The gaits of marsupials and the evolution of diagonal-sequence walking in primates
Funding information: National Science Foundation, Grant/Award Number: BCS-0137930; Boston University
Abstract
Objectives
Documenting the variety of quadrupedal walking gaits in a variety of marsupials (arboreal vs. terrestrial, with and without grasping hind feet), to aid in developing and refining a general theory of gait evolution in primates.
Materials and Methods
Video records of koalas, ringtail possums, tree kangaroos, sugar gliders, squirrel gliders, wombats, numbats, quolls, a thylacine, and an opossum walking on a variety of substrates were made and analyzed to derive duty factors and diagonalities for symmetrical walking gaits. The resulting distributions of data points were compared with published data and theories.
Results
Terrestrial marsupials' gaits overwhelmingly plot slightly below the theoretical “horse line” (Cartmill et al., Zoological Journal of the Linnean Society. 2002;136:401–420) typical of terrestrial mammals; arboreal marsupials' gaits overwhelmingly plot more decisively above it. Both distributions are roughly parallel to the horse line, but arboreal animals exhibit increased diagonality, so that their higher-speed walking gaits overlap with those of typical primates on the Hildebrand diagram of diagonality against duty factor.
Conclusions
Quadrupeds avoid gaits lying exactly on the (theoretically optimum) horse line, to avoid fore/hind limb interference (“forging”). This can be accomplished by either a slight reduction in diagonality (“downshifting”) or a more decisive increase (“upshifting”). Tree-dwellers adopt the second option to eliminate unilateral bipods of support from the gait cycle. The upshifted horse line represents an early phase in the evolution of primate-like diagonal-sequence gaits.
1 INTRODUCTION
In the very first comparative photographic studies of animal locomotion, Muybridge (1887) observed that different animal taxa have different characteristic footfall patterns, and that the walking gaits of primates differ from those of other quadrupeds. Camelids and a few other mammals have what are now called lateral-couplets (LC) gaits, in which the two legs on one side swing backwards and forwards more or less together as a coordinated pair during the gait cycle. All other terrestrial quadrupeds, including nonmammals (Ashley-Ross, Lundin, & Johnson, 2009; Jayes & Alexander, 2009; Reilly & DeLancey, 1997; Walker, 1972; Willey, Biknevicius, Reilly, & Earl, 2004; Zug, 1972), adopt diagonal-couplets (DC) gaits, in which diagonally opposite limbs move more or less together. Most of these animals use lateral-sequence (LS) walking gaits, in which the forefoot in each diagonal pair touches down first. Primates, however, usually adopt diagonal-sequence (DS) DC gaits, in which the hind foot in each diagonal pair touches down first. Typically, all these gaits are symmetrical, meaning that the second half of each cycle is a mirror image of the first half, with left and right sides exchanged. In a symmetrical gait, each footfall therefore follows its opposite-side counterpart by an interval equal to half the period of the cycle. At top speeds, most quadrupedal mammals switch to asymmetrical gaits like galloping or bounding (Gambaryan, 1974), in which there is no such mirror imaging (Figure 1d).
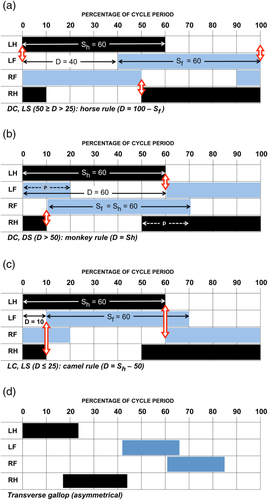
Analysts have sought to replace these verbal descriptions with more quantitative characterizations of gaits. Attempts to do so in terms of support sequences (Gray, 1944; Muybridge, 1887) or footfall sequences (Prost, 1965) were awkward, hard to follow, and unproductive. Building on A. B. Howell's (1944) distinction between symmetrical and asymmetrical gaits, Hildebrand (1965) devised a way of defining any symmetrical gait in terms of three numbers. (A similar analysis was developed independently by a Soviet researcher: Sukhanov, 1963, 1967). Hildebrand's three numbers are expressed as percentages of the stride period (the time that elapses between successive falls of a given foot). The three are (a) hindlimb duty factor (S h), meaning the percentage of the stride period during which a given hindlimb is on the ground; (b) forelimb duty factor (S f), meaning the percentage of the stride period during which a given forelimb is on the ground; and (c) diagonality (D), meaning the phase difference between the fore and hind limb cycles, expressed as the time (percentage of the stride period) that elapses between a hind footfall and the fall of the ipsilateral forefoot (Figure 1a–c).
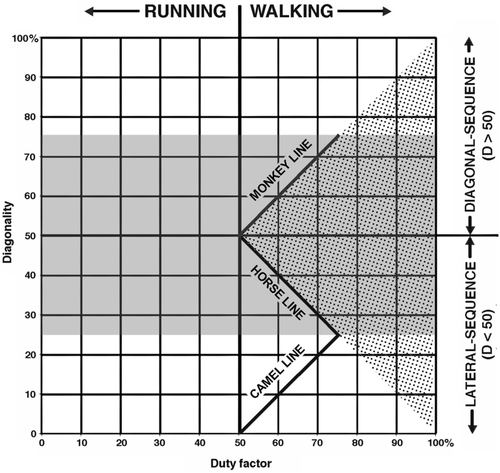
The differences between diagonal and lateral couplets or sequences are entirely a function of the third variable, diagonality. (For LS gaits, D < 50; for DS gaits, D > 50; for DC gaits, 25 < D < 75: Figure 1a–c). Duty factors (S) express the relative speed of movement. As an animal moves faster, each foot is in contact with the support during a smaller percentage of the gait cycle, and so the duty factor grows smaller. No matter what the speed, forelimb duty factors are usually nearly the same as those of the hindlimb (Cartmill, Lemelin, & Schmitt, 2002; Hildebrand, 1976). Therefore, the three variables defining a symmetrical gait can for most purposes be reduced to two: diagonality and duty factor. This allows us to represent most symmetrical gaits as single points on a bivariate plot. Such a plot is sometimes called a Hildebrand diagram (Figure 2).
