Sonography of diffuse thyroid disease
Abstract
Introduction/Purpose
This article aims to review of the common diffuse thyroid disease.
Methods
Thorough literature search and review was performed for each diffuse thyroid disease. The most recent and updated ultrasound images were obtained.
Results
Diffuse thyroid diseases discussed include multinodular goitre, Graves’ disease, Hashimoto thyroiditis, de Quervain thyroiditis, acute suppurative thyroiditis, anaplastic carcinoma, thyroid metastases, chronic lymphocytic leukaemia/small lymphocytic lymphoma, Langerhans cell histiocytosis, tuberculosis, plasmacytoma, IgG4-related disease and thyrolipoma
Discussion
The major clinical features and the sonographic features of each diffuse thyroid disease are reviewed.
Conclusion
This article serves as a synopsis of diffuse thyroid disease.
Introduction
The major indication for sonography of the thyroid gland is to evaluate focal nodules or masses to try to determine its nature. However, ultrasound is also useful in the assessment of diffuse thyroid disease. This article aims to review the common diffuse thyroid diseases and their sonographic features.
Multinodular goitre
Patients with multinodular goitre (MNG) are most commonly clinically euthyroid but may be hyperthyroid or borderline with suppressed thyroid-stimulating hormone (TSH) levels but normal free T4 and T3. It is the most common cause of tracheal/oesophageal compression and may even be the cause of laryngeal palsy.1
Non-toxic MNG is a common finding in both iodine-deficient and iodine-replete populations, with a prevalence of up to 12%.2 It is a result of recurrent episodes of hyperplasia and involution that combine to produce a more irregular enlargement of the thyroid gland, due to variations among follicular cells in their response to external stimuli such as trophic hormones. Nearly all long-standing simple goitres will convert into MNGs, and they produce the most extreme thyroid enlargements that are often mistaken for neoplasms. In the Plummer syndrome, an autonomously functioning nodule develops within a long-standing MNG and produces hyperthyroidism (toxic MNG). Clinically apparent autonomous nodules are estimated to develop in about 10% of MNGs during a 10-year follow-up, whereas the incidence of malignancy in long-standing MNGs is low (<5%).3, 4
The typical sonographic appearance of MNG5, 6 is a well-marginated, diffuse enlargement of the thyroid gland with a heterogeneous, nodular appearance (Figure 1). The presence of calcification, fibrosis, cystic change and haemorrhage contribute to the heterogeneous appearance. Most (87%) of the benign nodules are predominantly solid (<50% cystic component).7 13% of the benign nodules are predominantly cystic (>50% cystic component) with multiple internal septa. The cystic component is due to degeneration, haemorrhage or colloid content. A purely cystic nodule is rare (<2% of all nodules) but highly unlikely to be malignant.8 The spongiform appearance (aggregation of multiple microcystic components in >50% of the nodule volume) (Figure 2) is highly specific (99.7%) for benign hyperplastic nodule with a high negative predictive value (98.5%) for malignancy.7, 9, 10 The solid nodules are mostly isoechoic with a minority (5%) being hypoechoic. Although these nodules are unencapsulated, they remain sharply defined, haloed (the halo is comprised of compressed thyroid parenchyma and its associated blood vessels) and may be conglomerated. The heterogeneous internal echo pattern of the nodules is often seen with internal debris and septa from haemorrhage, and the solid components within cystic portions are due to blood clots (Figure 3). Calcifications (present in 15–25%) are commonly multiple, dense with posterior acoustic shadowing, and may be curvilinear/annular, dysmorphic or coarse. The presence of multiple, tiny, non-shadowing echogenic foci with ‘comet-tail’ artefacts (Figure 4) are suggestive of a colloid nodule,11 and should not be mistaken for microcalcifications. On Doppler these nodules show predominantly peripheral vascularity, while the septa and solid components within cystic portions due to organising blood and clots are avascular.12-15 The background thyroid parenchyma often shows coarse, heterogeneous echo pattern, unlike the fine, bright echoes in the normal thyroid gland.
