A case of marked bilateral asymmetry in the sacral alae of the Neandertal specimen Regourdou 1 (Périgord, France)
Funding information: French National Research Agency, Grant/Award Number: ANR-10-LABX-52; Grant Agency of Charles University, Grant/Award Number: 1088217; Irene Levi Sala CARE Archaeological Foundation; Leakey Foundation; Louisiana Board of Regents, Grant/Award Number: LEQSF(2015-18)-RD-A-22; Ministerio de Ciencia, Innovación y Universidades, Grant/Award Number: PGC2018-093925-B-C33; Ministerio de Economía y Competitividad, Grant/Award Number: CGL2015-65387-C3-2-P; Région Nouvelle Aquitaine, Grant/Award Number: 2016-1R40240-00007349-00007350; Universidad del País Vasco/Euskal Herriko Unibertsitatea, Grant/Award Number: 1044-16; Eusko Jaurlaritza-Gobierno Vasco, Grant/Award Number: IT1418-19
Abstract
Objectives
A marked asymmetry was previously reported in the sacral alae and S1-L5 facets orientation of the Neandertal individual Regourdou 1. Here, we provide a detailed description and quantification of the morphology and degree of asymmetry of this sacrum.
Material and methods
Regourdou 1 was compared to a modern human sample composed of 24 females and 17 males, and to other Neandertal individuals. Both traditional and geometric morphometric analyses were used in order to quantify the degree of sacral asymmetry of Regourdou 1.
Results
The asymmetry of both sacral alae and facets orientation substantially exceeds directional and absolute asymmetry of the healthy modern sample. Regourdou 1 shows a considerably shorter right ala, which is absolutely and relatively outside of the modern and Neandertal variations.
Conclusion
Regourdou 1 shows marked sacral asymmetry that probably originated in early ontogenetic development. An asymmetric sacrum reflects asymmetric load dissipation and could relate to other morphological abnormalities observed in the skeleton, especially the mild scoliosis of the spine and the asymmetry of the femoral diaphyses. Further investigation is necessary to elucidate the relationship between those morphologies as well as a potential impact on the life of the individual.
1 INTRODUCTION
The sacrum is important in weight transmission and posture (Pal, 1989) as its orientation within the pelvic girdle influences the lumbar lordosis (Been, Gómez-Olivencia, & Kramer, 2014; Been, Pessah, Peleg, & Kramer, 2013; Peleg et al., 2007). In addition, it has an impact on the shape and dimensions of the birth canal (Tague, 2000, 2007). It is generally assumed that Neandertals had similar sacral morphology to anatomically modern humans despite the presence of specific morphological traits described in several specimens: a very voluminous sacral canal (Meyer, Brůžek, Couture, Madelaine, & Maureille, 2011; Pap, Tillier, Arensburg, & Chech, 1996; Trinkaus, 1983), a less marked promontory with relatively flat vertical curvature (Bartucz & Szabó, 1940; Boule, 1911; Rak, 1991), and a relatively narrow sacral breadth (Bartucz & Szabó, 1940; Boule, 1911; Fraipont, 1927; Fraipont & Lohest, 1887). Unfortunately, there are only a few well-preserved Neandertal sacra (Supplementary Table S1), and no recent study has investigated potential differences between Neandertal sacra and contemporary or extant ones. With regard to the recent attempts at Neandertal body reconstruction (Chapman, 2017; Gómez-Olivencia et al., 2018; Sawyer & Maley, 2005), the sacrum is even more important as it provides information on the spinopelvic alignment (Been et al., 2017).
Based on this knowledge, we provide an analysis of the sacrum of the Regourdou 1 (R1) Neandertal that has a relatively well-preserved pelvis and thorax, but whose sacrum shows a peculiar morphology that demands further exploration before any attempts at reconstruction of the body.
2 THE REGOURDOU 1 NEANDERTAL
The Neandertal skeleton Regourdou 1 was discovered in September 1957 in Dordogne in the southwest of France (Piveteau, 1959) and the site was systematically excavated during 1961–1964. Some of the R1 skeletal pieces were recently found in faunal collections curated at the Musée National de Préhistoire, the Musée d'Art et d'Archéologie du Périgord and the Regourdou site Museum (Madelaine et al., 2008; Maureille, Gómez-Olivencia, Couture-Veschambre, Madelaine, & Holliday, 2015). Those new findings have nearly completed the trunk, lower limbs, feet, and pelvic girdle, and have thus allowed detailed analyses of certain anatomical regions, providing not only new data on Neandertal variation, but also on the paleobiology of this individual (Gómez-Olivencia, Couture-Veschambre, Madelaine, & Maureille, 2013; Gómez-Olivencia, Holliday, Madelaine, Couture-Veschambre, & Maureille, 2019; Meyer et al., 2011; Pablos et al., 2019). Despite not preserving the calvarium (i.e., skull without mandible following Martin, 1928), R1 is now one of the most complete adult Neandertal skeletons (Maureille et al., 2015). While direct dates have not yet been obtained, Layer 4 of the site, in which the skeleton was buried, is attributed to the latter part of MIS 5 (Bonifay, 1964; Pelletier et al., 2017; Turq, Jaubert, Maureille, & Laville, 2008).
Based on mandibular tooth wear and the closed medial clavicular epiphyses, R1 was a young adult (Vandermeersch & Trinkaus, 1995; Volpato et al., 2012) who may not have exceeded 30 years (Volpato et al., 2012). Some scholars have proposed that this skeleton belongs to a male individual based on either its overall morphology (Vallois, 1965), or specific features such as measurements of the axis (Gómez-Olivencia et al., 2007), canine breadth, or the proportion of the body and alae of the sacrum (Volpato et al., 2012). At present, the sex of the individual cannot be assessed from the pelvic remains, and metrics from other bones provides ambiguous information concerning sex assignment of the specimen (Meyer et al., 2011; Plavcan et al., 2014; Vandermeersch & Trinkaus, 1995).
The sacral base of R1 shows apparent asymmetry in the length of the alae, with the left more laterally pronounced than the right (Meyer et al., 2011). Additionally, the orientation of the facets that articulate with the fifth lumbar vertebra is also asymmetrical: the right shows a more dorsal orientation while the left shows a more dorsomedial orientation (Gómez-Olivencia et al., 2013). Asymmetry in size of the facets cannot be assessed due to slight erosion of the processes (Meyer et al., 2011). The sacrum is not the only region of the R1 skeleton showing a marked degree of asymmetry or morphological abnormality. High asymmetry was found in the upper limb bones (Vandermeersch & Trinkaus, 1995) and an unusual pattern of asymmetry was also noted for femora, tali, and thorax (Gómez-Olivencia et al., 2013; Maureille et al., 2015; Pablos et al., 2019). Particular periosteal abnormalities involving cortical bone were found on ribs and femoral diaphyses (Gómez-Olivencia et al., 2019; Maureille et al., 2016).