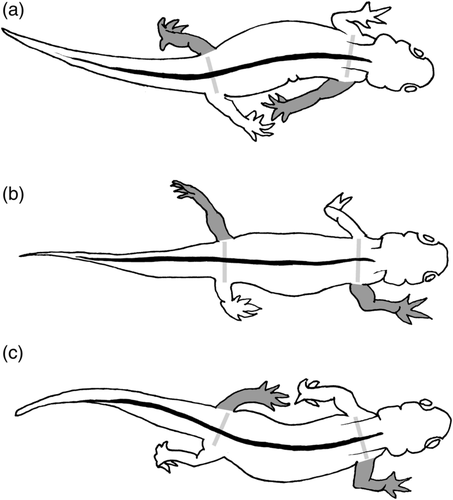
Ever since Muybridge, the distinctive gaits of different animal taxa have attracted scientific attention, and their functional, biomechanical, and adaptive significance has been extensively debated (Cartmill, Lemelin & Schmitt, 2002; Vilensky & Larson, 1989). Most of this debate has focused on the distinctive DC-DS walking gaits of primates. Muybridge (1887) contended that the habit of climbing has given monkeys peculiarly strong forelimbs, and that this somehow results in an aberrant footfall sequence. More recent authors (Kimura, Okada, & Ishida, 1979; Rollinson & Martin, 1981; Tomita, 1967) have advanced just the opposite argument—that primates have unusually big, strong hindlimbs; that this produces a caudal shift in the animals' center of mass; that a primate walking with a DC gait therefore tends to pitch backward at the moment of forefoot touchdown (whereas other animals tend to pitch forward); and that the contralateral hindfoot in the diagonal pair is set down earlier to prevent this, thus producing a DS gait. Subsequent studies have not borne out this idea (Cartmill, Cartmill, Schmitt, & Lemelin, 2005; Druelle, Berthet, & Quintard, 2019; Vilensky & Larson, 1989; Young, Patel, & Stevens, 2007). Prost (1965) argued that the DC-DS pattern, but not the DC-LS pattern, “allows an animal to use lateral spine bending to increase distance between successive contact points for the same leg,” thus increasing stride length. Hildebrand (1980) argued to the contrary that the lateral sequence “facilitates undulation of the spine, which rotates the [limb] girdles and lengthens the step.” But in fact, what chiefly facilitates lateral undulation of the spine (and corollary increase in stride length) is employing DC gaits, in which the forelimb on one side is protracted together with the opposite hindlimb, thus rotating the fore and hind limb girdles in opposite directions (Figure 3). Whether the forelimb in the diagonal pair strikes down just before the hindlimb (LS), or just after (DS), has little effect on girdle rotation.
Any satisfactory account of the peculiar gaits of primates has to do two jobs. First, it must explain why such gaits are adopted in spite of their demonstrated disadvantages, by pointing to something about them that is advantageous for primates. Second, it must also explain why any supposed advantages invoked to explain their presence in primates have not been exploited by most other mammals. If DS gaits are advantageous for primates for some reason, it must be shown that reason is not applicable to the great majority of mammalian quadrupeds, in which DS gaits rarely or never occur (Vilensky & Larson, 1989).
- The horse pattern (D = percentage duration of forelimb swing phase = 100 − S f: Figure 1a) results from following the rule “Lift each forefoot when the hindfoot on that side touches down.” This pattern, which is approximated in the symmetrical walking gaits of most quadrupeds, minimizes periods of bipedal support in LC-DS walking gaits, in which diagonality values lie between 25 (the LS amble or singlefoot; Schmitt, Cartmill, Griffin, Hanna, & Lemelin, 2006) and 50 (the trot). Its equation graphs a negative linear relationship of D against S (duty factor), with a slope of −1.
- The monkey pattern (D = S h: Figure 1b), characteristic of quadrupedal primates, results from following the rule “Lift each hindfoot when the forefoot on that side touches down.” This pattern minimizes periods of bipedal support in DC-DS walking gaits, in which diagonality values lie between 50 (the trot) and 75 (the DS amble).
- The camel pattern (D = S h − 50: Figure 1c), around which the gaits of camelids, giraffes (Basu, Wilson, & Hutchinson, 2018), pacing horses, and many carnivorans cluster, obeys the rule “Lift each hindfoot when the forefoot on the opposite side touches down.” This pattern minimizes periods of bipedal support in LC-LS walking gaits, in which diagonality values lie between 0 (the pace) and 25 (the LS amble). Both the camel and monkey equations generate lines with a positive relationship between D and S, with slopes of +1 on the Hildebrand diagram, displaced from each other by a phase shift of 180° (50%) on the diagonality axis.
Following Hildebrand (1976), Cartmill et al. (2002) suggested that the horse pattern (DC-LS) is the most commonly adopted because it yields larger, more stable support polygons overall throughout the gait cycle, and that the camel rule (LC-LS) is adopted by camels and other mammals with relatively long legs because the LC pattern helps to prevent each forefoot from hitting the hindfoot on the same side during the cycle. The monkey pattern (DC-DS) was explained with reference to the fact that most species with this gait pattern—including many marsupials as well as almost all primates—have grasping hind feet. In DC-DS gaits, each hindfoot strikes down shortly before the diagonally opposite forefoot. This footfall sequence allows these arboreal animals to grasp a safe support with the opposite hindfoot if the leading forefoot comes down on a support that fails. Cartmill and his collaborators identified this as the primary adaptive benefit of monkey-like gaits.
In subsequent publications, Cartmill, Lemelin, and Schmitt (2007a, 2007b) and Cartmill, Schmitt, et al., 2007 went on to elaborate this theory and test it with reference to the gaits of other mammals. In walking on poles, DS gaits were found to predominate in Caluromys, an arboreal didelphid marsupial with grasping hind feet, whereas LS gaits predominated in the more terrestrially adapted didelphid Monodelphis, which has less prehensile hind feet (Lemelin, Schmitt, & Cartmill, 2003). Marmosets (Callithrix jacchus), in which the power of pedal grasp is reduced, were found to use mainly LS gaits and to have difficulty walking on thin poles (Schmitt, 2003). Binturongs (Arctictis), large arboreal viverrids with semiprehensile tails but nongrasping hind feet, were likewise found to use LS gaits when walking on poles (Cartmill, Schmitt, Hartstone-Rose, & Lemelin, 2007). All these findings were in accord with the postulated association between grasping hind feet and DS walking gaits.
However, exceptions to the theory have also been noted. Lorisid primates, all of which have hind feet with exaggerated grasping adaptations, are found by some investigators to employ the DS gaits predicted by the theory; but others report LS gaits (Hildebrand, 1976; Schmitt & Lemelin, 2004; Stevens, 2008). Kinkajous (Potos), which are arboreal procyonids that (like binturongs) have prehensile tails and nongrasping hind feet, nevertheless employ primarily DS gaits on both poles and flat surfaces, both in running and walking (Cartmill, Schmitt, et al., 2007). Lemelin and Cartmill (2010) sought to explain this anomaly by noting that for kinkajous and other arboreal animals, DS walking gaits have two additional advantages: they minimize the part of the gait cycle when the animal is standing on only two legs on the same side, and they maximize the distance between front and rear support points during the much longer periods when the animal is supported by two diagonally opposite legs. Although that explanation accounts for the kinkajou data, it renders the LS gaits of binturongs and some other arboreal animals more puzzling.
Marsupials have also presented problems for the support-polygon theory. All tree-dwelling marsupials (apart from tree kangaroos, Dendrolagus) have grasping hind feet. If that theory is correct, these animals should prefer DS gaits, whereas their terrestrial relatives (and tree kangaroos) should adopt LS gaits. A preliminary study of marsupial gaits by Cartmill and colleagues (Cartmill, Schmitt, Lemelin, Cartmill, & Atkinson, 2008) showed that this expectation generally holds. However, there was one glaring exception: the koala (Phascolarctos), an arboreal animal with marked grasping specializations of the hands and feet, was found to use exclusively DS gaits on the ground but predominantly LS gaits on branches and other arboreal-type supports. Cartmill and his co-authors acknowledged that “This unexpected finding presents fundamental challenges to [our] theories of the distribution of gait patterns in mammals,” but they offered no solution to the puzzle.