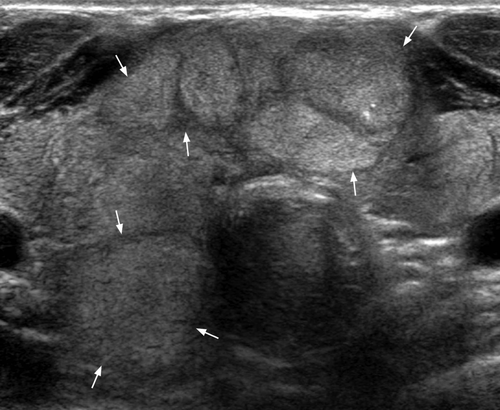
Studies have shown that patients with multiple thyroid nodules bear the same risk of malignancy as those with solitary nodules and multinodularity does not infer benignity.8, 12, 16 The most common cancer in MNG is papillary carcinoma. The role of ultrasound in MNG is to detect any suspicious nodule, evaluate the neck for metastatic nodes and to guide fine needle aspiration or biopsy of suspicious nodules and/or neck nodes.4 The sonographic features suspicious of papillary carcinoma in MNG are solid, ill-defined, taller than wide, markedly hypoechoic nodule, punctate microcalcification and chaotic intranodular vascularity. Typical sonographic appearances of papillary carcinoma metastatic nodes are solid/cystic, hyperechoic, ±punctate microcalcification, abnormal vascularity and particularly localised at the pre-/paratracheal and deep cervical chain regions.5, 6 Rapid growth of a thyroid nodule also indicates an increased risk of malignancy17, 18 and those thyroid nodules that demonstrate substantial growth on serial ultrasound should undergo fine needle aspiration or biopsy. However, recent data suggest fine needle aspiration cytology (FNAC) for nodules which grow >50% in volume is not likely to yield a diagnosis of malignancy. A positive FNAC yield is more likely in nodules with suspicious sonographic features.19
Toxic MNG refers to hyperthyroidism with MNG. Thyroid scintigraphy reveals functioning, hot nodules in suppressed extranodular thyroid tissues. It tends to occur in the elderly, with signs and symptoms of thyrotoxicosis being milder than that of Graves’ disease. On the other hand, toxic autonomous nodule refers to hyperthyroidism with one or two hyperfunctioning thyroid nodules.20 Such nodule, while functioning independently of the normal pituitary-thyroid control axis, produces excessive thyroid hormone and suppresses thyrotropin. Thyroid scintigraphy reveals toxic autonomous nodule as a hyperfunctioning, hot nodule while the surrounding extranodular thyroid tissues shows decreased tracer uptake (partial suppression) or absent tracer uptake (complete suppression).21
Graves’ disease
Graves’ disease is an autoimmune disorder characterised by the production of autoantibodies against multiple thyroid proteins, most importantly autoantibodies to the TSH receptor that mimic TSH action (long-acting thyroid-stimulating antibodies – LATS), which produce diffuse hyperplasia and hypertrophy of the thyroid gland.3, 22 It is the most common cause of endogenous hyperthyroidism, accounting for 60–80% of cases, with a 10:1 predilection for females and a peak incidence at age 20–50 years.2 It is characterised by the clinical triad of hyperthyroidism associated with diffuse enlargement of the gland, infiltrative ophthalmopathy with exophthalmos and localised, infiltrative dermopathy (pretibial myxoedema). Laboratory findings include elevated serum free T3 and T4 and decreased serum TSH.3
The cardinal sonographic features of Graves’ disease6, 22 are mild to moderate diffuse and symmetrical enlargement of the thyroid gland (including the isthmus) with rounded contour, hypoechoic, heterogeneous, ‘spotty’ parenchymal echo pattern and markedly increased parenchymal vascularity (turbulent flow with arterial-venous shunts) giving the appearance of ‘thyroid inferno’ (Figure 5). The parenchymal hypoechogenicity is due to decrease in colloid content and increased cellularity resulting in reduction of colloid–cell interface, and/or hypervascularity.23 It is noted that the