In this article, we explore the degree of asymmetry in the Regourdou 1 sacrum which is compared to healthy modern human individuals and all available Neandertal specimens using classical and geometric morphometric analysis enhanced with imaging techniques. Implications for the paleobiology of Regourdou 1 and further study of the specimen are proposed following the results.
3 MATERIALS AND METHODS
3.1 Regourdou 1 sacrum
The sacrum of R1 (Figure 1) is incomplete, preserving the upper part of the bone at the level of the first and second sacral vertebrae (S1, S2 respectively). The body of S1 is completely preserved and the sacral base is almost complete, missing only a small portion of the posterior left ala. The right auricular surface is complete, but the lower portion of the inferior arm is missing on the left side. The sacrum does not exhibit any sign of a trauma or healed fracture (Linstrom et al., 2009).

The R1 sacrum was micro-CT scanned (voxel size 0.065 mm) at the AST-RX platform of the Musée de l'Homme (Paris) using the microfocus tube of the micro-CT scanner “v|tome|x L 240” (GE Sensing & Inspection Technologies Phoenix X-ray). A 3D model semi-automatically segmented in Avizo 9.1 Lite (Visualization Sciences Group, SAS) was used in this study. To facilitate the manipulation, the micro-CT scan was reduced before the segmentation in ImageJ (Rueden et al., 2017) using a macrocreated by Renaud Lebrun (www.morphomuseum.com) to a resolution still sufficient for macroscopic analyses.
3.2 Comparative material
For this analysis, we used 3D models segmented from CT scans of Neandertal sacra of La Chapelle-aux-Saints 1 (LCAS; provided by Musée de l'Homme) and Kebara 2 (K2; provided by Tel Aviv University). Further data on later Neandertal specimens (LCAS, K2, Spy 2, Subalyuk 1, Shanidar 1, Shanidar 3) were culled from the literature (Supplementary Table S1).
For comparative purposes, we used 41 abdominal CT scans of 24 females and 17 males from the Hôpital Nord in Marseille (the data were collected with the approval of the ethics committee of the Faculty of Medicine in Marseille). Individuals between 20 and 40 years of age were used because of a high prevalence of sacroiliac degenerative defects in older patients. Patients with apparent vertebral or pelvic disorders (including scoliosis, sacralization, lumbarization, or sacroiliac joint fusion) were excluded from the study. 3D models of sacra were obtained by semi-automatic segmentation in Avizo. Five specimens (one male and four females) could not be digitized completely as the superior articular processes were not segmented properly on either one or both sides due to tight space between adjacent processes. These specimens were not included in the geometric morphometric analysis but were used in the analysis of linear measurements.
3.3 Data acquisition
In order to analyze the Regourdou 1 sacral base morphology, landmarks, linear measurements, and indices were acquired and compared with the ones of an extant modern human sample and Neandertal specimens. To collect data, each 3D model was positioned with the superior surface of the S1 body perpendicular to the z-axis of the virtual coordinate system and thus parallel to the screen (Figure 2) and from this view a set of 21 landmarks (Table 1) was digitized in the software Viewbox 4 (dHAL software, Athens, Greece). Landmark coordinates were subsequently projected onto a plane estimated from eight landmarks (Landmarks 1–5 in Table 1) lying on the border of the first sacral vertebra (Gunz, Mitteroecker, Neubauer, Weber, & Bookstein, 2009) (the plane estimation and projection of landmarks were performed following Gunz et al. (2009)). Ten linear dimensions were computed from projected coordinates (Table 2). For statistical testing of asymmetry, the modern comparative sample was digitized twice.
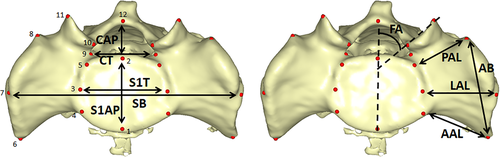
| Number | Abbr. | Name | Bilateral or medial | Definition | Reference |
|---|---|---|---|---|---|
| 1 | pr | Promontory | m | The most anterior point on S1 body on midsagittal plane | Rusk and Ousley (2016) and Schiess, Boeni, Rühli, and Haeusler (2014) |
| 2 | mp | Medial posterior | m | The most posterior point on S1 body on midsagittal plane | Rusk and Ousley (2016) and Schiess et al. (2014) |
| 3 | ls | Lateral S1 | b | Lateral point on the border of S1 body in the half of the distance from pr to mp | Rusk and Ousley (2016) |
| 4 | oa | Oblique anterior | b | The closest point on the S1 outline from the aa | Based on the AAL measurement |
| 5 | op | Oblique posterior | b | The closest point on the S1 outline from the ap | Based on the PAL measurement |
| 6 | aa | Ala anterior | b | The most anterior point on the superior ala | Based on the AAL measurement |
| 7 | al | Ala lateral | b | The most lateral point on the sacral ala in the extension of transverse diameter of S1 | Based on the LAL measurement |
| 8 | ap | Ala posterior | b | The most posterior point on the superior ala | Based on the PAL measurement |
| 9 | pb | Processus base | b | Base of the articular process | Schiess et al. (2014) |
| 10 | pm | Processus medial | b | Medial point on the articular process | Schiess et al. (2014) |
| 11 | pl | Processus lateral | b | Lateral point on the articular process | Schiess et al. (2014) |
| 12 | c | Canal | m | Medial point on the sacral canal wall | Schiess et al. (2014) |
| Number | Abbr. | Name | Bilateral or medial | Landmark boundaries | Reference |
|---|---|---|---|---|---|
| 1 | SB | Sacral breadth | m | al–al | Tague (2007) |
| 2 | S1AP | S1 A-P breadth | m | pr–mp | Tague (2007) |
| 3 | S1T | S1 transversal breadth | m | ls–ls | Tague (2007) |
| 4 | CAP | Canal A-P breadth | m | mp–c | Bräuer (1988) |
| 5 | CT | Canal transverse breadth | m | pb–pb | Bräuer (1988) |
| 6 | AB | Alar breadth | b | aa–ap | Plochocki (2002) |
| 7 | AAL | Anterior ala length | b | aa–oa | This study |
| 8 | LAL | Lateral ala length | b | al–ls | Tague (2007) |
| 9 | PAL | Posterior ala length | b | ap–op | Plochocki (2002) |
| 10 | FA | Facet angle | b | (pl–pm)-(pr–mp) | Noren, Trafimow, Andersson, and Huckman (1991) |
Seven indices were also computed from the measurements for a comparison of modern and fossil specimens (Table 3). Due to differential preservation of fossil remains and the availability of published data, a subset of these landmarks and linear variables were used to analyze the degree of R1 asymmetry and differences between Neandertals and modern humans. In order to include Kebara 2 and LCAS in the analysis, special treatment was necessary due to their taphonomic defects or deformation (Supplementary Figures S1 and S2).
