Multiple trauma and shock vital signs as potential for improved outcome in patients with severe head trauma
Yuya Imanishi, Makoto Ohtake and Taisuke Akimoto contributed equally to this work.
Abstract
Aim
To evaluate the prognostic factors in severe head trauma patients (Glasgow Coma Score (GCS) ≤ 8) with all trauma, including those with trunk injury as well as single severe head trauma (abbreviated injury scale (AIS) ≥ 3).
Methods
We included 152 consecutive patients with head trauma (AIS ≥ 3) and consciousness disorders (GCS ≤ 8) who were transported to our institute from January 2017 to October 2022. Data on the patients' background, vital signs at presentation, multiple trauma (AIS ≥ 3 in two or more locations), surgical intervention, and hematological findings were examined; a retrospective analysis was conducted with the modified Rankin Scale score after 3 months assigned as the primary outcome.
Results
The patients' mean age was 57.6 ± 23.4 years (0–89), 49 patients (32.2%) had multiple trauma, and 25 patients (16.4%) had accompanying shock vital signs. In the multivariate analysis of prognosis, age (p = 0.0007) and D-dimer levels (p = 0.0007) were independent poor prognostic factors. On the contrary, patients with multiple trauma (p = 0.027) and shock vital signs at presentation (p = 0.037) had a significantly better prognosis. In the non-shock group, 97.6% (41/42) of patients aged ≥50 years and with D-dimer level of 40 μg/mL or higher had a poor prognosis after 3 months.
Conclusion
Advanced age and high D-dimer levels are important independent associated factors in patients with severe consciousness disorder associated with head trauma; meanwhile, the prognosis is more favorable in patients whose consciousness disorders are associated with multiple trauma or circulatory failure, indicating that rapid improvement of circulatory failure may lead to better outcomes.
BACKGROUND
The Glasgow Coma Scale (GCS) score is commonly used to diagnose the severity of head trauma.1-3 The international head injury guidelines define severe head injury as head trauma with a GCS of 3–8, which accounts for 10% of all head injuries; ~35%–60% of patients with head trauma have concomitant injuries to other parts of the body.2, 4, 5 Although head trauma itself often causes consciousness disorder, when concomitant trunk injury occurs, consciousness disorder may develop for reasons other than intracranial causes, such as circulatory failure associated with shock vital signs. In such cases, even if the head injury was not severe, the condition would be classified as severe head trauma according to the definition of severity based on the level of consciousness. When there is concomitant trunk injury, a generalized examination and determination of factors directly related to the prognosis are necessary to distinguish the conditions that may cause consciousness disorder. Although many studies have evaluated the prognostic factors and mortality rates in single head trauma cases with a GCS of 8 or less, few studies have examined patients with various types of severe head trauma, including those with multiple trauma.6-8 In this study, we aimed to identify the prognostic factors in all patients with severe consciousness disorders associated with head trauma (Abbreviated injury score (AIS) ≥ 3).
MATERIALS AND METHODS
Study design and participants
This study protocol was reviewed and approved by the Institutional Review Board and the Ethics Committee of the Yokohama City University Medical Center (Approval no. B210400045). The requirement for written informed consent was waived, and the individual informed consent was opted out, due to the nature of the retrospective study design and as per the Personal Information Protection Law and National Research Ethics Guideline in Japan. This single-center, retrospective cohort study included 152 consecutive head trauma (AIS ≥ 3) patients with consciousness disorder (GCS ≤ 8) who were transported to our advanced critical care center from January 2017 to October 2022. Age, gender, trauma mechanism, vital signs at presentation, pupil findings, hematological findings, Injury severity score (ISS), AIS, Shock Index (SI), presence or absence of pelvic fracture, neurosurgery status, and other surgical procedures performed were examined. ISS is a severity rating method for patients with multiple trauma developed by Baker et al.9 in 1974 which is assessed from the anatomical aspects of multiple injury sites. In this study, ISS was used as an indicator of severe trauma, with ISS ≥ 16 defined as severe trauma, and AIS as an indicator of multiple trauma, with AIS ≥ 3 in two or more locations defined as multiple trauma. The SI is used for the initial assessment of hypovolemic shock proposed by Allgower et al. in 1967 and is defined as heart rate divided by systolic blood pressure; an SI of greater than 1.0 indicates hemorrhagic shock.10-12
