; NPWT, negative pressure wound therapy; SSI, surgical site infection.
DISCUSSION
This study showed that closed NPWT was significantly associated with a lower incidence of superficial and deep SSI after emergency laparotomy for gastrointestinal perforation. Several previous studies have suggested the prophylactic effect of closed NPWT for emergency contaminated surgery. However, to the best of our knowledge, this is the first study to show the prophylactic effect of closed NPWT against SSI after laparotomy for gastrointestinal perforation only. The results suggest that the use of prophylactic closed NPWT may be an option for the prevention of SSI after laparotomy for gastrointestinal perforation.
Closed NPWT exerts effects on wound healing through several mechanisms. Closed NPWT decreases lateral tension and dead space and reduces seroma formation. It also eliminates excess tissue edema, increases lymphatic drainage and blood flow around the closed incision, and releases local growth factors to promote healing of the surgical incision and prevent complications.12-15 On the basis of an extensive literature review, an international interdisciplinary consensus recommends the use of closed NPWT in patients at high risk for SSI.16 Previous studies have reported that closed NPWT is useful in elective and emergency colorectal surgery with a high risk of SSI, but some randomized controlled trials have reported no effect, so it is important to identify patients for whom closed NPWT will be beneficial.3, 7-9, 17, 18 High risk for SSI is defined in terms of wound class, patient comorbidities, and preoperative condition. In this study, the CDC target wound class was that of 3 or higher. Furthermore, patients with ASA-PS class of 3 or higher are considered to be at high risk of developing SSI, but in this study, more than 70% of the patients were of ASA-PS class 4.19 The present study showed that closed NPWT may contribute more to a reduction in SSI in patients at highest risk for SSI than was shown in previous studies.
Due to the higher rate of SSI in contaminated and dirty wounds, many surgeons opt to leave the wound open for healing by secondary intention or by delayed primary closure.20 Management of open surgical wounds with NPWT has now become commonplace in the treatment of contaminated wounds. Open NPWT and delayed suturing have been reported to be effective in emergency surgery for colorectal perforation.6, 21 However, several problems have been pointed out in regard to open NPWT and delayed primary closure. The use of open NPWT may lead to longer wound healing and hospitalization times and patient discomfort until the open wound is closed. In addition, open NPWT results in additional healthcare costs, and delayed primary closure requires wound closure under local anesthesia, with dressing changes needed two or three times a week. Frazee et al., in a prospective, randomized trial comparing open NPWT to closed NPWT for contaminated surgery, showed that closed NPWT healed wounds faster than open NPWT without wound complications, and in terms of cost, they stated that closed NPWT may be less expensive than open NPWT.7 We were not able to examine cost in the present study, and we could not find any difference in the length of hospital stay because of the high ASA-PS class and the severity of the study patients' conditions, as many of them required transfer to other hospitals, making the length of hospital stay not easily comparable. However, closed NPWT offers the possibility of rapid wound healing while minimizing short-term discomfort for the patient and potentially reducing medical costs compared to open NPWT.
The optimal duration of therapy for closed NPWT and the optimal negative pressure to be applied on a closed incision have yet to be determined because there are few data on closed NPWT after contaminated surgery. There are two main devices that can be used for closed NPWT, but each device uses different pressure settings: for example, PREVENA™ (3M Company, San Antonio, TX, USA) recommends a pressure setting of −125 mmHg, but that of the PICO 7 is −80 mmHg. The duration of closed NPWT in this study was not constant as it ranged from 3 to 7 days because some patients required earlier-than-planned replacement due to poor application of negative pressure. We used the PICO 7 device because device set-up and use are easy, its size is suitable for the size of the laparotomy wound, and the canister is free and easy to carry. In addition, the work of the medical staff may be reduced because the dressing only needs to be changed once before the end of closed NPWT, and it is easy to change and manage. However, the disposable canister has a fixed capacity and may cause early failure of negative pressure in severe cases with large amounts of leachate. This study excluded surgeries requiring open abdominal management in conjunction with damage control surgery, and it is unclear whether closed NPWT would be useful in such very severe cases. This will require further study.
LIMITATIONS
Several limitations of this study should be considered when interpreting its results. First, this study is of a retrospective observational design performed at a single center with a small sample size. For a more thorough analysis, a larger multicenter study is required. Second, although the diagnosis of SSI was based on CDC criteria, it was made subjectively by the attending surgeon and evaluated retrospectively from the patient's electronic medical record. This might have resulted in some risk of bias. Third, the duration of application of closed NPWT was not constant, and the method of general wound management, such as dressing and washing, was not protocolized but was determined based on the preference of the attending surgeons. Finally, we did not perform an economic evaluation to determine the cost-effectiveness of NPWT compared with standard surgical dressing.
CONCLUSION
The application of closed NPWT in patients undergoing laparotomy for gastrointestinal perforation was associated with a lower incidence of SSI compared with that of standard surgical dressing. Closed NPWT may be an option for the prevention of SSI after gastrointestinal perforation. Further prospective analyses with a larger number of patients are required.
ACKNOWLEDGMENTS
We would like to thank Rise Japan LLC for English language editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Dr. Yasuyuki Kuwagata is an editorial board member of the Acute Medicine & Surgery journal and a co-author of this article. To minimize bias, this author was excluded from all editorial decision-making related to the acceptance of this article for publication.
ETHICS STATEMENT
Approval of the research protocol: The Ethics Committee of Kansai Medical University approved this retrospective study (approval no.: 2024193).
Informed consent: The need for informed consent was waived because of the observational nature of the study and the requirement for no treatment beyond daily clinical practice (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labor and Welfare, Japan; Ethical Guidelines for Medical and Health Research Involving Human Subjects).
Registry and the registration no. of the study/trial: Not applicable.
Animal studies: Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author, TO, upon reasonable request.