| Number | Abbr. | Name | Unilateral or bilateral | Computation |
|---|---|---|---|---|
| 1 | SBi | Sacral base index | u | S1T/SB |
| 2 | S1i | S1 index | u | S1AP/S1T |
| 3 | Ci | Canal index | u | CAP/CT |
| 4 | LALi | Lateral ala length asymmetry | u | LAL_R/LAL_L |
| 5 | FAi | Facet angle asymmetry | u | FA_R/FA_L |
| 6 | LALSBi | LAL to SB | b | LAL/SB |
| 7 | LALS1Ti | LAL to S1T | b | LAL/S1T |
3.4 Data analysis


Mixed model analysis of variance (ANOVA) was used to statistically test DA in the linear measurements as it also considers the effect of measurement error (Palmer & Strobeck, 1986; Van Dongen, Molenberghs, & Matthysen, 1999). The model was fit with R package lme4 (Bates et al., 2018). The index of asymmetry using the same equation as for %DA was also computed for R1 in order to know the amount of asymmetry compared to the healthy sample.
Traditional metric analysis only provides information about asymmetry in size, but geometric morphometrics also evaluates asymmetry in shape. To test asymmetry in shape, projected landmarks were superimposed using generalized Procrustes analysis (GPA) in order to filter out different positions, rotations, and size (Bookstein, 1991). Presence of DA and FA in shape was tested by Procrustes ANOVA (Klingenberg, 2015) and DA was visualized. As direction of FA in individuals is not of biological interest, the amount of FA can be computed as individual deviations from the mean asymmetry in units of Procrustes distance (Klingenberg & Montero, 2005). The R1 configuration was corrected for the mean asymmetry of the modern comparative sample and a resulting FA score was compared to the amount of FA in the sample.
Differences between modern and Neandertal samples were tested for raw measurements and indices by nonparametric two-tailed Wilcoxon rank-sum test. For those variables that may be pathologically affected in R1, the test was repeated without this specimen.
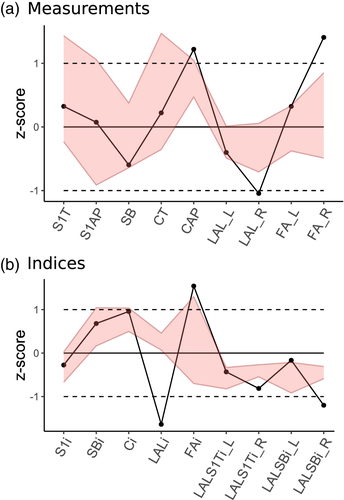
Finally, to analyze the degree of asymmetry in R1, the raw measurements and indices were transformed into adjusted z-scores that were first used in Maureille, Rougier, Houet, and Vandermeersch (2001) (see also Scolan, Santos, Tillier, Maureille, & Quintard, 2012). R1 variables were compared with 95% confidence intervals of the modern sample's variability and minimum and maximum values of Neandertal sample. Shape of the R1 sacrum was analyzed relative to the comparative sample by principal component analysis (PCA) performed on the covariance matrix of Procrustes coordinates. Special attention was given to the asymmetric component of landmark configurations that result after subtraction of symmetrized configuration from each specimen (Klingenberg, Barluenga, & Meyer, 2002).
Virtual imaging has proved to be a very useful tool in assessing abnormal morphological conditions in skeletal remains (Milella, Zollikofer, & Ponce de León, 2015; Zurmühle et al., 2017). Sacral asymmetry was visually inspected using mirror imaging, volume rendering, and cross sections in defined positions (see details in the Supplementary Information). Visualization using volume rendering allows us to explore the trabecular system of the sacrum. For this, only a subregion of the sacrum demarcated by the S1-S2 transition was considered as it was not affected by post-mortem trabecular loss in the caudal portions.
Data processing, analyses, and visualizations were performed in RStudio IDE (RStudio Team, 2016) using a set of R packages geomorph (Adams & Otárola-Castillo, 2013), Morpho (Schlager, 2016), and tidyverse (Wickham, 2017). Visualizations of shape changes were created in MorphoJ (Klingenberg, 2011) and processed in Inkscape (www.inkscape.org).
3.5 Error of measurement
The intraobserver error was computed for projected landmarks and linear dimensions. To evaluate the error related to projected landmarks acquisition, they were superimposed using GPA without scaling the landmarks configurations (von Cramon-Taubadel, Frazier, & Lahr, 2007). The measurement error of individual landmarks was then represented by the median of Euclidian distances between pairs of homologous landmarks. It ranged between 0.27 mm for landmark pbL (the base of the left articular process) and 0.83 mm for landmark alR (lateral margin of the right sacral ala), with an overall error of 0.5 mm (Supplementary Table S2). The maximum deviation was 3.44 mm in landmark pmL (medial point on the articular process), but no other specimen exceeded 2.5 mm for other measurements (Supplementary Table S2).
Absolute and relative measurement errors were computed to evaluate the intraobserver error of linear measurements and angles (Supplementary Table S3). Concerning linear measurements, relative error ranged between 0.22% for sacral breadth and 2.33% for canal anteroposterior diameter. Facet angles had slightly higher intraobserver error (around 2.6%).
4 RESULTS
4.1 Asymmetry in the modern sample
DA was tested on four linear measurements and one angle in the reference sample by mixed model ANOVA considering the effect of measurement error. Table 4 summarizes the results providing also the amount of %AA. This analysis showed that the mean of our healthy modern human sample sometimes does not conform to perfect bilateral symmetry. This was demonstrated in posterior ala length and facet angle (Table 4) which show significant %DA. Posterior ala length is on average 1 mm greater (%DA is 3.7) on the right side which means that the right ala tends to be posteriorly longer than its left counterpart. On the other hand facet angle is 4.2° larger (%DA = 9.5) on the left side, meaning a more coronal orientation of the left articular facet in our comparative sample.
| Variable | Mean (right)a | Mean (left)a | Side difference | p-Value | Mean %DA | Mean %AA | R1%DA scorea |
|---|---|---|---|---|---|---|---|
| AAL | 33.6 ± 4.9 | 33.9 ± 5.3 | −0.38 ± 2.5 | .372 | −1 ± 7.1 | 5.7 ± 4.3 | −14.7 |
| LAL | 33.2 ± 4 | 32.9 ± 4.5 | 0.33 ± 1.6 | .076 | 1.1 ± 5.0 | 4.1 ± 3.0 | −16.8 |
| PAL | 31.7 ± 3.6 | 30.6 ± 3.8 | 1.11 ± 1.9 | <.001 | 3.7 ± 6.4 | 6.4 ± 3.5 | – |
| AB | 45.7 ± 4.9 | 45.4 ± 4.5 | 0.29 ± 2.5 | .524 | 0.5 ± 5.5 | 4.5 ± 3.3 | – |
| FA | 48.5 ± 11.1 | 52.1 ± 9.3 | −4.24 ± 8.2 | .001 | −9.5 ± 16.3 | 14.5 ± 11.9 | 31.8 |
- Note: Significant p-values are in bold.