Treatment protocol
Our facility is a tertiary emergency facility/severe trauma center in Japan. When a severe trauma patient is transported to our facility, a physiological evaluation is usually performed to identify conditions that require urgent intervention, in accordance with the international guidelines for the initial treatment of trauma.1, 2, 13 Resuscitation should be performed in the following order: airway and respiratory management, circulatory management, and to confirm the dysfunction of the central nervous system (CNS). The level of consciousness was assessed after airway stabilization, respiratory and circulatory management of the primary survey, and blood tests were performed during the primary survey. In cases of head trauma accompanied by trunk injury, rapid hemodynamic stabilization is performed before intracranial intervention, especially for non-responders to fluid replacement and blood transfusions, by performing hemostasis, resuscitative endovascular balloon occlusion of the aorta, or transcatheter arterial embolization depending on the case immediately after transport. Although the dysfunction of the CNS is difficult to manage during the initial evaluation phase, hypotension associated with hypoxia and circulatory failure should be avoided to prevent secondary brain injury. After these management measures are taken to ensure maintenance of life immediately after transport, anatomic evaluation is carried out to systematically assess for injuries in each body part and to determine the need for radical treatment. A computed tomography (CT) scan of the head is performed at this stage, and emergency surgery is carried out if intracranial conditions requiring surgical evacuation are identified.
Outcome measurement
The primary endpoint was modified Rankin Scale (mRS) at 3 months, and the secondary endpoint was the 3-month mortality rate. For the prognostic evaluation, patients with an mRS of 0–2 were classified as the good prognosis group, while those with an mRS of 3–6 were classified as the poor prognosis group. The patient's condition 3 months post-injury was evaluated through phone calls, letters, and outpatient findings.
Statistical analysis
To account for biases, the analysis was performed after the completion of the study period. Results are presented as the mean and standard deviation for quantitative data and as frequencies (percentages) for categorical data. Data were not normalized due to the limited number of enrolled patients. For comparisons between groups, Pearson's Chi-square (or Fisher's exact test) and Wilcoxon tests were performed. Kaplan–Meier analysis and log-rank tests were employed to assess the association of time to death in the 3-month acute phase with various factors such as age, D-dimer value, multiple traumas, and pupil distraction. Additionally, multivariate Cox proportional hazards models were used to identify independent factors of time to death. Statistical significance was set at p < 0.05. All statistical analyses were performed using JMP® 15 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The characteristics of 152 head trauma (AIS ≥ 3) patients with consciousness disorder (GCS ≤ 8) are shown in Table 1. The patients' mean age was 57.6 ± 23.4 (0–89) years, and 106 patients (69.7%) were men; with regard to the trauma mechanism, 76 patients (50.1%) had fall-related injuries, while 63 patients (41.4%) had traffic injuries. The median GCS was 4,3-8 131 patients (86.2%) had severe injuries (ISS ≥ 16), 49 patients (32.2%) had multiple injuries with trunk injuries (AIS ≥ 3), and 25 patients (16.4%) had shock vital signs (SI > 1). Skull fractures were observed in 80 patients (52.6%) and pelvic fractures in 25 patients (16.4%). The majority of the patients (139, 91.4%) had focal brain injury with subdural hematoma, epidural hematoma, or cerebral contusion at hospitalization, of whom 81 (53.3%) required neurosurgery; meanwhile, no surgery-related deaths were reported. The mean length of hospital stay was 31.2 ± 27.8 days, and an mRS of 0–2 at discharge was observed in 110 patients (65.1%). There was no significant difference in head and neck AIS between single and multiple head traumas (p = 0.299).