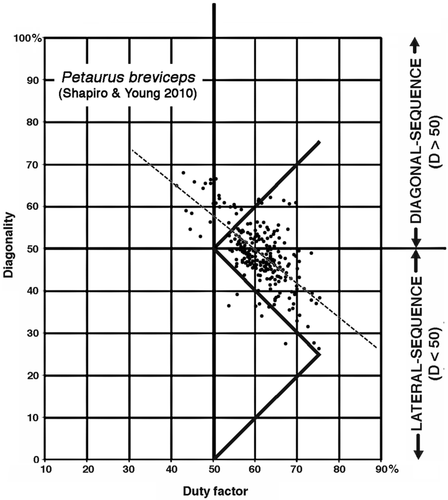
Two gliding arboreal marsupials with prehensile hind feet have also been reported to violate the predictions of the support-polygon model. The tiny (10–15 g) feathertail glider (Acrobates) shows a preponderance of DS gaits, but with a significant admixture of LS gaits (Karantanis, Youlatos, & Rychlik, 2015); and in a study conducted by Shapiro and Young (2010), the sugar glider Petaurus breviceps displayed a preponderance of LS gaits, on both poles and flat surfaces (Figure 4).
To assess the meaning of these anomalies, we undertook a comparative study of symmetrical gaits in marsupials, including a variety of terrestrial and arboreal species with both grasping and nongrasping hind feet.
2 MATERIALS
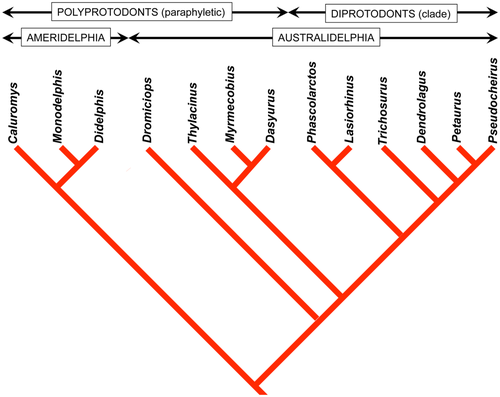
Our primary sample (Table 1) comprises new data on adults of 11 marsupial species belonging to 9 families, representing the three major orders of living marsupials and broadly distributed across the phylogenetic tree of marsupials (Figure 5).
| Superorder Australidelphia |
| Order Diprotodontia |
| Suborder Vombatiformes |
| Family Phascolarctidae |
| Phascolarctos cinereus: 11, 38 |
| (San Diego, Miami, Melbourne, Perth, and Edinburgh Zoos) |
| Family Vombatidae |
| Lasiorhinus latifrons: 3, 21 |
| (Perth Zoo) |
| Suborder Phalangeriformes |
| Family Petauridae |
| Petaurus breviceps: 5, 27 |
| (Henry Doorly Zoo, Omaha; Gladys Porter Zoo, Brownsville) |
| Petaurus norfolcensis: 1, 3 |
| (Perth Zoo) |
| Family Pseudocheiridae |
| Pseudocheirus occidentalis: 1, 11 |
| (Perth Zoo) |
| Suborder Macropodiformes |
| Family Macropodidae |
| Dendrolagus goodfellowi: 1, 8 |
| (San Diego Zoo) |
| Dendrolagus matschiei: 5, 9 |
| (Miami Zoo; Henry Doorly Zoo, Omaha) |
| Order Dasyuromorphia |
| Family Dasyuridae |
| Dasyurus maculatus: 2, 51 |
| (Perth Zoo; Henry Doorly Zoo, Omaha) |
| Family Thylacinidae |
| Thylacinus cynocephalus: 1, 2 |
| (Hobart Zoo, Tasmania: Online footage) |
| Family Myrmecobiidae |
| Myrmecobius fasciatus: 2, 9 |
| (Perth Zoo) |
| Superorder Ameridelphia |
| Order Didelphimorphia |
| Family Didelphidae |
| Didelphis virginiana: 1, 11 |
| (Memphis Zoo) |
- Note: Numbers following each species name indicate number of individuals, number of gait cycles included. Filming locations follow in parentheses. Taxa and nomina from May-Collado, Kilpatrick, and Agnarsson (2015).
Short video clips of the last surviving marsupial wolf or thylacine (Thylacinus cynocephalus), a captive that died in the Hobart (Tasmania) Zoo in 1936, are available on several internet sites. These clips were downloaded and analyzed, yielding two symmetrical walking cycles for this extinct animal. The other animals in our primary sample were housed at zoos and other animal facilities in the United States, Australia, and the United Kingdom (see Acknowledgments). Our comparative secondary sample comprises data drawn from previous studies (Cartmill et al., 2002, Cartmill, Lemelin, & Schmitt, 2007a, 2007b; Cartmill, Schmitt, et al., 2007, Cartmill et al., 2008; Schmitt et al., 2006; Lemelin & Cartmill, 2010) for a wide variety of therian mammals. Tables 1 and 2 list the species in both the primary and secondary samples, with the numbers of individuals recorded and number of gait cycles analyzed for each species. Some of the species in our primary sample are represented by only a few individuals and cycles—as few as two cycles for one individual, in the case of the thylacine. Nevertheless, they are included here for the sake of documenting gaits for a wide spectrum of marsupials.