- Abbreviations: %DA, percentage directional asymmetry. %AA, percentage absolute asymmetry.
- a Underlined values are beyond 2 SD from the modern human sample mean.
Landmark analysis of the whole structure showed significant DA and FA. Table 5 presents results of Procrustes ANOVA relative to measurement error as a confounding effect. Magnitude of measurement error is much lower than the magnitude of FA as indicated by mean square and F ratio (Klingenberg, 2015) that evaluates that the magnitude of FA is approximately 12 times larger than the measurement error. Mean differences between the original and mirrored configurations are illustrated in Figure 3. Concordantly with the analysis of measurements, asymmetry of the sacrum is more pronounced in the dorsal region. Sacral alae are more symmetric anteriorly while the posterior portion tends to be more developed on the right. Likewise, articular facets are more coronally oriented on the left.
| Effect | Sum of squares | Mean squarea | Degrees of freedom | F |
|---|---|---|---|---|
| Individual | 0.4260 | 0.420 | 1,015 | 10.39* |
| Side | 0.0037 | 0.135 | 27 | 3.34* |
| Ind × side | 0.0382 | 0.040 | 945 | 12.04* |
| Error | 0.0068 | 0.003 | 2,016 |
- Note: Individual effect represents the variation between individuals in the symmetric component of shape. Side represents directional asymmetry. Ind × side quantifies fluctuating asymmetry. Error is the residual variation due to measurement error.
- Abbreviation: ANOVA, analysis of variance.
- a Mean square was multiplied by 1,000.*p < .0001.

4.2 Analysis of Neandertal sacra
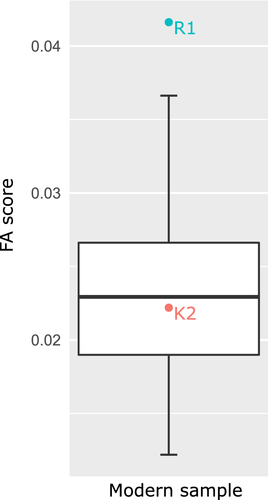
With regard to the small number of available Neandertal specimens, the Neandertal range of variation was indicated by minimum, maximum, and mean values for each of the linear variables (Figure 4; individual values for Neandertal specimens in Table 6). Most of the Neandertal variation intervals for sacral measurements overlap with modern human variation, but there is a tendency toward wider S1 and greater canal diameters. All Neandertal sacra also have rather short alae. The indices confirm the tendency toward wider S1 relative to sacral breadth and voluminous sacral canal opening. The alae are not markedly asymmetrical and they are relatively short compared to modern sample, which may be due to the assumed male sex of majority of the Neandertal individuals. The FAi also deviates from the mean of healthy modern individuals which is biased by Kebara 2.
| Variable | N | Mean | SD | 95% inferior | 95% superior | R1a | K2a | LCASa | Su1b | Sh1b | Sh3b | Spy 2b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurements | ||||||||||||
| SB | 41 | 112.8 | 7.5 | 97.5 | 128 | 103.7 (107.9)c | 118.5 | 103.3 | 107.8 | 104.0 | 117.0 | 103.0 |
| S1AP | 41 | 29.4 | 2.7 | 24.1 | 34.8 | 29.8 | 30.4 | 24.5 | 32.8 | – | 35.1 | – |
| S1T | 41 | 46.6 | 4.6 | 37.3 | 56.0 | 49.6 | 53.0 | 44.4 | 49.3 | 47.0 | 60.0 | 48.0 |
| CAP | 39 | 17.0 | 2.5 | 11.9 | 22.1 | 23.2 | – | 22.3 | 19.0 | – | – | – |
| CT | 41 | 31.9 | 2.4 | 27.0 | 36.7 | 32.9 | 30.1 | 31.1 | 31.2 | 38.0 | 39.0 | – |
| LAL_R | 41 | 33.2 | 4.0 | 25.1 | 41.4 | 24.8 | 33.7 | 28.8 | 30.25 | – | – | 27.5 |
| LAL_L | 41 | 32.9 | 4.5 | 23.9 | 42.0 | 29.3 | 33.0 | – | 28.55 | 28.5 | 28.5 | – |
| AB_R | 41 | 45.7 | 4.9 | 35.7 | 55.7 | 38.2 | 41.2 | 34.9 | – | – | – | – |
| AB_L | 41 | 45.4 | 4.5 | 36.3 | 54.5 | – | 36.3 d | – | – | – | – | – |
| FA_R | 38 | 48.5 | 11.1 | 25.9 | 71.0 | 80.2 | 58.8 | 67.7 | 37.4 | – | – | – |
| FA_L | 38 | 52.1 | 9.3 | 33.3 | 70.9 | 58.2 | 45.0 | 58.4 | 52.5 | – | – | – |
| Indices | ||||||||||||
| SBi | 41 | 0.41 | 0.05 | 0.32 | 0.51 | 0.48 | 0.45 | 0.43 | 0.46 | 0.45 | 0.51 | 0.47 |
| S1i | 41 | 0.63 | 0.06 | 0.51 | 0.76 | 0.60 | 0.57 | 0.55 | 0.67 | – | 0.59 | – |
| Ci | 39 | 0.54 | 0.09 | 0.36 | 0.71 | 0.71 | – | 0.72 | 0.61 | – | – | – |
| LALi | 41 | 1.01 | 0.05 | 0.91 | 1.12 | 0.85 | 1.02 | – | 1.06 | – | – | – |
| FAi | 37 | 0.92 | 0.15 | 0.62 | 1.22 | 1.38 | 1.31 | 1.16 | 0.71 | – | – | – |
| LALSBi_R | 41 | 0.29 | 0.02 | 0.25 | 0.34 | 0.24 | 0.29 | – | 0.28 | – | – | 0.27 |
| LALSBi_L | 41 | 0.29 | 0.03 | 0.24 | 0.34 | 0.28 | 0.28 | – | 0.27 | 0.27 | 0.24 | – |
| LALS1Ti_R | 41 | 0.72 | 0.14 | 0.45 | 1.00 | 0.50 | 0.64 | 0.65 | 0.61 | – | – | 0.57 |
| LALS1Ti_L | 41 | 0.72 | 0.15 | 0.42 | 1.01 | 0.59 | 0.62 | – | 0.58 | 0.61 | 0.48 | – |
- Note: Variable definitions are in Table 2. Underlined values are at the limit or outside the 95% confidence interval of the modern human sample.
- Abbreviations: K2, Kebara 2; LCAS, La Chapelle-aux-Saints 1; R1, Regourdou 1; Su1, Subalyuk 1; Sh1 and Sh3, Shanidar 1 and 3.