| Patients characteristics | n = 152 | Single head trauma, n = 103 | Multiple trauma, n = 49 | p-Value |
|---|---|---|---|---|
| Mean age ± SD (years) | 57.6 ± 23.4 | 57.5 ± 22.7 | 48.4 ± 23.9 | 0.033 |
| Sex male (%) | 106 (69.7) | 73 (70.9) | 33 (67.4) | 0.658 |
| Trauma mechanisms (%) | ||||
| Traffic injuries | 63 (41.4) | 32 (31.1) | 31 (63.3) | <0.001 |
| Fall-related injuries from height | 46 (30.2) | 29 (28.2) | 17 (34.7) | 0.412 |
| Fall-related injuries | 30 (19.9) | 30 (29.1) | 0 | ー |
| Others | 13 (8.5) | 12 (11.7) | 1 (2.0) | 0.135 |
| Findings at the time of arrival | ||||
| GCS median (range) | 4 (3–8) | 4 (3–8) | 4 (3–8) | 0.756 |
| Shock Index > 1 (%) | 25 (16.4) | 12 (11.7) | 13 (26.5) | 0.022 |
| ISS mean ± SD | 24.1 ± 9.9 | 19.5 ± 7.2 | 33.7 ± 7.7 | <0.001 |
| Head and nNeck AIS (range) | 4 (3–6) | 4 (3–6) | 4 (3–5) | 0.299 |
| Skull fracture (%) | 80 (52.6) | 51 (49.5) | 29 (59.2) | 0.265 |
| Pelvis fracture (%) | 25 (16.4) | 4 (3.9) | 21 (42.9) | <0.001 |
| Neurosurgical surgery (%) | 81 (53.3) | 53 (51.5) | 28 (57.1) | 0.511 |
| TAE (%) | 16 (10.5) | 4 (3.9) | 12 (24.5) | <0.001 |
| Antiplatelet and anticoagulants | 10 (6.6) | 9 (8.7) | 1 (2.0) | 0.169 |
| Blood test results at the time of visit | ||||
| Platelet mean ± SD (×103/μL) | 213.5 ± 84.7 | 215.4 ± 88.4 | 209.6 ± 76.2 | 0.689 |
| Hemoglobin mean ± SD (g/dL) | 12.4 ± 2.5 | 12.5 ± 2.5 | 12.1 ± 2.3 | 0.221 |
| D-dimer median (range) (μg/mL) | 40.2 (0.8–951.2) | 31.35 (0.8–951.2) | 57.7 (8–930.9) | 0.0021 |
| Fibrinogen mean ± SD (μg/mL) | 236.7 ± 113.7 | 251.3 ± 124.6 | 205.9 ± 86.4 | 0.027 |
| Lactate median (range) (mmol/L) | 3 (0.5–79.7) | 3 (0.5–79.7) | 2.9 (0.6–19) | 0.987 |
| Period of hospitalization (range) (days) | 24.5 (1–136) | 16 (1–136) | 36 (1–101) | 0.0021 |
| 90 days mRS 0–2 (%) | 49 (32.2) | 26 (25.2) | 23 (46.9) | 0.0075 |
| Death(mRS 6) at discharge (%) | 37 (24.3) | 29 (28.2) | 8 (16.3) | 0.112 |
- Note: Data are presented as the number (%) or the mean ± standard deviation (SD). Bold letters mean significant data with p < 0.05.
- Abbreviations: GCS, Glasgow Coma Scale; ISS, Injury Severity Score; mRS, modified Ranking Scale; RTS, Revised Trauma Score; TAE, transarterial embolization.
In the multivariate analysis of the prognosis, age (p = 0.0007) and D-dimer levels (p = 0.0007) were independent poor prognostic factors (Table 2). By contrast, patients with multiple trauma (p = 0.027) and shock vital signs at presentation (p = 0.037) had a significantly better prognosis. In the multivariate analysis of mortality rates, age (p = 0.037), head and neck AIS (p = 0.0032), low GCS score (p = 0.0031), bilateral pupil dilation (p = 0.004), D-dimer levels (p = 0.0014), and lactate levels (p = 0.026) were independent associated factors (Table 3). Kaplan–Meier analysis followed by log-rank tests showed that D-dimer ≥40 (log-rank: p < 0.001), age ≥50 years (log-rank: p = 0.023), and bilateral mydriasis (log-rank: p = 0.012) were associated with shorter time to death in the acute phase within 3 months (Figure 1).