| Order Didelphimorphia |
| Family Didelphidae |
| Caluromys philander (4, 114) |
| Monodelphis brevicaudata (1, 5) |
| Order Proboscidea |
| Family Elephantidae |
| Loxodonta africana (1, 1) |
| Order Rodentia |
| Family Sciuridae |
| Sciurus carolinensis (1, 1) |
| Family Muridae |
| Rattus norvegicus (1, 2) |
| Order Primates |
| Family Cheirogaleidae |
| Cheirogaleus medius (3, 8) |
| Microcebus sp. (2, 7) |
| Mirza coquereli (1, 7) |
| Family Lemuridae |
| Eulemus fulvus (1, 7) |
| Eulemur mongoz (1, 8) |
| Hapalemur griseus (1, 8) |
| Lemur catta (1, 19) |
| Varecia variegata (1, 5) |
| Family Daubentoniidae |
| Daubentonia madagascariensis (3, 13) |
| Family Galagidae |
| Otolemur garnetti (1, 4) |
| Family Lorisidae |
| Loris tardigradus (3, 51) |
| Nycticebus coucang (6, 83) |
| Perodicticus potto (1, 2) |
| Family Callitrichidae |
| Callithrix jacchus (2, 3) |
| Family Cebidae |
| Cebus capucinus (1, 8) |
| Family Atelidae |
| Ateles geoffroyi (1, 9) |
| Family Cercopithecidae |
| Papio anubis (1, 7) |
| Erythrocebus patas (1, 10) |
| Macaca fascicularis (1, 10) |
| Family Hominidae |
| Pan troglodytes (1, 5) |
| Order Carnivora |
| Family Canidae |
| Canis familiaris (2, 37) |
| Family Ursidae |
| Ursus thibetanus (1, 5) |
| Family Procyonidae |
| Potos flavus (2, 99) |
| Procyon lotor (1, 2) |
| Family Mustelidae |
| Galictis vittata (1, 4) |
| Family Viverridae |
| Arctictis binturong (4, 18) |
| Family Herpestidae |
| Suricata suricatta (1, 1) |
| Family Felidae |
| Felis caracal (1, 2) |
| Felis catus (1, 7) |
| Puma concolor (1, 2) |
| Leptailurus serval (1, 1) |
| Panthera tigris (1, 8) |
| Order Artiodactyla |
| Family Camelidae |
| Camelus dromedarius (1, 13) |
| Lama glama (1, 10) |
| Family Giraffidae |
| Giraffa camelopardalis (1, 7) |
| Family Bovidae |
| Capra hircus (1, 1) |
| Ovis aries (1, 1) |
| Family Cervidae |
| Muntiacus muntjak (1, 3) |
| Order Perissodactyla |
| Family Rhinocerotidae |
| Ceratotherium simum (1, 4) |
| Family Equidae |
| Equus caballus (6, 55) |
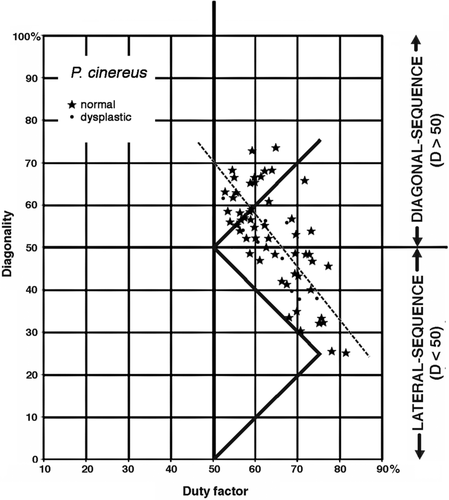
Some of the koalas (Phascolarctos cinereus) bred and housed at the San Diego Zoo suffer from a genetically conditioned dysplasia of the hip and shoulder joints, which deteriorate as the animals grow older (Pye, 2009; Pye, Hamlin-Andrus, & Moll, 2008). In its severest form, the hip dysplasia may affect a koala's gait. Animals that the San Diego Zoo's veterinarians identified as severely afflicted were excluded from our study. Four animals suffering from mild to moderate hip dysplasia were retained in our study after statistical tests revealed no significant differences between them and normal koalas in the slopes or intercepts for least-squares regressions of diagonality against duty factor (Figure 6). Data for potentially dysplastic San Diego koalas currently housed in other zoos fell inside the San Diego cluster on the Hildebrand diagram, and were also retained in our primary sample.
3 METHODS
Videorecordings of the animals in our primary sample walking without constraints on horizontal cylindrical surfaces (poles or branches) and flat surfaces (floor or ground) were made using a Sony HDR-HC5 videocamera. Animals housed at the Perth (Australia) Zoo under nocturnal lighting conditions were filmed using the monochromatic “NightShot” feature on the camera and an auxiliary infrared spotlight. The recordings were imported into video-editing software and analyzed using procedures described in previous publications (Cartmill et al., 2002, 2006). Each cycle chosen for analysis was cropped beginning and ending at touchdown for the same hind foot. The frame number was then noted for the touchdown and liftoff of each foot during the cycle. The nine frame numbers thus identified were entered in a spreadsheet to calculate the duration of the cycle (stride period), duration of the stance and swing phases for each foot, and diagonality. Gait cycles were discarded if they deviated from perfect symmetry by more than 10%—that is, if successive hind footfalls or successive fore footfalls were found to be separated by less than 40% or more than 60% of the cycle's period (Schmitt et al., 2006; Young, 2012). All procedures involving living animals were approved in advance by administrators and institutional animal care, use and ethics panels at the institutions housing the animals. No animal was anesthetized, deprived of food or water, hurt or harmed in the course of data collection. Some of the gait cycles of the koalas, sugar gliders, and opossum used in this study were generated on substrates chosen by the experimenters. All other videorecordings were obtained simply by filming animals moving around in their home enclosures at their own discretion on supports of their own choosing. No animals displaying stereotyped “pacing” behavior were encountered or included in this study.
4 RESULTS
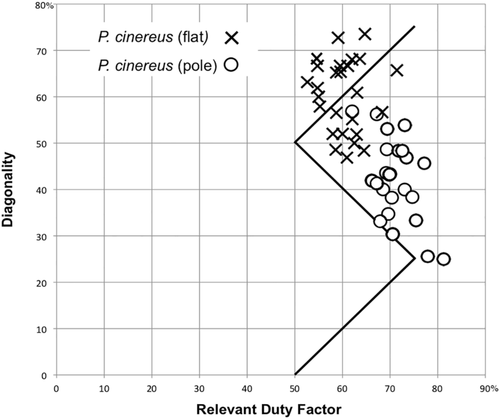
1. Phascolarctos cinereus (koala). Koalas are moderately large (4–15 kg) arboreal folivores with pronounced grasping specializations of the hind feet. Plotted on a Hildebrand diagram, their symmetrical gaits were distributed along a diagonal scatter running roughly parallel to the horse line and consistently above it on the diagonality axis (Figure 7). The koala gaits fell into two nearly discrete clusters. In walking on flat surfaces, the animals adopted gaits with relatively low duty factors and high diagonalities, clustering around the monkey line; in walking on poles and branches, they exhibited higher duty factors and lower diagonalities, as reported by Cartmill et al. (2008).
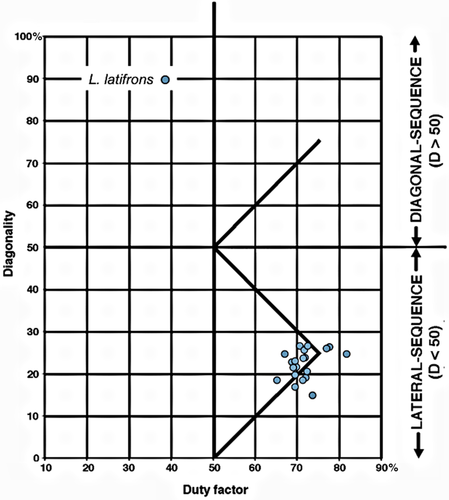
2. Lasiorhinus latifrons (southern hairy-nosed wombat). L. latifrons is a moderately large (20–32 kg) burrowing animal more closely related to koalas than to other marsupials in this study (Figure 5). Like other wombats, it is exclusively terrestrial and has nongrasping hind feet. The wombat gaits that we recorded were all slow LS walks on the ground. On the Hildebrand diagram, they fell in the ambiguous “singlefoot” area near the intersection of the horse and camel lines, where D ≅ 25 and S ≅ 75 (Figure 8).