- a Measured on the 3D model.
- b From the literature (Bartucz & Szabó, 1940; Chapman, 2017; Meyer, 2013; Pap et al., 1996; Trinkaus, 1983; Trinkaus, 2011). Values for Su1 calculated from z-scores for LAL (Meyer, 2013) and for FA they were measured on a photograph from superior view (Bartucz & Szabó, 1940) which does not show any marked distortion and moreover an angle does not rely on scale. LAL values for Sh1, Sh3, and Spy 2 calculated from SB and S1T.
- c The value in parentheses is an estimation based on a reflection of the left side.
- d This value is not reliable even after the slight correction of the taphonomic distortion which has most affected anteroposterior dimensions of the left ala.
Statistical testing confirms observed differences between Neandertal and modern human sacra (Table 7). Significant differences are observed in variables related to absolute and relative alae dimensions and sacral canal opening even after excluding R1 in cases where R1 could bias the comparison.
| Variable | N (Neandertals) | p (with R1) | p (without R1)a |
|---|---|---|---|
| Measurements | |||
| SB | 7 | NS | NS |
| S1AP | 5 | NS | – |
| S1T | 7 | NS | – |
| CAP | 3 | * | – |
| CT | 6 | NS | – |
| LAL_R | 5 | * | NS |
| LAL_L | 5 | NS | NS |
| AB_R | 3 | ** | * |
| FA_R | 4 | NS | NS |
| FA_L | 4 | NS | NS |
| Indices | |||
| SBi | 7 | ** | * |
| S1i | 5 | NS | – |
| Ci | 3 | ** | – |
| LALi | 3 | NS | NS |
| FAi | 4 | NS | NS |
| LALSBi_R | 4 | * | NS |
| LALSBi_L | 5 | * | * |
| LALS1Ti_R | 5 | * | NS |
| LALS1Ti_L | 5 | * | * |
- Abbreviations: NS, nonsignificant (p > .05); R1, Regourdou 1.
- a Variables that could be affected by pathology in R1 were additionally tested without R1.*p < .05. **p < .01.
This comparison suggests the presence of certain directional differences between Neandertal sacra studied here and the modern sample: all specimens have a larger S1 body relative to sacral breadth, relatively more anteroposteriorly spacious sacral canal and relatively smaller alae, that is, mediolaterally short and anteroposteriorly narrow.
4.3 Asymmetry of the Regourdou 1 sacrum
The Regourdou 1 sacrum was metrically compared to modern humans (Tables 4 and 6). Due to preservation problems, we were able to compare R1 to the modern sample in only three of the five calculated asymmetry indices given in Table 4. Two of these indices yielded results beyond two SD from the modern human sample: lateral ala length and facet angle, while the third one (anterior ala length) is 1.93 SD from the mean (Table 4).
Table 6 compares R1 and other Neandertals to our modern human comparative sample. The R1 sacrum fell outside the 95% confidence interval of the sample for three linear measurements (anteroposterior canal diameter, right lateral ala length, and right facet angle) and four indices (LALi, FAi, right LALSBi), and one index (Ci) is at the limit of the variability (Table 6, Figure 4). The majority of those variables relate to the asymmetry of the alae length or the articular facets orientation. The other measurement is the anteroposterior diameter of the canal which is greater than in the modern sample, and which could also be related to the significantly larger anteroposterior dimension of the canal present in the fifth lumbar vertebra (L5) (Gómez-Olivencia, Arlegi, Barash, Stock, & Been, 2017). Right ala length is significantly smaller than our modern human sample. In contrast, the right facet angle falls far above the modern sample's upper limit. Finally, while not significant, the total sacral breadth slightly deviates toward the lower margin (Figure 4a).
Regarding variables expressing asymmetry (LALi, FAi), the degree of asymmetry in both ala length and facet orientation is far beyond the range observed in healthy modern human sacra (Table 6, Figure 4b). The right ala is very short relative to SB and S1T diameters.
Despite the differences observed between modern and Neandertal sacra affecting the alae and facets, R1 has an even shorter right ala and more asymmetric alae length and facet orientation. Values for R1 are also outside the Neandertal range of variation.
Projected coordinates of landmarks (except landmarks apR, apL, and c due to their absence in fossil specimens) were superimposed using GPA. The PCA was subsequently performed on the Procrustes coordinates and on their asymmetric component to analyze the overall shape of Neandertal sacra relative to modern human variability, and the asymmetry of R1. In the PCA without symmetry control, R1 was represented by three coordinate configurations: the original asymmetric version and two symmetrized versions based on the right or left side. Neandertal specimens K2 and symmetrized LCAS fall within modern human variation for the first four PCs which comprise 79% of total variation (Figure 5a,b) while R1 is within the variation for the first two PCs only. The first two PCs mainly express shape differences in the anterior alar region and relative dimensions of alae and S1 body while PC3 and PC4 reflect orientation of articular facets. Both K2 and LCAS tend to have more negative values along PC1 which means they have less prominent anterior portion of the alae. LCAS also has lower value for PC3 which reflects higher facet angle.
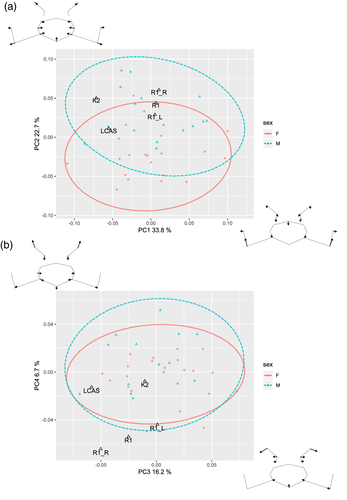
Different configurations change the position of R1 particularly along PC2, reflecting sexual differences in the modern human sample. As one would expect, the symmetrized configuration based on the longer left ala shifts R1 more into the female variability and vice versa. On the other hand, different versions of R1 move along both the PC3 and PC4 axes, reflecting a greater difference between right and left facet orientation. The R1 original configuration falls outside the modern sample's variation, as does the symmetrized version based on the right side. On the contrary, the left-side mirrored configuration falls at the edge of the sample variation (Figure 4), suggesting this side is closer to the normal morphology.
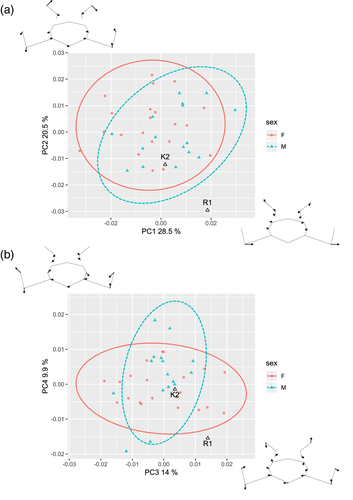
Performing PCA on the asymmetric component of landmark configurations (Figure 6a,b), R1 falls on the edge of or outside the healthy modern sample for first four PCs that account for 73% of total variation. In contrast, K2 is again well inside the 95% confidence ellipses of both males and females. The first two PCs reflect the asymmetry in anterior alae and facet orientation, respectively, while PC3 and PC4 reflect mixed effects of alar anteroposterior development and facet orientation.