| Variate | 90 days mRS0-2, n = 49 | 90 days mRS3-6, n = 103 | Univariate | Multivariate | |
|---|---|---|---|---|---|
| p-Value | Odd (95%CI) | p-Value | |||
| Age mean ± SD (years) | 41.2 ± 22.9 | 60.9 ± 20.9 | <0.001 | 1.02 (1.01–1.04)a | 0.0007 |
| Sex male (%) | 34 (69.4) | 72 (69.9) | 0.949 | ||
| Skull fracture (%) | 27 (55.1) | 53 (51.5) | 0.674 | ||
| Neurosurgical surgery (%) | 23 (46.9) | 58 (56.3) | 0.279 | ||
| Head and neck AIS (range) | 4 (3–5) | 4 (3–6) | 0.0041 | 1.34 (0.79–2.31)a | 0.277 |
| Shock Index > 1 (%) | 13 (26.5) | 12 (11.7) | 0.018 | 0.30 (0.09–0.93) | 0.037 |
| GCS median (range) | 6 (3–8) | 4 (3–8) | 0.135 | ||
| Anisocoria (%) | 6 (12.2) | 24 (23.3) | 0.110 | ||
| Bilateral mydriasis (%) | 6 (12.2) | 17 (16.5) | 0.493 | ||
| D-dimer median (range) (μg/mL) | 24.3 (0.8–315) | 49.8 (1–951.2) | <0.001 | 1.01 (1.00–1.02)a | 0.0007 |
| Lactate median (range) (mmol/L) | 2.5 (0.6–70.7) | 3.1 (0.5–79.7) | 0.071 | ||
| Pelvis fracture (%) | 11 (22.5) | 14 (13.6) | 0.169 | ||
| TAE (%) | 5 (10.2) | 11 (10.7) | 0.929 | ||
| With two or more AIS 3 or more locations (%) | 23 (46.9) | 26 (25.2) | 0.0075 | 0.38 (0.15–0.89) | 0.027 |
| ISS mean ± SD | 24.7 ± 9.3 | 23.7 ± 10.2 | 0.466 | ||
- Note: Data are presented as the number (%) or the mean ± standard deviation (SD). Bold letters mean significant data with p < 0.05.
- Abbreviations: GCS, Glasgow Coma Scale; ISS, Injury Severity Score; mRS, modified Ranking Scale; RTS, Revised Trauma Score; TAE, transarterial embolization.
- a Unit Odd ratio.
| Variate | 90 days mRS6, n = 37 | 90 days mRS0-5, n = 115 | Univariate | Multivariate | |
|---|---|---|---|---|---|
| p-Value | Odd (95%CI) | p-Value | |||
| Age mean ± SD (years) | 63.2 ± 18.7 | 51.8 ± 24.1 | 0.0084 | 1.03 (1.00–1.05)a | 0.037 |
| Sex male (%) | 24 (64.9) | 82 (71.3) | 0.458 | ||
| Skull fracture (%) | 20 (54.1) | 60 (52.2) | 0.842 | ||
| Neurosurgical surgery (%) | 16 (43.2) | 65 (56.5) | 0.159 | ||
| Head and Neck AIS (range) | 4 (3–6) | 4 (3–5) | <0.001 | 3.14 (1.47–6.71)a | 0.0032 |
| Shock Index > 1 (%) | 7 (18.9) | 18 (15.7) | 0.656 | ||
| GCS median (range) | 3 (3–8) | 6 (3–8) | 0.0031 | 0.55 (0.37–0.82)a | 0.0031 |
| Anisocoria (%) | 8 (21.6) | 22 (19.1) | 0.741 | ||
| Bilateral mydriasis (%) | 10 (27.0) | 13 (11.3) | 0.033 | 7.2 (1.88–27.6) | 0.004 |
| D-dimer median (range) (μg/mL) | 123.1 (2–951.2) | 32.9 (0.8–502.1) | <0.001 | 1.01 (1.00–1.01)a | 0.0014 |
| Lactate median (range) (mmol/L) | 4.6 (0.9–79.7) | 2.7 (0.5–70.7) | 0.0028 | 1.06 (1.01–1.12)a | 0.026 |
| Pelvis fracture (%) | 6 (16.2) | 19 (16.5) | 0.965 | ||
| TAE (%) | 5 (13.5) | 11 (9.6) | 0.496 | ||
| With two or more AIS 3 or more locations (%) | 8 (21.6) | 41 (35.7) | 0.112 | ||
| ISS mean ± SD | 26.4 ± 9.8 | 23.3 ± 9.8 | 0.098 | ||
- Note: Data are presented as the number (%) or the mean ± standard deviation (SD). Bold letters mean significant data with p < 0.05.
- Abbreviations: GCS, Glasgow Coma Scale; ISS, Injury Severity Score; mRS, modified Ranking Scale; RTS, Revised Trauma Score; TAE, transarterial embolization.
- a Unit Odd ratio.