3, 4. Petaurus breviceps (sugar glider), P. norfolcensis (squirrel glider). Almost all of our data points for these two species of small (110–230 g) gliding arboreal marsupials fell within the range of those collected for P. breviceps by Shapiro and Young (Figures 3 and 9a). Both DS and LS gaits were recorded. Average diagonality for P. breviceps was somewhat higher in our study than in that of Shapiro and Young (2010). The distribution of gaits produced in walking on poles and branches overlapped almost completely with that of walking gaits on flat surfaces, and the difference between the means fell short of 95% significance (p = .071; two-tailed t test).
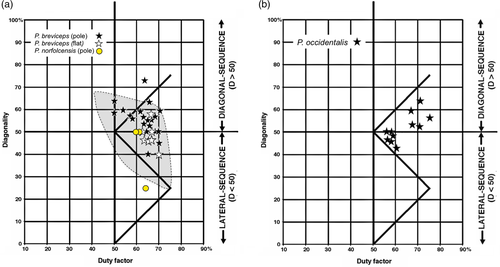
5. Pseudocheirus occidentalis (western ring-tailed possum). P. occidentalis is a medium-sized (~1 kg) arboreal herbivore with a prehensile tail and grasping hind feet. The symmetrical gaits that we recorded for this animal (Figure 9b) were mostly trotting walks (D = 50) and DS walks, executed on branches and arrayed on the Hildebrand diagram in an elongated cluster running roughly parallel to and below the monkey line, so that the slower walks—that is, those with duty factors exceeding 65—displayed higher diagonalities than the faster walks.
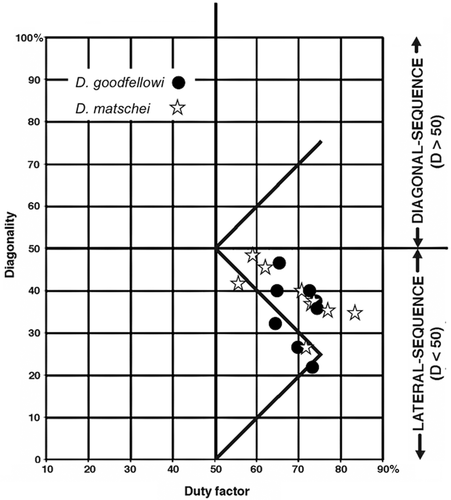
6, 7. Dendrolagus goodfellowi (Goodfellow's tree kangaroo), D. matschiei (Matschie's tree kangaroo). Tree kangaroos (Dendrolagus) are relatively small (7–11 kg) macropodids that have adapted to living in trees by evolving broad hands and feet armed with powerful claws. They lack grasping specializations of the hind feet, and climb using a claw grip. Like terrestrial kangaroos, they prefer to use asymmetrical hopping or bounding gaits on supports of all types. We were able to record only a few symmetrical walking gaits (exclusively on flat supports) for the two Dendrolagus species in our sample. These gaits approximated the horse line, though none fell exactly on it (Figure 10).
8. Dasyurus maculatus (spotted quoll). D. maculatus, the largest of the quolls, is a cat-sized (2–4 kg) carnivorous marsupial. It climbs well and has a well-developed hallux, and a high percentage of its prey species are arboreal; however, more than 80% of its locomotion is on the ground, and its short metapodials suggest that it is a slow runner (Jones, Rose, & Burnett, 2001). The locomotor cycles that we recorded for this animal were mainly rather slow LC walks, executed on both branches and flat surfaces and concentrated around the upper end of the camel line (Figure 11a)—an area of the Hildebrand diagram where a number of eutherian Carnivora also tend to cluster.
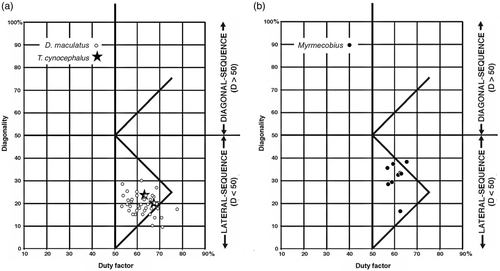
9. Thylacinus cynocephalus (thylacine). T. cynocephalus was a terrestrial carnivore resembling a canid of medium size (20–30 kg) in habits and external appearance. The two cycles representing all that we will ever know about the gaits of this animal were executed on the floor of a zoo enclosure. They lay entirely within the cluster of our Dasyurus data on the Hildebrand diagram (Figure 11a).
10. Myrmecobius fasciatus (numbat). This small (500–700 g), endangered termite-eating marsupial is exclusively terrestrial and lacks a hallux (Cooper, 2011; Thomas, 1888). The cycles that we recorded were executed on the ground. Apart from one LC outlier, they were all DC-LS walks, mostly distributed below the horse line (Figure 11b).
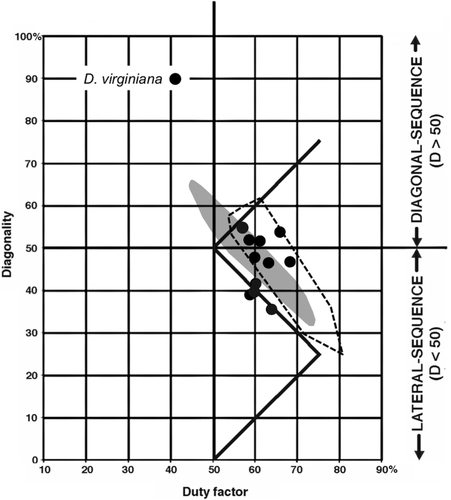
11. Didelphis virginiana (Virginia opossum). D. virginiana is a largely terrestrial animal, but it often feeds, travels, and shelters in trees (Allen, Marchinton, & Lentz, 1985; Lemelin, 1999). It has a prehensile tail and a moderately divergent and opposable hallux, both of which it employs in arboreal locomotion. Eleven symmetrical cycles (Figure 12) were recovered from a single female walking on a flat surface. All 11 were DC walking gaits. Four fell on the horse line or very near it; the other seven fell about 15–20% above the horse line on the diagonality axis. Of these seven, four were marginally DS. All the other cycles were LS (D < 50).