persistence of parenchymal hypoechogenicity after cessation of medical therapy is associated with relapse of hyperthyroidism.24 The increased parenchymal vascularity does not correlate with the thyroid function and is merely a reflection of inflammatory activity. However, the increased vascularity tends to decrease in response to therapy and to appear again in relapse. It should be noted that in Graves’ disease the peak systolic velocity in the inferior thyroid artery might be increased up to 120 cm/s. No such increased in the peak systolic velocity is seen in Hashimoto thyroiditis.22, 25
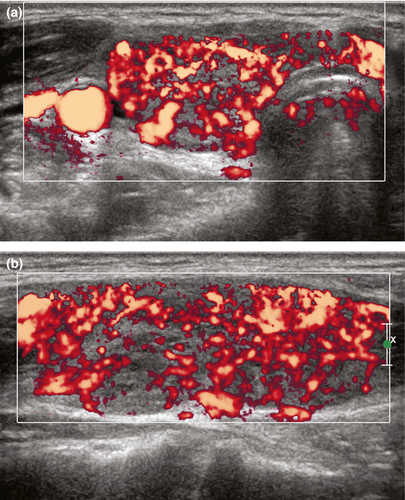
The diagnosis of Graves’ disease is based on clinical and laboratory findings and ultrasound is usually not routinely required. However, ultrasound of thyroid may be useful in patients who fail medical therapy and in whom other causes of thyroiditis is suspected. Thyroid ultrasound is also indicated for thyroid volume estimation in patients about to undergo radioactive iodine treatment.6, 22
Thyroid scintigraphy in Graves’ disease shows the thyroid gland is usually enlarged, with glandular activity increased with respect to the background due to increased stimulation and glandular function.21
Hashimoto thyroiditis (chronic lymphocytic thyroiditis)
Hashimoto thyroiditis is a chronic, autoimmune-mediated lymphocytic inflammation of the thyroid gland,25 resulting in destruction of the thyroid gland with gradual, progressive thyroid failure.3 It is the most common cause of hypothyroidism in iodine-replete regions, has a female predilection of 10–20:1 and is most prevalent at age between 45 and 65 years. The pathogenesis is a breakdown in self-tolerance to thyroid autoantigens. Circulating autoantibodies against thyroglobulin and thyroid peroxidase are present in most patients with Hashimoto thyroiditis, and the induction of thyroid autoimmunity results in progressive depletion of thyroid epithelial cells by apoptosis with replacement of the thyroid parenchyma by mononuclear cell infiltration and fibrosis.3, 26 The most common presentation is gradual, painless enlargement of the thyroid. The goitre in Hashimoto thyroiditis is usually firm and rubbery, but may vary from soft to hard. Thyroid peroxidase antibodies are often in very high titres (>1000 IU/L). Patients are usually euthyroid with normal T3 and T4 hormones. 20% of cases present with hypothyroidism while 5% of cases show an initial toxic phase of ‘Hashitoxicosis’.1, 23 The diagnosis of Hashimoto thyroiditis is mainly based on positive serology for thyroid autoantibodies in symptomatic patients. As such the diagnosis may be missed when the thyroid autoantibodies are negative. Studies have shown that ultrasound is useful in the diagnosis of Hashimoto thyroiditis and other autoimmune thyroid diseases with typical sonographic appearances.6, 27-29
The classical sonographic appearance of Hashimoto thyroiditis is a diffuse, moderately enlarged, hypoechoic gland with lobulated contours, heterogeneous echo pattern and fine, echogenic fibrotic streaks within. The vascularity varies with the stage and type of involvement.25 In fact the sonographic appearances of Hashimoto thyroiditis vary in different phases of the disease.6, 25
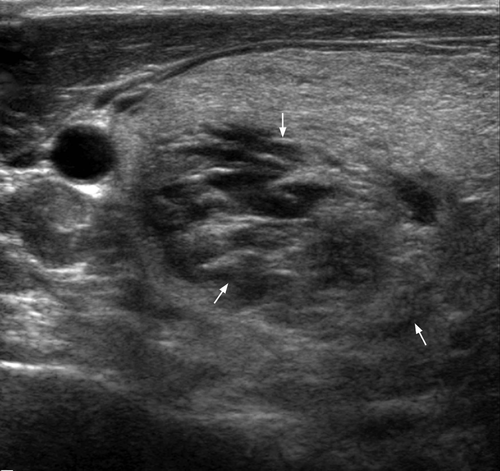