Comparing the amount of FA in the modern sample with the FA score for R1 and K2, R1 greatly exceeds the sample variation, whereas K2 is close to the mean value (Figure 7).
External morphology of the R1 sacrum has already been described by Meyer et al. (2011). Using virtual imaging methods allowed us to better visualize the object asymmetry. A 3D model of the R1 sacrum was mirror imaged and superimposed with the original model using landmarks lying on the S1 body (Table 1, Figure 8). The asymmetry in the length of the sacral alae is clearly visible, but the visualization also shows different development of the alae in the superoinferior direction. When the sacral base is positioned horizontally, the shorter right ala rises above the mirrored left ala and thus reaches relatively more cranially, suggesting an asymmetrical alignment of coxal bones in the coronal plane.
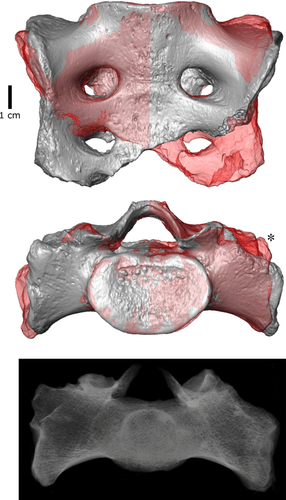
Trabecular bone was inspected in the micro-CT scan of R1 sacrum using volume rendering (Figure 8) and cross sections (Supplementary Figure S3). Volume rendering allowed us to see the density of trabecular bone and showed that the trabecular network is denser on the left side than on the right side. The same pattern was observed in the sagittal and transversal slices of the sacrum (Supplementary Figure S3).
High-resolution imaging allows better observation of the external morphology of the auricular surfaces without the confounding effect of surface coloring (Figure 1). Both auricular surfaces show relatively smooth transition between cranial and caudal arms without great constriction (Nishi et al., 2017). The preserved upper portion of the left auricular surface is very rugose and exhibits conspicuously deep pits. On the other hand, the right auricular surface displays only slight rugosity, but a deep groove caudally. This relief does not correspond to the morphological changes related to age described on the iliac auricular surface (Lovejoy, Meindl, Pryzbeck, & Mensforth, 1985), although the sacral auricular surface does not follow the same changes (Passalacqua, 2009) probably due to differentially thick cartilage (Schunke, 1938). However, greater number of surface irregularities was linked to greater stability of the joint (Vleeming, Volkers, Snijders, & Stoeckart, 1990).
5 DISCUSSION
In our study, we performed a metric and morphological analysis of the Neandertal sacrum which confirms the presence of some significant differences compared to extant modern humans. We also visualized and quantified the asymmetry of Regourdou 1 sacral alae and articular facets. In comparison with healthy individuals, R1 shows a substantial asymmetry in both mentioned dimensions exceeding the normal variation of the comparative sample. Furthermore, based on our univariate and multivariate comparisons with healthy individuals, the length of the ala is absolutely and relatively very short on the right side. In the discussion, we are going to analyze these results and propose potential interpretations concerning the origin and consequences of this morphology.
5.1 Biomechanics and sacral asymmetry
Skeletal asymmetry has been studied mostly on limb bones in association with behavioral lateralization. Habitually repeated biomechanical stress is usually associated with bone growth and therefore structural enlargement of the side experiencing greater loading (Carlson & Judex, 2007; Ruff, Holt, & Trinkaus, 2006; Shaw, Hofmann, Petraglia, Stock, & Gottschall, 2012). Bone functional adaptation is relevant especially for the long bones of upper and lower limbs. However, the interpretation of limb girdle and axial bones asymmetry is more complicated due to different biomechanical functions and development (Auerbach & Raxter, 2008). For example, an opposite pattern to that expected from the biomechanical law has been noted in clavicles, which exhibit enlarged diaphyseal diameters in the context of shorter total length in the dominant upper limb, indicating that different factors influence the clavicle vs. other bones of the arm (Auerbach & Raxter, 2008). Asymmetry in the sacrum has not been extensively studied, but several studies have shown left-side dominance of the sacral dimensions and put the result in relation to predominant right-handedness and crossed asymmetry of lower limbs (Akman et al., 2008; Plochocki, 2002; Tobolsky, Kurki, & Stock, 2016).
The sacrum provides gravity-driven transmission of weight from the upper body to the lower limbs (Pal, 1989) and transmits shock waves leading from the lower limbs during gait (Mizrahi, Verbitsky, & Isakov, 2000). In order to resist shear forces in upright posture, the sacrum is wedged in between the coxal bones, and additional resistance is provided by the gluteus maximus muscle and iliosacral ligaments (Barker et al., 2014; Steinke et al., 2010) that generate perpendicular compressive force closure of the sacroiliac joint to overcome gravity (Vleeming et al., 2012). Therefore, the sacrum must resist two different simultaneous pressures: vertical shear and tensile forces of the muscles and ligaments. It has been shown that shear stress in the growth plate promotes degradation of the cartilage, accelerates ossification, and thus causes cessation of growth (Carter, 1987). The sacrum undergoes a high degree of shear stress; therefore, the left-side asymmetry could be caused by earlier cessation of alar growth on the more loaded right side (Plochocki, 2002).
In our analysis, R1 shows left-sided asymmetry in sacral alae which is in concordance with the predominant %DA published elsewhere. However, we did not confirm this type of %DA in our sample. In contrast to other studies analyzing alar asymmetry, we evaluated %DA in relation to intraobserver error and we obtained nonsignificant results except for the posterior ala length which showed right-side asymmetry. Even if %DA in the sacrum exists, the degree of asymmetry is very low in modern humans (our results and Akman et al., 2008; Plochocki, 2002; Tobolsky et al., 2016). It probably originates in the second decade of life during the adolescent growth spurt when the sacroiliac apophyses emerge and fuse (Bollow et al., 1997; Nissinen et al., 2000; Plochocki, 2002). Therefore, the sacrum does not have a long period in which it may be sensitive to lateralized habitual behavior or loading. However, Neandertals and Late Pleistocene humans exhibited stronger asymmetry in the upper limbs than do the majority of extant humans (Sparacello, Villotte, Shackelford, & Trinkaus, 2017; Trinkaus, Churchill, & Ruff, 1994, but see Kubicka, Nowaczewska, Balzeau, & Piontek, 2018). There is no evidence of similarly strong compensatory asymmetry in the lower body (Ruff, Trinkaus, Walker, & Larsen, 1993; Trinkaus et al., 1994), which given the small number of Neandertal sacra preserved, prevents any assumptions about sacral asymmetry in Neandertals.
