Placental and Fetal Microbiota in Rhesus Macaque: A Case Study Using Metagenomic Sequencing
Qiao Du and Xu Liu contributed equally to this work and share the first authorship.
ABSTRACT
Recent evidence challenging the notion of a sterile intrauterine environment has sparked research into the origins and effects of fetal microbiota on immunity development during gestation. Rhesus macaques (RMs) serve as valuable nonhuman primate models due to their similarities to humans in development, placental structure, and immune response. In this study, metagenomic analysis was applied to the placenta, umbilical cord, spleen, gastrointestinal tissues of an unborn RM fetus, and the maternal intestine, revealing the diversity and functionality of microbes in these tissues. Additionally, gut metagenomic data of adult Rhesus macaques from our previous study, along with data from a human fetus obtained from public databases, were included for comparison. We observed substantial microbial sharing between the mother and fetus, with the microbial composition of the placenta and umbilical cord more closely resembling that of the fetal organs than the maternal intestine. Notably, compared with other adult RMs, there was a clear convergence between maternal and fetal microbiota, alongside distinct differences between the microbiota of adults and the fetus, which underscores the unique microbial profiles in fetal environments. Furthermore, the fetal microbiota displayed a less developed carbohydrate metabolism capacity than adult RMs. It also shared antibiotic resistance genes with both maternal and adult RM microbiomes, indicating potential vertical transmission. Comparative analysis of the metagenomes between the RM fetus and a human fetus revealed significant differences in microbial composition and genes, yet also showed similarities in certain abundant microbiota. Collectively, our results contribute to a more comprehensive understanding of the intrauterine microbial environment in macaques.
Summary
-
Characterized diverse microbial diversity in placental, umbilical, and fetal gastrointestinal and spleen tissues of RMs, revealing distinct microbiomes compared to adult macaques, highlighting the unique microbial environment of the RM fetus.
-
Identified significant microbial similarities between fetal and maternal microbiomes in RMs, highlighting a trend toward vertical transmission and its potential implications for fetal immunity and development.
-
Conducted comparative metagenomic analysis between RM and human fetal microbiomes, highlighting both distinct and shared microbial features, which supports the use of RMs as a model to explore human fetal microbial influences.
1 Introduction
Activation of the fetal immune system during gestation significantly impacts fetal health and pregnancy outcomes (Romero et al. 1998; Zhivaki and Lo-Man 2017). A well-developed immune system with a diverse immune repertoire is essential for the fetus to respond effectively to potential antigens and other threats (Leavy 2015). Fetal inflammatory response, closely linked to immune system activation, is a key indicator of overall fetal health (Romero et al. 1998; Rackaityte and Halkias 2020; Gotsch et al. 2007). Additionally, diverse immune cells present in the fetal gut may initiate immune activation, highlighting the gut as a critical site for immune responses (Stras et al. 2019). Initial studies predominantly supported the sterile womb hypothesis (Walter and Hornef 2021), suggesting that the placenta functions as an immune organ protecting the fetus from exposure to external antigens and microbes during pregnancy (Robbins and Bakardjiev 2012; Delorme-Axford et al. 2013). Furthermore, the placenta's role extends beyond mere barrier protection, encompassing maternal-fetal cell communication and regulation of immune interactions (PrabhuDas et al. 2015). However, an increasing number of studies provided evidence of low-biomass microbes in human placental and fetal tissues (Younge et al. 2019; Seferovic et al. 2019; Rackaityte et al. 2020; Perez-Muñoz et al. 2017; Kundu et al. 2017). Notably, early exposure to microbes can influence the developing immune system, subsequently affecting reproduction and pregnancy outcomes (Jain 2020; Mishra et al. 2021). Amid concerns about potential contamination, robust research methods, including the use of negative controls, culture techniques, and scanning electron microscopy, have successfully validated the presence of microorganisms in the intrauterine environment (Mishra et al. 2021; Aagaard et al. 2014; Leon et al. 2018; Parnell et al. 2017, 2019). These studies challenged prior beliefs, reshaping our understanding of microbial vertical transmission and its role in developing the immune system and reproductive outcomes.
The primary limitation in current research on human placental and fetal microbiota is the difficulty in obtaining placental and fetal tissue before delivery. Post-delivery tissue collection introduces confounding variables that can potentially skew experimental results (Romero et al. 1993). To address these challenges, researchers have increasingly turned to animal models, with nonhuman primates (NHPs) proving particularly valuable due to their close physiological, anatomical, reproductive, genetic, behavioral, and immune similarities to humans (Carlsson et al. 2004; Carter 2007). Among NHPs, rhesus macaques (RMs), noted for their developmental patterns, placental structures, and immune responses highly similar to those of humans, are especially significant, making them an effective model for studying microbial infection and fetal immunity in pregnancy (Carlsson et al. 2004; Carter 2007). However, research on intrauterine microbiota in RMs remains relatively scarce, largely due to ethical and welfare concerns associated with collecting fetal samples from NHPs (Prescott 2010). Several studies using 16S rRNA sequencing to investigate microbial signals in fetal and placental tissue of RMs and Japanese macaques have reached controversial conclusions regarding the presence of intrauterine microbiota (Chu et al. 2017; Prince et al. 2017; Theis et al. 2020; Chu et al. 2018; Prince et al. 2018, 2019). Given these challenges and the existing controversies, further targeted research is essential.