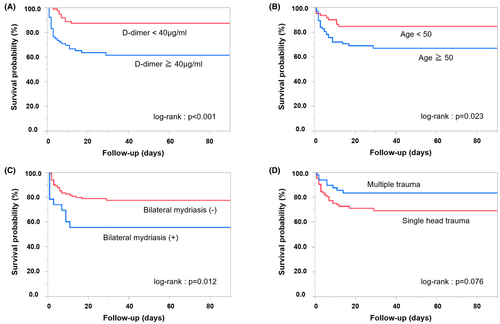
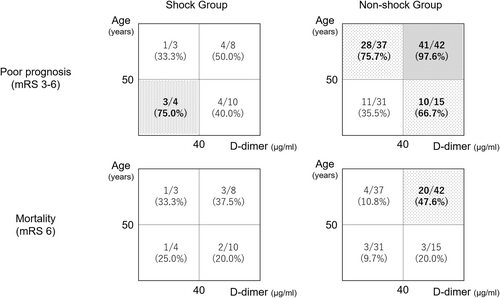
The receiver operating characteristic curve analysis showed that the optimal cutoff age for prognosis was 50 years (area under curve: 0.740, sensitivity: 0.748, specificity: 0.673). The prognostic values calculated based on the sensitivity and specificity rates showed that 97.6% (41/42) of patients in the non-shock group aged 50 years or older and with a D-dimer level of 40 μg/mL or higher had a poor 3-month prognosis (Figure 2). On the contrary, 75.0% (3/4) of patients in the shock group were aged <50 years and had a D-dimer level of 40 μg/mL or less. With regard to the mortality rates, the shock and non-shock groups had relatively high mortality rates among patients aged >50 years and with a D-dimer level of 40 μg/mL or higher.
DISCUSSION
Various prognostic and mortality-related factors have been reported for single head trauma cases, including advanced age, low GCS levels, coagulation/fibrinolytic system disorders, abnormal findings on CT, anisocoria, trunk injury, hypoxemia, fever, intracranial pressure, and cerebral perfusion pressure.3, 14-16 In particular, advanced age, low GCS, and high D-dimer levels were frequently reported as independent mortality-related factors. In the present study, only patients with severe consciousness disorder (GCS ≤ 8) were included, and the results were consistent with those of previous studies, which reported that advanced age and higher D-dimer levels were poor prognostic factors of head trauma patients. The results were the same in a sub-analysis excluding patients who used antithrombotic drugs (data not shown). In addition to advanced age and D-dimer levels, bilateral pupil dilation, head and neck AIS, and lactate levels were independent factors associated with mortality. Lactate levels were not assessed in previous studies, but this may be due to the fact that this study included many patients with circulatory failures associated with trunk injury as well as those with head trauma alone.
Previous studies have established multiple trauma or shock vital signs as vital factors associated with poor prognosis and mortality in trauma care.12, 17 In contrast, in the present study limited to patients with severe head trauma, those with multiple trauma or shock vital signs showed a good prognosis. This result is very interesting and indicates that when multiple trauma and shock vital signs are involved as causes of consciousness disorder in head trauma patients, it is relatively easy to improve the patients' general condition by improving the hemodynamics and providing appropriate therapeutic intervention for the trunk injury following the patient's arrival. In our sub-analysis that separated the single head trauma and multiple trauma groups (data not shown), the independent factors associated with prognosis in the single head trauma group were age (p = 0.015) and D-dimer (p = 0.012), which is consistent with previous reports. In the multiple trauma group, only age (p = 0.016) was associated with prognosis. The absence of vital signs of shock as a prognostic factor in this sub-group analysis could be attributed to the few cases with shock vital signs in the single head trauma group, which were relatively high in the multiple trauma group. Taken together, some patients with multiple trauma may have impaired consciousness due to circulatory failure rather than intracranial damage, indicating that rapid improvement of circulatory failure may improve the prognosis of such patients.