In acute focal Hashimoto thyroiditis, discrete nodules occur in nearly equal frequency against a background of diffuse Hashimoto thyroiditis or normal thyroid parenchyma. Those nodules with background Hashimoto thyroiditis tend to be solitary, solid, non-calcified, hyperechoic and haloed, while those nodules without background of Hashimoto thyroiditis may have cystic change and eggshell calcifications. The vascularity of nodular Hashimoto thyroiditis varies widely and may simulate both benign and malignant thyroid nodules.30 In acute diffuse Hashimoto thyroiditis, there is diffuse hypoechoic goitre that may show lobulated contour.6 Ill-defined, patchy hypoechoic areas separated by echogenic fibrous septa may be seen (Figure 6).31 There may also be multiple small (~2–6 mm) hypoechoic nodules giving a micronodular echo pattern (representing lymphocyte infiltration and echogenic rims due to fibrous septa) that involves the entire gland.32 The parenchymal vascularity may vary from hypovascular to diffuse hypervascularity.33 The hypervascularity is caused by the hypertrophic action of thyroid-stimulating hormone (TSH), and decreases when the TSH level returns back to normal.34 It should be noted that the hypervascularity in Hashimoto thyroiditis is never as marked as in Graves disease, and the flow velocities are within normal limits.25
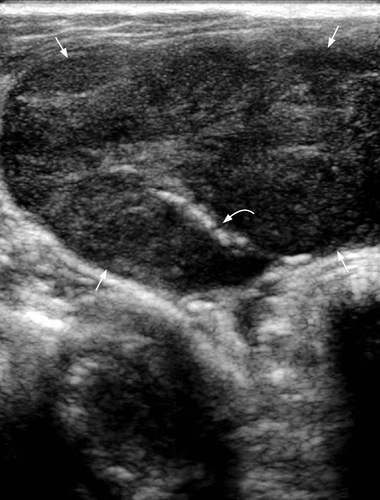
In chronic Hashimoto thyroiditis, sonography shows an enlarged, hypoechoic, micronodular gland with lobulated contours. Diffuse, hypoechoic parenchymal echoes giving the appearance of ‘ghost-like thyroid’ may be seen, with or without echogenic fibrous septa.25
In the atrophic/end stage, Hashimoto thyroiditis appears as a small, hypoechoic gland with heterogeneous echo pattern, and usually hypovascular on Doppler.6, 25
Patients with Hashimoto thyroiditis are at increased risk for developing other autoimmune diseases, including endocrine (type 1 diabetes, autoimmune adrenalitis) and non-endocrine (systemic lupus erythematosus, myasthenia gravis, Sjogren syndrome).3 There is also increased incidence of thyroid malignancy in Hashimoto thyroiditis. A strong association is identified between thyroid non-Hodgkin lymphoma and antecedent Hashimoto thyroiditis.35, 36 There are also reports suggesting increased risk of papillary carcinoma in Hashimoto thyroiditis.37-39 Therefore, during sonographic examination of patients with Hashimoto thyroiditis, care must be taken to evaluate for lesions suspicious of thyroid malignancy. In particular, the presence of calcification of any pattern in nodules associated with Hashimoto thyroiditis should be regarded as suspicious of malignancy. It is reported that calcification of any kind in nodules associated with Hashimoto thyroiditis has a 50% risk of being malignant compared to 4.7% in non-calcified nodules.40
de Quervain thyroiditis (subacute granulomatous thyroiditis)
de Quervain thyroiditis is a self-limited, non-suppurative inflammatory disease of the thyroid gland.41 It is likely to be triggered by a viral infection, as most patients have a preceding history of upper respiratory infection just before the onset of thyroiditis. The viral infection may lead to exposure to a viral or thyroid antigen secondary to virus-induced host tissue damage, and the antigen in turn stimulates cytotoxic T lymphocytes that damage thyroid follicular cells. Unlike autoimmune thyroid disease, this immune response is virus-initiated and not self-perpetuating, and therefore the process is limited.3 Patients most commonly present with preceding flu-like symptoms, high-grade fever and tender goitre. The goitre may be symmetric or asymmetric, diffuse or nodular. The thyroid pain is initially unilateral but then spreads to the contralateral lobe, and half the patients present with thyrotoxic