5 DISCUSSION
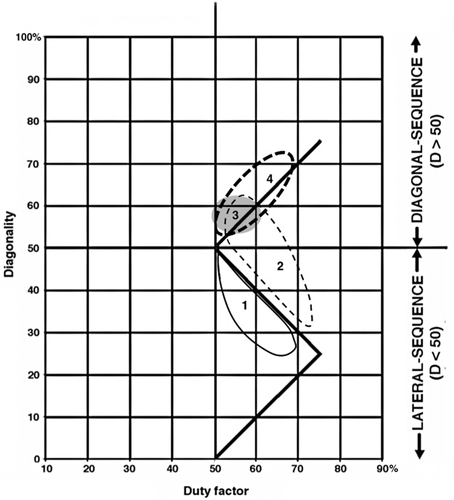
Figure 13 shows all the symmetrical gaits for the species in our primary sample plotted on a single Hildebrand diagram. Inspection of this data scatter reveals three general facts:
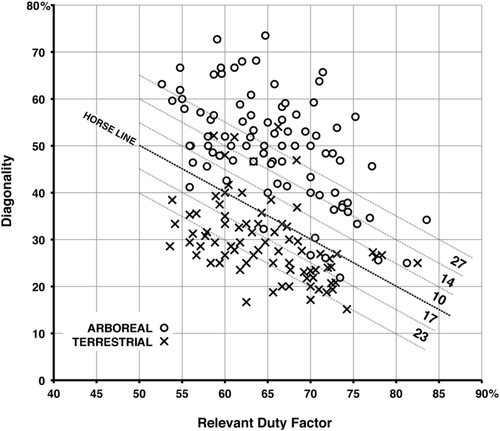
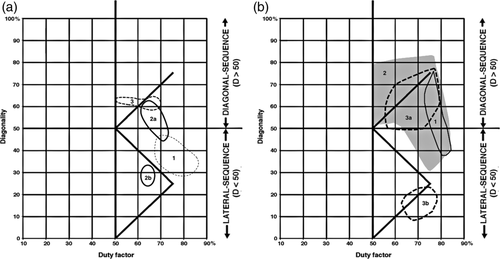
1. Although there is some overlap between the arboreal and terrestrial sets, almost all of the terrestrial marsupials' walking gaits fall below the horse line, and almost all the gaits of the arboreal marsupials fall above it. Most of the exceptions are data for Didelphis virginiana and the two species of Dendrolagus. Didelphis virginiana is a semiarboreal animal descended from more consistently arboreal tropical didelphids, and might with almost equal justice have been scored as arboreal. Walking gaits reported for this species by Hildebrand (1976) and White (1990) resemble those of typical arboreal marsupials in our study (Figure 13) and of Petaurus breviceps in that of Shapiro and Young (2010): Figure 4). (Similar remarks apply to the largely terrestrial South American didelphid Monodelphis brevicaudata: Lemelin et al., 2003). Conversely, Dendrolagus is a kangaroo-up-a-tree evolutionary makeshift that is less than ideally adapted to arboreal locomotion. The anomalous diagonality values recorded for Didelphis and Dendrolagus may be due to phylogenetic inertia, reflecting the arboreal antecedents of the former and the terrestrial antecedents of the latter. When these two taxa are excluded from our sample (Figure 14), the separation between the arboreal and terrestrial groups is nearly complete.
2. In both the arboreal and terrestrial groups overall, diagonality goes up as duty factor goes down. Since duty factor is inversely correlated with speed, this means that both groups adopt more diagonal gaits as they walk faster, following the horse-line pattern. (In the monkey and camel patterns, animals adopt less diagonal gaits as they walk faster.) Throughout the scatter, most of the arboreal animals exhibit markedly higher diagonalities than the terrestrial ones (Figure 14).
These facts appear to explain the puzzling disjunction in our data for koalas, which unexpectedly adopted mostly DS gaits on the ground and mostly LS gaits in walking on poles and branches (Figure 7). Our videorecordings reveal the reason for this: namely, that koalas hate to find themselves on the ground. On poles and branches, they tend to move slowly and cautiously; but when placed on the ground, they make rapidly for the nearest tree, sometimes even breaking into an asymmetrical canter in an effort to get back up a tree as quickly as possible. Because koalas display a horse-like inverse relationship between diagonality and duty factor, the fast walking gaits that they adopt on the ground (in heading for a tree) are DS.
3. Although the support-polygon model implies that the horse line should be the theoretical optimum for DC-LS gaits, both arboreal and terrestrial marsupials appear to be avoiding it, leaving a poorly populated gap in the data just above the horse line, separating the arboreal group from the terrestrial group (Figure 13).
A broader comparison, pooling the gait data for all the species in our primary and secondary samples (Figure 15), exhibits a similar gap above the horse line in the scatter of data points. Contrary to the predictions of the support-polygon model, avoidance of this narrow strip on the Hildebrand diagram appears to be a general phenomenon in mammalian locomotion. The basic shape of the support-polygon zigzag is evident in the pooled data; but the wedge-shaped cloud of points above the horse line is shifted upward, with its apex about 9% higher on the diagonality axis than expected. There is no such avoidance of either the camel or monkey lines.
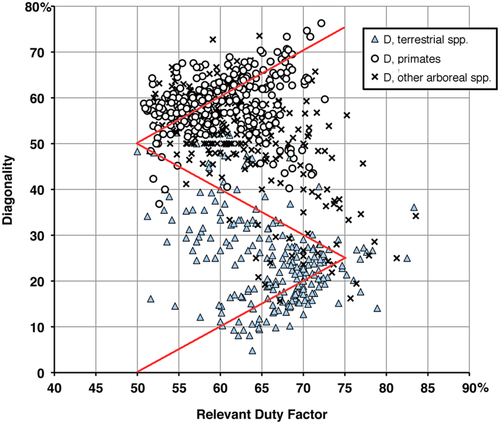
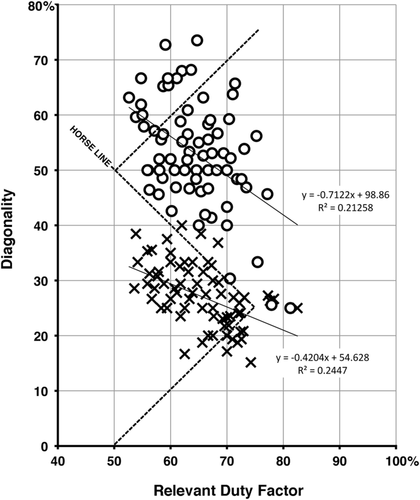
Why do quadrupedal mammals avoid the horse line? We suggest that they do so in order to avoid interference between fore and hind limbs.
In all gaits that obey the horse rule, each hindfoot comes down at the moment when the forefoot on the same side is lifted (Figure 1a). This is also the moment of that forefoot's maximum retraction, and of that hindfoot's maximum protraction. Therefore, the probability that the descending hindfoot will strike the rising forefoot is greatest at such moments, which occur twice in each symmetrical gait cycle. Some authors have made contrary assertions. Young (2012, p. 581) writes that “in a DS gait, hindlimb touchdowns coincide with maximal retraction of the ipsilateral forelimb, increasing the potential that the two limbs will physically collide.” However, in a DS walking gait, the hind footfall necessarily precedes the ipsilateral forefoot liftoff (which is the point at which forelimb retraction is maximal) by a percentage of the gait cycle equal to D + S f − 100 (dimension “p” in Figure 1b). Schmitt (2003, p. 34) proposed that “The use of LS gaits where the hindfoot does not land until the forefoot-contact period is almost over may help avoid problems of interference” between fore- and hindlimbs. Again, we believe that the reverse is the case. Bringing the hindfoot down at the moment when the ipsilateral forefoot is fully retracted and about to be lifted maximizes the chances that the descending hindfoot will hit the forefoot.