On the other hand, the sacrum has a high FA compared to other bones (Storm, 2009; Tobolsky et al., 2016), which implies a greater susceptibility to developmental stress. This is concordant with the fact that the sacrum and lumbosacral junction are regions of frequent developmental abnormalities (Dzupa et al., 2014; Masnicová & Beňuš, 2003). R1 substantially exceeds the range of %DA and even %AA (Table 4). Regarding the short period and rather small amount of growth from apophyseal sacroiliac plates during the second decade of life, a possible origin of sacral asymmetry in the earlier developmental period will now be explored.
5.2 Development and sacral asymmetry
The mesenchymal template (i.e., paraxial mesoderm) from which the vertebral column originates is formed in the third week of intrauterine development and consists of paired somites that develop simultaneously but independently of each other (Scheuer & Black, 2000). After union of these paired structures, they become precursors of individual vertebrae. Lateral parts of the sacrum are considered developmentally homologous structures to ribs and costal processes of other vertebrae (Scheuer & Black, 2000; Tague, 2007, but see O'Rahilly, Müller, & Meyer, 1990) which are formed from a portion of somites (Barnes, 2012a). Subsequently, a chondrification starts in each blastemal vertebra from two paired centers (Scheuer & Black, 2000). Final ossification starts at the eighth to ninth weeks generally from one center in the vertebral centrum (Barnes, 1994; Scheuer & Black, 2000). During ossification, a sacral ala develops from paired anterior and posterior ossification centers at the level of each of the first three sacral vertebrae. The lateral parts fuse with sacral bodies between 2 and 6 years of age (Scheuer & Black, 2000).
Given the degree of sacral asymmetry in R1 and no evidence of a direct injury on the sacrum, this morphology most likely originated earlier in development (for similar cases see Pfeiffer, 2011; Pitre & Lovell, 2010). Regarding the fact that the period of origin of such a pronounced sacral asymmetry extends from the intrauterine to early postnatal period or even early childhood, two possible explanations can be proposed: a developmental abnormality or an indirect post-traumatic consequence. Unilateral hypoplastic vertebral defects are commonly associated with failure of paraxial mesoderm formation or chondrification (Barnes, 1994, 2012b; Scheuer & Black, 2000); that is, they originate after an insult during the period from the third to eighth weeks of intrauterine life. However, as the asymmetrical appearance is restricted to the sacral alae and is not pronounced in Regourdou 1 S1 body, the morphology could also have originated a few years after birth, which does not rule out a trauma to the lower limbs as a possible cause leading to asymmetric weight transmission. To assess these possibilities, it is helpful to review other abnormalities in the skeleton.
5.3 Other skeletal abnormalities in the R1 skeleton
A deviation from bilateral symmetry is, apart from the sacrum, also apparent on upper limb bones, vertebrae, sternum, femora, and tali of the R1 individual. Other abnormalities of different types are present on the ribs and femoral shafts.
The asymmetry of upper limb bones of R1 was first studied by Vandermeersch and Trinkaus (1995). They found marked asymmetry in diaphyseal dimensions of the clavicles, humeri, radii, and ulnae with the right side consistently more robust than the left one concluding it was a physiological asymmetry caused by right-hand dominance. The degree of asymmetry was substantial in comparison with modern humans, but it was closer to the lower margin in the Neandertal range (Vandermeersch & Trinkaus, 1995). More evidence of right-handedness in R1 comes from the direction of scratches on the front teeth which is direct evidence of right-hand dominance (Volpato et al., 2012).
Upper limb asymmetry is often attributed to high levels of habitual behavioral lateralization; however, there are also cases of pathologically induced alterations in the diaphyseal properties of upper limbs that can lead to a similar asymmetric appearance (Churchill & Formicola, 1997; Tesorieri, 2016; Trinkaus et al., 1994). Such asymmetry is caused by muscular or neural dysfunction that leads to a secondary effect of a global or localized bone atrophy, and the cause of the primary dysfunction may not be apparent from the skeleton (Churchill & Formicola, 1997; Trinkaus et al., 1994). Resulting limb asymmetry is not always extreme and could therefore be assessed as a sign of handedness (Churchill & Formicola, 1997). However, if the insult occurred during growth, it would also lead to shortening of the impacted limb bones. In addition, the impaired limb would also show periosteal atrophy in the areas of muscular attachments (Ortner, 2003).
Regourdou 1 does not preserve any pair of complete upper limb long bones allowing measurement of their total length, but the bones seem morphologically congruent and do not show any marked discrepancy in length (Vandermeersch & Trinkaus, 1995). In addition, cross-sectional characteristics do not exhibit an abnormal mechanical pattern (Volpato, Couture, Macchiarelli, & Vandermeersch, 2011), and areas of muscular attachment are of similar extent in both upper limbs, indicating that each was capable of normal physical activity with regard to contemporary fossil humans (Vandermeersch & Trinkaus, 1995). Considering these facts, the asymmetry of R1 upper limbs can be considered as physiological.
Three vertebrae exhibit mild scoliotic signs: the spinous process of the seventh cervical vertebra is mildly rotated, and the larger right articular facets of the second thoracic vertebra and spinous process of the ninth thoracic vertebra are twisted to the left (Gómez-Olivencia et al., 2013). Several vertebrae also exhibit mild osteophytes on their articular facets and bodies, especially in the cervical region (Gómez-Olivencia et al., 2013). The sternum is slightly longer on its right side (Gómez-Olivencia, Franciscus, Couture-Veschambre, Maureille, & Arsuaga, 2012).
The first right rib exhibits a prominent bump on its cranial surface close to its dorsal end. In contrast, the seventh right rib shows an area of porous bone propagation inside the compact bone external surface close to the sternal end (Gómez-Olivencia et al., 2018).
The femoral shafts were described as highly asymmetrical in cross-sectional shape, longitudinal curvature, and expression of the linea aspera: the left diaphysis is more circular, less curved and the linea aspera is more marked (Maureille et al., 2015). Moreover, on the two femoral diaphyses, we observe abnormal relief (in three areas on the right and two on the left) pushing out the surface of the bone. These two pieces look similar and their unusual morphology is probably related to the same origin. Based on their external appearance, it has been considered that these features could represent calcified subperiosteal hematomas (Madelaine et al., 2008; Maureille et al., 2015). While this hypothesis is unlikely, we must nonetheless question if these areas of femoral bone external relief have the same cause(s) as those observed on the two ribs. Bone remodeling is common in metabolic and infectious diseases and uncommon in developmental abnormalities (Aufderheide & Rodríguez-Martín, 1998; Pinhasi & Mays, 2008), which may imply presence of two different defects. Despite its rare occurrence, it can be the case that an individual had more than one disease affecting the skeleton (Ortner, 2008).
The right talocalcaneal articulation has also been described as asymmetric. In particular, it appears that the tali may have differed in the orientation of their heads (Maureille et al., 2015; Pablos et al., 2019; Vandermeersch & Trinkaus, 1995).