symptoms. Laboratory findings typically show elevated erythrocyte sedimentation rate and C-reactive protein. The white cell count is usually normal or mildly elevated. In patients with thyrotoxicosis, the T3 and T4 hormones are elevated and the TSH is decreased initially. Thyroglobulin is elevated due to follicular destruction and thyroid antibodies are usually absent. This initial thyrotoxic phase may last from weeks to 2 months, is followed by a brief euthyroid phase lasting from 1 to 3 weeks, then a hypothyroid phase lasting from weeks to months. In the final recovery phase the thyroid gland returns to normal function and the patient is euthyroid.41
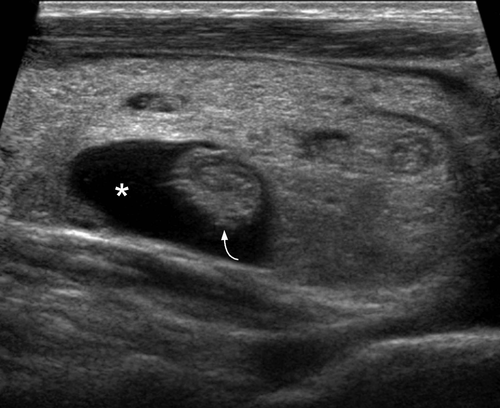
Sonographically in the acute phase there is typically a focal, ill-defined, hypoechoic nodular area in the subcapsular region, with normal or heterogeneous, hypoechoic adjacent thyroid parenchyma. The involved nodular area is avascular or hypovascular on Doppler ultrasound (Figure 7). Tenderness of the thyroid is elicited by transducer pressure. Inflammatory nodes are often seen in the central compartment or the lower internal jugular chain. In the subacute phase, there is progression of glandular enlargement to involve the whole lobe or even the entire gland (Figure 8). The parenchyma remains hypovascular and shows diffuse patchy or confluent, ill-defined hypoechoic echo pattern. There is usually residual tenderness on transducer pressure. Adjacent inflammatory nodes may still be seen. In the recovery phase, there is return to normal appearance but occasionally glandular atrophy or residual nodule may be seen.6, 41, 42 There are reports that the decreased flow seen in the affected areas during the acute phase of the disease is usually followed by increased flow during the recovery phase of the disease.43, 44
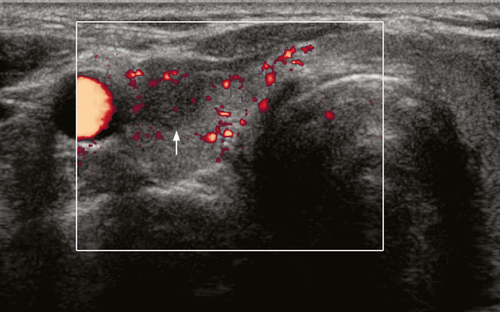
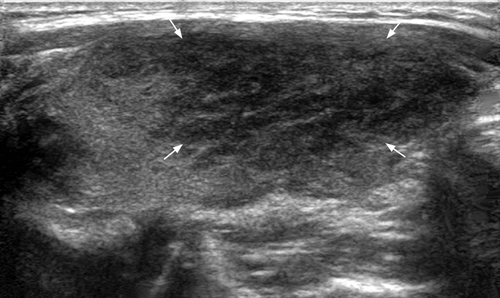
The sonographic appearance in the acute phase of de Quervain thyroiditis may be mistaken for a thyroid nodule raising the concern of malignancy. Nevertheless, the clinical inflammatory symptoms and signs and the thyroid tenderness serve as useful clues to the diagnosis. A follow-up ultrasound in 7–10 days will show the progression of the inflammation to the rest of the thyroid and obviate the need of an unnecessary fine needle aspiration or biopsy.6
Most patients with de Quervain thyroiditis recover uneventfully but permanent hypothyroidism is seen in 4–22% of cases and may be late in onset. Thus, patients may need annual follow-up of thyroid function to exclude subclinical hypothyroidism. 41
Acute suppurative thyroiditis
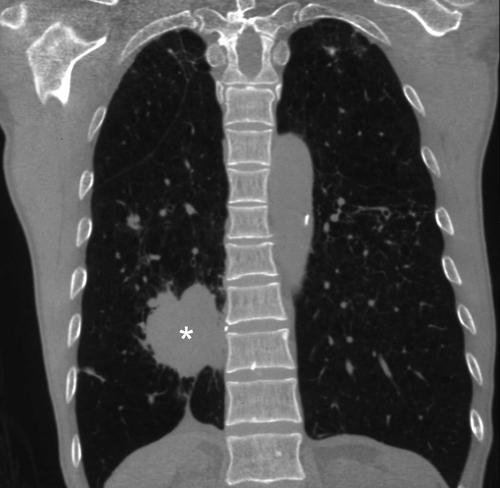
The thyroid gland is an organ highly resistant to infection, due to its thick fibrous capsule, high vascularity, high iodine content and abundant lymphatics. When the rare suppurative infection of the thyroid does occur, it usually affects children and tends to be recurrent and on the left side and is due to the presence of a pyriform fossa sinus associated with a fourth branchial cleft anomaly.45, 46
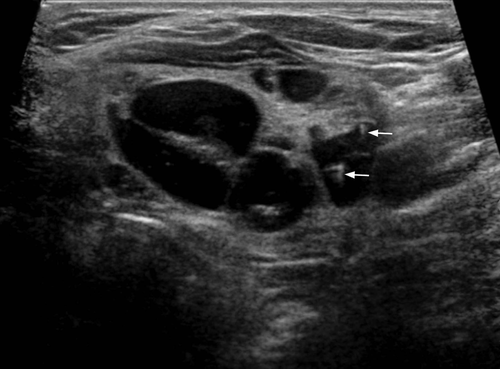
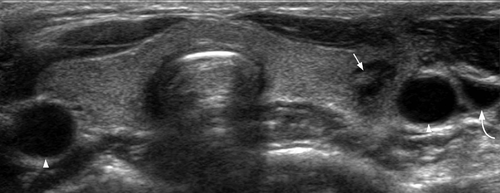
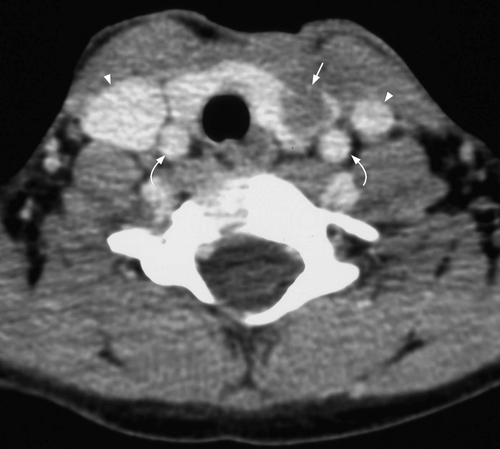
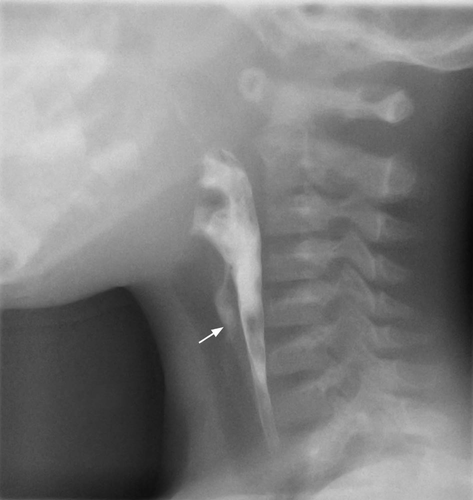