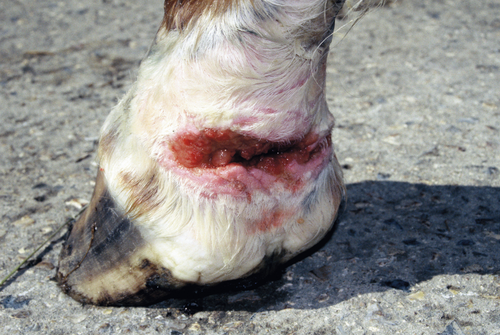
Such hits may result in missteps or stumbling, or even in injury from the impacts of hind nails, claws, or hooves against the backs of the forefeet. In young horses, this sort of interference is known as “overreaching” or “forging” (from the rhythmic metallic sounds produced when the toes of the hind horseshoes strike the backs of the ipsilateral front horseshoes). Such impacts can produce wounds that may be life-threatening if they become infected or penetrate a tendon sheath (Figure 16). Forging is most likely to occur at the trot, when diagonality and duty factor both approach 50; but the problem affects any gait that exactly obeys the horse rule (Figure 17a). Factors that slightly delay or retard the lifting of the front hoof at the end of stance phase—for example, heavy horseshoes, fatigue, or deep sand underfoot—can be expected to promote and exacerbate forging (Armistead & Patterson, 1957; Ross & Dyson, 2010, pp. 301, 1004). Conversely, lifting the forefoot earlier advances the forelimb cycle with respect to the hindlimb cycle, thus reducing the probability of forging. This results in a reduction in diagonality (Figure 17b) and therefore a downward displacement of the datum for that gait on the Hildebrand diagram.
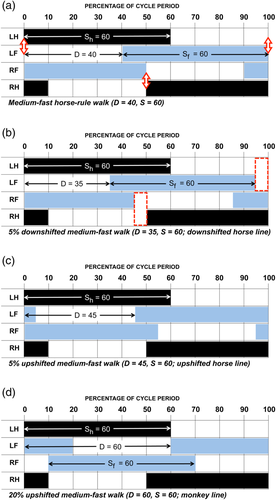
We will refer to this sort of deviation from the horse rule (by advancing the timing of the forelimb cycle, reducing diagonality) as downshifting (with reference to the vertical D axis on the Hildebrand diagram). Downshifting reduces the risk of forging. But any DC-LS gait that falls below the horse line has the disadvantage of incorporating two periods of exclusively unilateral bipedal support—that is, support by only the two legs on one side (boxes, Figure 17b; cf. Figures 5 and 7 in Cartmill et al., 2002). Brief periods of unilateral bipedal support pose no problems for a walking horse, but they are more disadvantageous for an arboreal animal balancing on a branch (Cartmill, Lemelin, & Schmitt, 2007b; Lemelin & Cartmill, 2010). Downshifting is not an optimum way for an arboreal animal to avoid forging.
Such an animal can avoid both unilateral bipedality and forging by increasing the diagonality of its gait—that is, retarding the timing of the forelimb cycle relative to that of the hindlimb cycle (Figure 17c). We will call this sort of deviation from the horse rule upshifting. Downshifting avoids forging by ensuring that the forefoot is already starting to swing forward when the ipsilateral hindfoot strikes down; upshifting avoids forging by bringing the hindfoot down earlier, while the ipsilateral forefoot is still planted far enough forward (relative to the trunk) to be out of reach of the descending hindfoot. Slight downshifting will eliminate forging, but slight upshifting (as occurs in the cases involving deep sand or heavy horseshoes) may make it worse. To be effective, upshifting must be more marked than downshifting. This explains why the gap between the upshifted and downshifted data lies above the horse line (Figures 13-15). Exactly how far a horse-rule gait needs to be upshifted to avoid forging depends on the animal's configuration and behavior (joint angulations at hind footfall, the linear extent of forelimb retraction and hindlimb protraction, and the lengths of the fore and hind limbs relative to the trunk), but there will always be some minimum level of upshifting that is required to accomplish the job.
The facts presented here suggest that typical quadrupedal mammals follow the horse rule, but modify it to avoid forging (overreaching interference), by either a slight downshifting or a more pronounced upshifting. The gap opened up by these opposite modifications generates the sparsely populated region running above and parallel to the horse line in the scatter of our data points on the Hildebrand diagram. Because any LS-LC gait that is upshifted above the horse-line falls in a region of the Hildebrand diagram where periods of unilateral bipedality do not occur (Figure 2), arboreal mammals with horse-like walking gaits tend to upshift rather than downshift, in order to eliminate unilateral bipedality while minimizing the chances of fore-hind limb interference. Animals conforming to an upshifted horse-line pattern will exhibit primate-like DS gaits at high walking speeds (lower duty factors), and LS gaits when they move more slowly (higher duty factors). In our data, this pattern is evident in the arboreal marsupials as a whole (Figure 13), and in the intraspecific gait distributions for Phascolarctos cinereus, Petaurus spp., and Dendrolagus matschei. Other investigators report similar upshifted horse-line patterns—DS at low duty factors (high speeds), LS at high duty factors (lower speeds)—for symmetrical walking gaits in a wide variety of arboreal mammals: Petaurus breviceps (Shapiro & Young, 2010, 2012), the didelphids Didelphis virginiana (White, 1990), and Caluromys philander (Cartmill, Lemelin, & Schmitt, 2007b; Lemelin et al., 2003), the basal australidelphian marsupial Dromiciops australis (Pridmore, 1994), the tamarin Saguinus oedipus (Nyakatura, Fischer, & Schmidt, 2008), the sloths Bradypus variegatus, Choloepus didactylus, and C. hoffmani (Mendel, 1985; Nyakatura et al., 2010), the kinkajou Potos flavus (Lemelin & Cartmill, 2010), the acacia rat Thallomys paedulcus (Karantanis, Rychlik, Herrel, & Youlatos, 2017a), and the climbing mice Apodemus agrarius and Myodes glareolus (Karantanis, Rychlik, Herrel, & Youlatos, 2017b).
When such an animal's locomotion exhibits, or is studied across, a narrow range of speeds (and hence of duty factors), its gaits may be restricted to only a short segment of the upshifted horse-line pattern on the Hildebrand diagram. Such consistently slow-moving arboreal animals as chameleons, pottos, and slow lorises may have gaits that are largely or exclusively LS (Cartmill, Schmitt, & Lemelin, 2004) because they are restricted to the lower part of an upshifted horse line. Conversely, consistently fast-moving animals like Acrobates (Karantanis et al., 2015), Saguinus mystax (Garber & Pruetz, 1995), and Potos (Lemelin & Cartmill, 2010) may appear monkey-like because their gaits are concentrated on, or restricted to, the upper, DS end of an upshifted horse line.
Diagonal-sequence gaits are not peculiar to arboreal animals with grasping hindfeet. Such gaits are also adopted by arboreal animals that lack grasping specializations of the hindfoot (e.g., Potos). Conversely, many animals with grasping hindfeet adhere to an upshifted horse-line pattern, and therefore adopt LS gaits at slow speeds. These facts contradict the representation of DS gaits as simple corollaries of grasping hindfeet (Cartmill et al., 2002). Nevertheless, all the animals that obey an upshifted horse rule have arboreal habits; and all the animals that cleave to downshifted variants of the horse rule appear to be terrestrial.