5.4 Possible associations of the skeletal abnormalities and consequences
Sacral asymmetry certainly relates to asymmetrical load dissipation and therefore the mild scoliosis observed in the presacral vertebrae of R1 could be associated with the sacral asymmetry. In addition, the articular facets of S1 show an asymmetric orientation known as facet tropism that occurs relatively often in modern humans (Kalichman, Suri, Guermazi, Li, & Hunter, 2009)—this is in fact true for our comparative sample. Facet joint orientation is critical in maintaining overall stability of the spine (Noren et al., 1991); superior articular facets face dorsomedially in the lumbosacral region, restricting the range of rotation and thus providing stability with respect to shear forces. Normal variation of the facet angle is approximately between 30 and 70° (Mahato, 2011; Noren et al., 1991), and facet tropism occurs when one of the facets is oriented more mediolaterally. It is not clear how facet tropism originates (Samartzis et al., 2016), but it is a probable risk factor for degenerative diseases such as spondylolisthesis and disc herniation (Dai, 2001; Kim et al., 2013; Lai et al., in press; Noren et al., 1991; Schleich et al., 2016). Coronal orientation of one facet causes increased torsional stress because of abnormal rotation of the lumbar spine (Noren et al., 1991). This leads to greater interfacet shear forces in the more sagitally oriented facet (Giles, 1987) leading to degenerative osteoarthritic changes occurring predominantly on this facet (Giles, 1987; Kalichman et al., 2009). Moreover, trabecular bone is organized in a network that is adaptively remodeling in response to changes in mechanical loading (Chappard, Baslé, Legrand, & Audran, 2008). Therefore, the preserved trabecular structure may contain direct information about the forces that act on the bone. There are two main trabecular bundles in the sacral base that transfer weight from the S1 body and articular facets to the auricular surfaces (Pal, 1989). Asymmetric pattern of trabecular distribution in R1 sacral base indicates that the longer left sacral ala was loaded more that the right.
It was observed that even mild sacral lateral hypoplasia is associated with pelvic obliquity and thus load transfer imbalance (Ortner, 2003; Pfeiffer, 2011). Pelvic obliquity is defined following the Terminology Committee of the Scoliosis Research Society as a “deviation of the pelvis from the horizontal in the frontal plane” (A Glossary of Scoliosis Terms, 1976, pp. 57–58) which goes hand in hand with leg length discrepancy, and importantly, one can be either a cause or a consequence of the other (Gurney, 2002; Winter & Pinto, 1986). Given that the longitudinal growth of long bones is constrained, leg length discrepancy originates during development and may persist into adulthood when the pelvic obliquity is fixed by muscle contractures either above or below the pelvis (Hefti, 2015; Winter & Pinto, 1986) which should be apparent in femoral cross-sectional geometry. The pelvis of R1 after reassembly also shows certain asymmetry (Meyer, 2013; Trinkaus, 2018), which has not been studied in detail due to the fragmentary preservation of coxal bones.
The skeletal abnormalities of R1 do not exclude one of the proposed mechanisms of origin (developmental or traumatic), but we were able to provide reasonable associations between several of the abnormal asymmetries of lower limbs and spine. Unilateral developmental vertebral anomalies have varying degrees of expression from mild hypoplasia to total aplasia (Barnes, 2012b). Lateral vertebral hypoplasia generally affects thoracic or lumbar vertebrae, but it also appears in one or more sacral segments forming an asymmetrically shaped sacrum (Barnes, 2012b). However, regarding the biomechanics in the sacroiliac joint described in Section 4.1, a trauma causing leg length discrepancy could have led to the same effect and thus a traumatic origin in early childhood cannot be excluded, especially due to the state of preservation of the lower limb long bones of R1. Both developmental and traumatic pathologies are well documented in Late Pleistocene individuals, and are in fact quite abundant (Berger & Trinkaus, 1995; Trinkaus, 2011; Trinkaus, 2012, 2018). However, following Berger and Trinkaus (1995), lower limb pathologies are relatively infrequent in the Neandertal fossil record. These authors hypothesize that this bias could result from the necessity for mobility and the fact that most of the Neandertal fossil record comes from caves and rock shelters (Bar-Yosef, 2013): those not able to keep up (in this case due to leg injuries) would be left behind, and thus chances to be incorporated into the fossil record would decrease. Therefore, rather mild defects of lower limbs are found in the Paleolithic record (Trinkaus, 2012) which raises the question about the necessity of provision of care in the Late Pleistocene societies and in this specimen in particular (Spikins et al., in press; Tilley, 2015).
6 CONCLUSION
We have demonstrated a high degree of sacral asymmetry in the Regourdou 1 Neandertal individual. The right side of its sacrum shows an abnormal morphology that resulted in an asymmetric conformation of the sacral base. Based on our univariate and multivariate comparisons with modern healthy individuals and preserved Neandertal sacra, the asymmetry is caused by a relatively short ala and a high facet angle on the right side of the sacrum. A high degree of alar asymmetry probably originated in early development. An asymmetric sacrum reflects asymmetric load dissipation, indicated by trabecular bone density, and could relate to other morphological abnormalities observed in the skeleton, especially the mild scoliosis of the spine and the asymmetry of the femoral diaphyses. Additional information on other asymmetries on this skeleton will allow us to test our proposed hypotheses.
ACKNOWLEDGMENTS
This research was supported by the Grant Agency of Charles University (no. 10882) and by Irene Levi Sala CARE Archaeological Foundation. Further support comes from the project “NATCH” convention 2016-1R40240-00007349-00007350 of the Région Nouvelle Aquitaine (B.M., C.C.V.), and the ANR (French National Research Agency) projects: LabEx Sciences archéologiques de Bordeaux, no ANR-10-LABX-52, subproject “NéMo” (B.M., C.C.V.). A.G.O. received support by the Research Group IT1418-19 (Eusko Jaurlaritza-Gobierno Vasco), and by the Spanish Ministerio de Ciencia, Innovación y Universidades (project PGC2018-093925-B-C33). T.H. received support from the Louisiana Board of Regents LEQSF(2015-18)-RD-A-22. The micro-CT of the sacrum was funded by a Leakey Foundation grant thanks to the generous donation by Gordon Getty and Cole Thompson. The authors would like to thank Jean-Jacques Cleyet-Merle, head of the Musée national de Préhistoire (Les Eyzies-de-Tayac), and Véronique Merlin-Anglade, head of the Musée d'Art et d'Archéologie du Périgord (Périgueux), for the authorization to study the collections conserved at their institutions. Also, the authors would like to thank the curators of anthropological collections of the Sackler Medical Faculty (Tel Aviv) and Musée de l'Homme (Paris) for providing comparative material. Finally, the authors thank Frederic Santos (PACEA) for his statistical help.