Infection commonly starts in the perithyroid soft tissues. Abscesses, both intra- and extrathyroid in locations, appear as ill-defined, hypoechoic heterogeneous lesions with internal debris. Internal septa or gas may be present, and adjacent inflammatory/reactive nodes are usually seen. The fascial planes between the thyroid and soft tissues will be preserved if the infection is confined to the perithyroid tissues, but the facial planes will be obliterated if the infection extends to involve the thyroid. Ultrasound is used to confirm the presence of an abscess, to see whether the thyroid is involved and to assess the status of adjacent vital structures such as the major neck vessels (Figures 9 and 10). It is also useful for guiding needle aspiration of the abscess, and to assess treatment response. After the infection has subsided, a barium study or computerised tomography should be performed to identify the commonly associated pyriform fossa sinus (Figure 11).46 It has been reported that suppurative thyroiditis may be complicated by thyrotoxicosis.47
Anaplastic carcinoma of thyroid
Anaplastic carcinomas account for less than 5% of thyroid tumours. These are undifferentiated tumours of the thyroid follicular epithelium, and are aggressive with a nearly 100% mortality rate. Patients with anaplastic carcinoma are older (mean age of 65 years) than those with other types of thyroid malignancy. About 25% of patients with anaplastic carcinoma have prior history of well-differentiated thyroid carcinoma, and another 25% of patients harbour a concurrent well-differentiated tumour in the resected specimen.3
The most common presentation is a rapidly growing, large (usually >5 cm), painful thyroid-area mass. Half of the patients have associated symptoms from local invasion/compression to the upper aerodigestive tract, such as dyspnoea, dysphagia and hoarseness (present in 30% of cases and due to recurrent laryngeal nerve palsy). Distant metastases (to lungs, bones, liver and brain) are not uncommonly seen at presentation. The natural course is rapidly fatal, with a mean survival of 6 months after diagnosis.48, 49
On ultrasound, anaplastic carcinoma appears as an ill-defined hypoechoic tumour diffusely infiltrating the entire lobe or gland.48-50 Necrotic areas and dense amorphous calcifications are common, and seen in 78% and 58% of cases respectively (Figure 12).51 Background of nodular goitre is seen in 47%.48 Extracapsular spread to invade the adjacent structures including the larynx, trachea, oesophagus and recurrent laryngeal nerve, and vascular invasion to the common carotid artery and internal jugular vein, is seen in a third of the patients. Nodal (Figure 13) or distant metastases are present in 80% of patients.52 The metastatic nodes are hypoechoic and show cystic necrosis in 50% of cases.53 Doppler ultrasound shows multiple, small, chaotic intratumoral vascularity but necrotic tumours may be avascular or hypovascular due to vascular infiltration/occlusion. Abnormal vascularity is also seen within the metastatic nodes and the tumour thrombus in the infiltrated vessels. 48, 49
Thyroid metastases
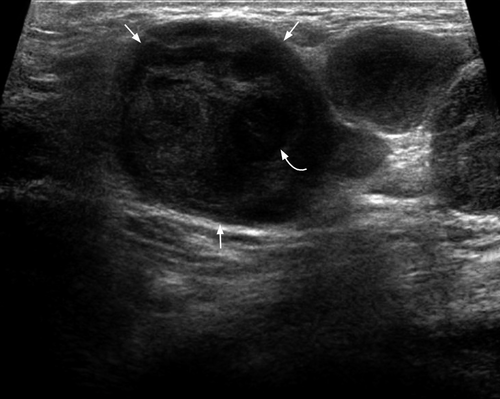
Primary malignancies that most often result in metastatic deposits to the thyroid are those tumours that are prone to haematogenous spread, such as malignant melanoma (39% of cases), renal (10% of cases), breast (21% of cases)54 and lung carcinomas. Pancreatic and gastrointestinal malignancies may also metastasise to the thyroid.54-57 Although the thyroid is one of the most vascular organs, the incidence of thyroid metastases from various clinical and surgical series is only 3%.58 However, at autopsy thyroid metastases may be identified from 2% to 24% of patients with known malignant tumours. 59
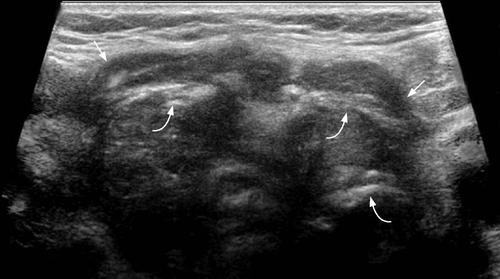