To the best of our knowledge, few studies have managed trunk injury and shock vital signs associated with head trauma, since most of the previous studies have only examined head trauma “alone.” Wafaisade et al. reported that even a head injury alone can result in hypotension associated with coagulopathy, and the presence of hypotension causes a twofold increase in the fatality rate of head injury; moreover, a systolic blood pressure below 90 mmHg is a critical factor in causing secondary brain damage.18 However, the actual frequency of hypotension in single head trauma cases is relatively low, and a previous study using the Los Angeles trauma database reported that hypotension with a systolic blood pressure of 90 mmHg was observed only in 3% of patients with head trauma alone19; this finding suggests that factors other than intracranial injury strongly trigger the shock vital signs. Shock vital signs in cases where high intracranial pressure is expected are likely to lead to early cerebral ischemia due to an extremely low cerebral perfusion pressure. In an effort to prevent cerebral hypoperfusion, the aim is to transport severely injured patients to the hospital as quickly as possible and to improve their hemodynamics as soon as possible after they arrive at the hospital. In summary, since the improvement of shock vital signs maintains cerebral perfusion and leads to a favorable outcome, in cases of head trauma accompanied by trunk injury, it is important to quickly improve hemodynamics immediately after transport.
Figure 2 shows the number of patients with poor prognosis and deaths, divided into shock and non-shock groups. Almost all non-shock patients aged >50 years and with high D-dimer levels have a poor mRS at 3 months. It is noteworthy that the trend of poor prognosis in shock and non-shock patients was opposite. The results suggest that the consciousness disorder in non-shock patients may be due to intracranial injury, and the prognosis is worse in patients who developed consciousness disorders because of the dysfunction of CNS. Fernández et al. examined trauma patients with a GCS of 3 and bilateral pupil dilation and reported an in-hospital mortality rate of 91%, with head and neck AIS score of 3 or higher being a particularly relevant factor for in-hospital mortality.17 This result supports the finding that intracranial injuries have a greater prognostic impact compared with trunk injuries. By contrast, the prognosis of shock cases was opposite to that of non-shock cases, with a relatively poor prognosis in patients aged <50 years and with a D-dimer levels of less than 40 μg/mL (Figure 2). The small number of patients in this group (4 cases) may be one of the factors; however, this group had the most severe disease, with a GCS of 3 in all patients. The reasons why the D-dimer levels did not get that bad could be that the injuries were witnessed, and the time from injury to blood test was less than 60 minutes in most cases.
There was some bias in the characteristics of the patients as our facility specializes in the management of severe trauma. It is important to acknowledge that our study population comprised a limited cohort of patients who were transported and admitted to a high-level trauma center. Most of the studies to date have only investigated patients with head trauma alone, and only a few studies have examined head trauma patients accompanied by trunk injury or shock; therefore, there has been an insufficient discussion about this condition in the literature. In the presence of shock vital signs, it is important to consider that the assessment of consciousness level at the primary survey stage may be unreliable and mislead severity evaluation. Accordingly, among patients with head trauma accompanied by circulatory failure who were diagnosed as severe in the initial stage, some patients may lack severe brain damage and show improved outcomes with early hemodynamic stabilization. In addition, the level of consciousness does not remain constant after improvement of circulatory status, which can be considered a limitation of this study. Nonetheless, estimating the prognosis at the primary survey stage may contribute to the rapidity of subsequent treatment intervention. Therefore, the results of this study are meaningful in examining the prognosis and treatment strategies for patients with these cases.
CONCLUSION
Advanced age and high D-dimer levels are important independent associated factors for both poor prognosis and mortality in patients with severe consciousness disorder associated with head trauma. By contrast, patients with multiple trauma or shock vital signs are more likely to have a good prognosis, indicating that when patients with head trauma have impaired consciousness partially due to circulatory failure, aiming to improve circulation early may lead to a better prognosis.
ACKNOWLEDGMENTS
We would like to thank Editage for English language editing.
FUNDING INFORMATION
This work was supported by a grant from Japan Society for the Promotion of Science (JSPS) KAKENHI to MO (Grant No. 24K12228).
CONFLICT OF INTEREST STATEMENT
The authors (YU, MO, TA, TK, MY, KS, JS, TK, KS, IT, and TY) declare that they have no financial or non-financial competing interests.
ETHICS STATEMENT
Approval of the research protocol: This study protocol was reviewed and approved by the Institutional Review Board Ethics Committee of the Yokohama City University Medical Center (Approval no. B210400045). The study procedures were performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Informed consent: The Ethics Committee of the Yokohama City University Medical Center also waived the requirement for written informed consent, and individual informed consent was obtained through an opt-out approach, per the Personal Information Protection Law and National Research Ethics Guideline in Japan. Consent for the publication of this study was obtained through an opt-out approach.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