We suggest that DS gaits originated as a side effect of retardation of the forelimb cycle relative to that of the hindlimb (upshifting). Most mammals using DC walking gaits shift them upward or downward with respect to the horse line to avoid forging; but the terrestrial ones downshift, which makes DS walking gaits impossible. Arboreal mammals almost invariably upshift. Upshifting opens a window for the evolution and development of DS gaits.
We conjecture that in the ancestors of both primates and marsupials, the upshifted horse line represented an easily attained initial phase in the evolution of DS gaits from a horse-like ancestral pattern. This transformation would have proceeded through four stages (Figure 18):
5.1 Stage 1
The symmetrical walking gaits of the ancestral therian mammals would have approximated the horse line, with slight downshifting to avoid fore-hind limb contact.
5.2 Stage 2
An intermediate, marsupial-like stage of primate locomotor evolution would have coupled an upshifted horse-line distribution—an opossum-like or koala-like gait pattern, combining fast DS gaits with slower LS gaits—with the emergence of more pronounced grasping specializations of the hindfoot. Such specializations appear to have evolved independently in carpolestids and in the lineage leading to early euprimates (Bloch, Silcox, Boyer, & Sargis, 2007). This convergence suggests that the (plesiadapiform) last common ancestor of both had an upshifted horse-line gait pattern, adopted in connection with arboreal habits and a limited, Ptilocercus-like prehensility of the hindfoot (Sargis, 2004).
5.3 Stage 3
In early euprimates, the lower, LS end of the upshifted horse line was dropped from the locomotor repertoire. The result was the sort of gait pattern seen in many primates, in which all symmetrical walking gaits are DC cycles concentrated in the lower left corner of the upper right quadrant of the Hildebrand diagram.
5.4 Stage 4
The final innovation was moving from the upshifted horse-line pattern, in which diagonality decreases as duty factors rise (that is, in slower gaits), to the monkey-line pattern, in which diagonality varies directly with duty factor. This pattern is not seen in lorises, and it may have evolved in parallel in different groups of euprimates. Antecedents of this final transformation can also be found among marsupials—in our data for Pseudocheirus (Figure 9b), and arguably in the gait pattern reported for the brush-tailed possum Trichosurus vulpecula, in which walking gaits are almost exclusively DS and diagonality goes down as duty factors decline (White, 1990). A pattern of direct covariation between diagonality and duty factor makes it possible to eliminate LS gaits entirely from the locomotor repertoire at all walking speeds, as in typical primates today.
This reconstruction of gait phylogeny finds suggestive parallels in the ontogeny of gaits in some anthropoids. In a longitudinal study of gait ontogeny in squirrel monkeys (Saimiri), Young (2012) found that as squirrel monkeys mature, changes in mass distribution and relative limb proportions result in an increased probability of “forging” interference between the fore and hind limbs. Animals reduce the incidence of such interference by reducing limb excursions and by increasingly shifting to camel-like LC-LS gaits, especially on flat surfaces. Similar patterns of ontogenetic shifts in gait patterning, from LS-DC upshifted horse-line gaits through an intermediate stage featuring both DS gaits and transient “islands” of camel-like LC-LS gaits to a nearly exclusive adult concentration on monkey-pattern DC-DS gaits, are also seen in macaques (Figure 19; Hildebrand, 1967; Nakano, 1996) and baboons (Shapiro & Raichlen, 2005). We endorse Young's interpretation of these facts:
Although LSDC is the primitive walking pattern across tetrapods (Hildebrand, 1976), it may be that among most primates LSDC is primarily an “infant gait”—a transitory phenomenon associated with somatic immaturity—whereas LSLC gaits continue to be used at later ages and even into adulthood, perhaps as a means of mitigating the problem of limb interference … as Shapiro and Raichlen (2005) noted in another study of baboon gait ontogeny, LSLC gaits share with DSDC gaits the possible advantage of ensuring that a hindlimb is firmly planted near the animal's midline at the moment of fore limb touchdown, thus promoting stability in the event that a precarious substrate is encountered (Cartmill et al., 2002, Cartmill, Lemelin, & Schmitt, 2007a, 2007b). However, LSLC gaits also increase the amount of time the animal must spend on ipsilateral limb bipods, likely compromising mediolateral stability, particularly in an arboreal context. (Young, 2012)
We do not suggest that this recurring pattern of gait ontogeny represents some sort of Haeckelian recapitulation of phylogeny. The upshifted horse-line pattern seen in infant anthropoids may indeed be adopted because of some phylogenetically acquired innate propensity, fixed in the ancestral anthropoids or primates and representing an apomorphic departure from the ancestral mammals' (slightly downshifted) horse-line pattern. But subsequent modifications in developing anthropoids appear to be learned as departures from the innate pattern. As the growing juvenile's limbs grow longer relative to its trunk and its strides become more confident and excursive, the animal modifies its gait patterns in a way that compensates for the increasing incidence of “forging” interference between its fore and hind limbs. To accomplish this, a transient camel-like LS-LC gait may be adopted temporarily during development (and occasionally in adult life), especially on flat surfaces; but the attendant disadvantages of lateral instability in arboreal locomotion eventually cause camel-pattern gaits to be largely or entirely abandoned in favor of a monkey-like DS-DC pattern. The amount of intraspecific variability seen in this sequence of behavioral shifts suggests that it is learned rather than innate. Nevertheless, the functional exigencies that drive developing monkeys through this series of alterations in their gait behavior would presumably have applied throughout early phases of primate evolution. Although few arboreal marsupials exhibit monkey-like gait patterns, their quadrupedal gaits can be plausibly interpreted as representing an early stage in this process. It may be that, as Jenkins (1974) famously observed about primate arboreality, the important innovation of euprimates was not the adoption of a DS gait, but their successful restriction to it.
ACKNOWLEDGMENTS
We are grateful to the officers, staff, and animal caregivers of the Melbourne and Perth Zoos in Australia, the Miami MetroZoo, the Gladys Porter Zoo (Brownsville, TX), San Diego Zoo, the Riverbanks Zoo (Columbia, SC), the Memphis Zoo, the Albuquerque Zoo, the Edinburgh Zoo in Scotland, and the Duke University Lemur Center for their generous cooperation and assistance in studying the locomotion of the wonderful animals in their care. We are grateful as well to Hannah Lemieux, Richard Tole, Efi Mandrides, and Clare Shepherd at TI Media Ltd. for providing the photograph used in Figure 17. We thank Pierre Lemelin and Daniel Schmitt for giving us their kind permission to incorporate data from our earlier joint publications. A preliminary version of this study (Cartmill, 2009) was presented at the annual meetings of the American Association of Physical Anthropologists. Our work was supported by NSF Grant BCS-0137930 and by research funding from Boston University.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