Most thyroid metastases appear as ill-defined, heterogeneous hypoechoic lesions (Figures 14 and 15). Occasionally they may be relatively circumscribed, iso- or hypo-echoic, and show cystic/necrotic components. They are often multiple, unilateral or bilateral. The absence of microcalcification is useful to distinguish from primary papillary carcinoma of thyroid.58, 60 Ultrasound guided fine needle aspiration was shown to provide diagnostic results specific for metastases in up to 95.5% of cases, and is effective in preventing unnecessary surgery. 60
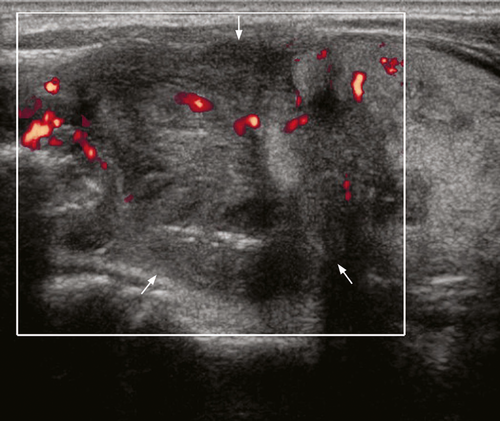
Patients with thyroid metastases often have extensive metastatic disease in the rest of the body, including metastatic neck nodes. As such the presence of thyroid metastases is associated with a poor prognosis.58, 60, 61
Chronic lymphocytic leukaemia/small lymphocytic lymphoma
Diffuse large B-cell lymphoma and mucosa associated lymphoid tissue lymphoma are the two most common histological subtypes of primary thyroid lymphoma. Initial presentation of chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) as thyroid lesion is exceedingly rare.59 Moreover, SLL-B lymphoma of the thyroid associated with CLL-B may not be preceded by autoimmune thyroiditis.62, 63 In general this disease shows poor response to therapy. In particular the presence of 17p deletion is a poor prognostic indicator.63
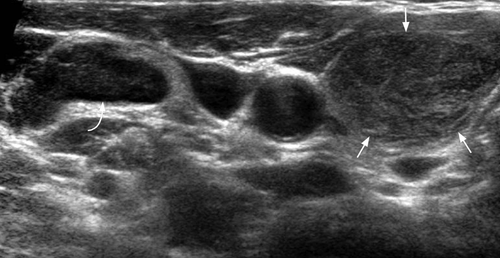
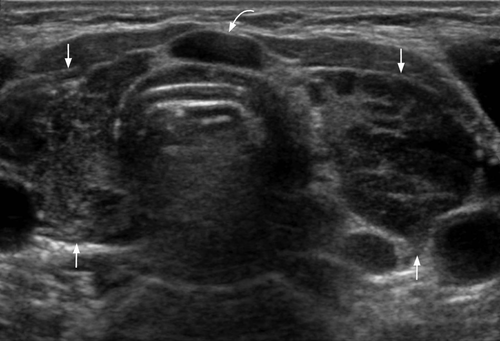
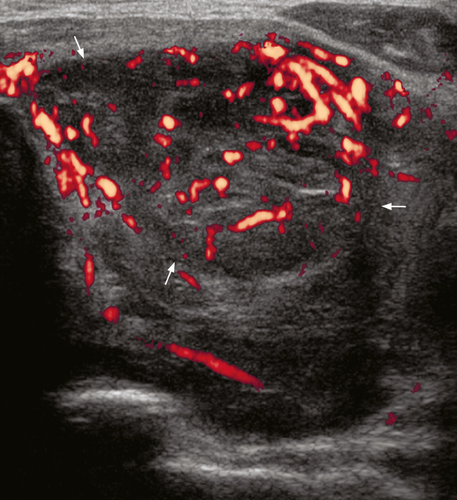
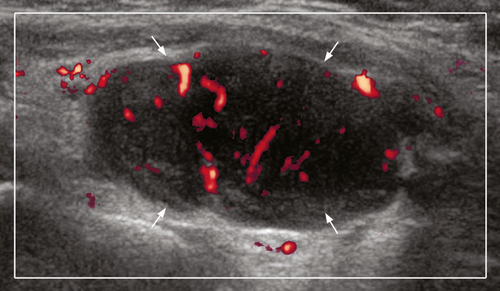
On ultrasound, CLL/SLL of the thyroid appears as heterogeneous, hypoechoic goitre with nodules. There are often hypoechoic, enlarged neck nodes with loss of normal architecture and abnormal increased vasculature on Doppler (Figures 16-19).63 It is reported that a central blood flow pattern is highly suggestive of the diagnosis of primary thyroid lymphoma rather than nodular goitre.64
Langerhans cell histiocytosis
Langerhans cell histiocytosis is a rare disease with an annual incidence of 4–5.4 per million.65 Involvement of the thyroid by LCH is very uncommon, even when there is extensive disease in the body.66, 67
Langerhans cell histiocytosis in the thyroid may manifest as goitre and precede subsequent multisystem involvement.68 Biochemically there is low free T4 and elevated TSH. Occasionally the thyroid function may be normal.69, 70
The pathology typically shows diffuse or focal histiocytic infiltrates, Birbeck granules (invaginations of the cell membrane that are pentalaminar with cross striations and often characteristic vesicular expansions), prominent eosinophilic component and destruction of follicles in a background of lymphocytic thyroiditis. On immunohistochemistry, it stains strongly positive for CD1a- and S-100-, and for lysozyme & KP-1.71 Histologically, it may resemble poorly differentiated carcinoma of thyroid.72
It should be noted that when there is thyroid involvement by LCH, parathyroid involvement should also be considered especially when the serum calcium level is depressed with inappropriate serum parathyroid hormone level.73
Langerhans cell histiocytosis confined to the thyroid frequently shows a relatively indolent clinical course.74 On the other hand, systemic disease with thyroid involvement usually indicates poor prognosis.71
Langerhans cell histiocytosis in the thyroid appears as diffuse or nodular hypoechoic goitre with heterogeneity on ultrasound,75 and may present as heterogeneous thyroid enlargement with anterior prominent projections.76 FNAC may be useful in confirming the diagnosis.
Tuberculosis
Thyroid tuberculosis is very rare even in populations with high prevalence of other forms of tuberculosis.77 It was estimated that 0.1–0.4% of all new cases of tuberculosis involve the thyroid gland.78-80
There were scanty case reports on the ultrasound findings of thyroid tuberculosis. The sonographic appearances of thyroid tuberculosis may range from multifocal, ill-defined, heterogeneous hypoechoic lesions involving both lobes of the thyroid,81 to solitary well-defined, heterogeneous, predominantly anechoic lesion with internal echoes and irregular margins/walls.82 There is a recent case report on the dynamic monitoring of the sonographic features of thyroid tuberculosis diagnosed by ultrasound, confirmed by ultrasound guided core-needle biopsy and followed up serially by ultrasound during the entire course of treatment. This case demonstrated the evolution of the thyroid tuberculosis from a heterogeneous, fluid-filled nodule to become an ill-defined, hyperechoic solid nodule with tiny calcified foci consistent with a calcified tubercular goitre. Doppler study revealed persistent punctate and strip-shaped blood flow around and within the lesion. 83
The differential diagnosis for thyroid tuberculosis may include thyroid malignancies, suppurative infections and haemorrhagic cysts.83 Ultrasound guided biopsy seems to be a valuable tool for obtaining a timely and accurate diagnosis of thyroid tuberculosis,83-85 so as to avoid unnecessary thyroidectomy and initiate appropriate antitubercular treatment.
Plasmacytoma
Solitary extramedullary extramucosal plasmacytomas are exceedingly rare and mostly occur in the fifth to sixth decade with a male preponderance of 3 to 1. More than 90% of these are found in the head and neck region and accounts for 0.4% of all head and neck neoplasms.86-90 Among these patients, 14–25% show serum para protein while <5% have urine Bence-Jones proteins. 90, 91
Solitary extramedullary plasmacytoma is a focal accumulation of monoclonal plasma cells. Approximately 5–20% of solitary extramedullary plasmacytoma and more than 50% of solitary bone plasmacytoma will subsequently progress to multiple myeloma on long-term surveillance.92
Solitary plasmacytoma of the thyroid is a rare tumour that makes up 1.4% of the extramedullary plasmacytomas.93 Less than 75 cases of solitary extramedullary plasmacytoma of the thyroid have been reported up to 2011.94
Ultrasound features of thyroid plasmacytoma have been reported as heterogeneous hyperechoic/hypoechoic nodule, with high elastography index on shear wave elastography, and increased vascularity around and within the nodule on Doppler study.95
IgG4-related disease
Hashimoto's thyroiditis with increased IgG4-positive plasma cells is likely a subtype of thyroiditis closely related to IgG4-related disease, known as IgG4 thyroiditis, distinctive from the non-IgG4 thyroiditis. IgG4 thyroiditis tends to involve the younger age group, with lower female to male ratio, higher levels of thyroid autoantibodies, diffuse low echogenicity and prompt deterioration requiring surgery as well as more subclinical hypothyroidism. 96
It was found that there is a significant correlation of IgG4 thyroiditis subtype with diffuse low echogenicity on ultrasound, while the non-IgG4 thyroiditis is associated with diffuse coarse echogenicity.96 The ultrasound finding was also noted to be related to the degree of thyroid function impairment,28, 29 suggesting that low echogenicity of the thyroid is indicative of hypothyroidism and severe follicular degeneration.
Lipoma
Thyrolipomas are rare well-circumscribed and capsulated mass lesions comprising of fat and thyroid tissue, and are benign and usually biologically inactive.
Fatty lesions may be isoechoic on ultrasound and cannot be distinguished from normal thyroid parenchyma.
The diagnosis of thyrolipoma is usually based on the identification of fat component on CT or MRI as well as FNAC.97