The fetal microbiota is influenced by various maternal sources, with multiple pathways enabling microbial translocation to the intrauterine environment (Koleva et al. 2015; Schoenmakers, Steegers-Theunissen, and Faas 2019). Among these sources, the maternal gut microbiota is a key potential source, with microbes crossing the intestinal barrier to enter the bloodstream and ultimately reaching the placenta (Schoenmakers, Steegers-Theunissen, and Faas 2019). The placenta and umbilical cord are vital in maternal-fetal interactions, possibly mediating microbial transfer (Koleva et al. 2015; Schoenmakers, Steegers-Theunissen, and Faas 2019). The fetal spleen, a critical immune organ, aids immune cell development before birth (Nunez, Réot, and Menu 2021), while the fetal gastrointestinal (GI) tract likely serves as the initial site of microbial colonization (Schoenmakers, Steegers-Theunissen, and Faas 2019), essential for preparing innate immunity in early life. In this study, we performed a cesarean section to collect the placenta, umbilical cord, spleen, GI tissues of an unborn RM fetus, and maternal intestine from a deceased pregnant RM and conducted a comprehensive metagenomic analysis. Despite the implementation of stringent criteria to exclude environmental contamination and low-quality sequences, we observed a variety of metabolically active microbes in the placenta, umbilical cord, and fetal organs. To explore potential vertical transmission in RMs, we compared the microbial profiles of the mother and the fetus. Comparisons of microbial composition, function, and antibiotic resistance genes (ARGs) were conducted between fetal, maternal, and other adult RMs and provided further evidence supporting the possibility of vertical transmission. Separately, we compared the microbiota between the RM fetus and a human fetus, revealing key differences and similarities and suggesting the potential of RMs as a model for studying human fetal microbiomes. This study aims to contribute valuable information to the understanding and research of fetal microbiota and its potential impact on fetal immunity and overall health.
2 Materials and Methods
2.1 Sample Collection
Maternal intestinal contents, as well as placental, umbilical cord, fetal spleen, and fetal GI tissues, were obtained from a captive female RM housed at the Sichuan Green-House Biotech Co. Ltd. (Meishan, Sichuan, China). The company was established by West China Hospital of Sichuan University and has gained the License for Experimental Animals and Production of Experimental Animal Feed (NO: SCXK(Chuan)2024-0013) and License for Use of Experimental Animals (No: SYXK(Chuan)2024-0192). All the macaques for reproduction were kept in continuous full contact groups with sex ratios of 1:5–1:6 (male: female). Each group stayed in a separate cage around 25m2 in area and was provided with necessary welfare equipment, such as flying rings and toys. The macaques were fed three times a day at a regular time. In the morning and afternoon, the amount of food was provided about 100–150 g/individual. At noon, fruits or vegetables were provided 150–200 g/individual. All the macaques were allowed to feed and drink freely. The dietary composition of the macaque in this experiment was as follows: 50% corn, 14% wheat, 15% soybean meal, 5% wheat bran, 4.5% fish meal, 5.5% sucrose, 5% bone meal, 0.1% multivitamins, 0.1% vitamin C powder, 0.1% trace elements, 0.1% lysine, 0.2% methionine, and 0.1% vitamin B complex powder. The cage inspection was conducted four times a day, twice in the morning and twice in the afternoon. The whole cage sterilization was applied 2–3 times a week to prohibit any infection to the animals.
The subject of our study was an 8-year-old maternal macaque, who stayed in a continuous full contact group with five other individuals. The macaque died on August 10, 2022, at her late stage of pregnancy around 19–20th weeks of gestation. No evident signs of illness were observed during her pregnancy. A qualified veterinarian conducted an autopsy on the maternal macaque less than 2 h after its death, revealing no obvious pathological anomalies in the tissues. The death of the maternal macaque was probably caused by the stress from extremely high temperatures during that period. The fetus's death was a consequence of the mother's death.
Before the dissection of the maternal macaque, the entire ultraclean workbench was disinfected thoroughly. Intestinal contents from the mother were collected using aseptic cotton swabs. The fetus was a male individual, who did not pass through the birth canal. It was retrieved after the excision of the entire sterilized uterus and was placed in an ultraclean workbench for ultraviolet irradiation. Placental tissue, umbilical cord, fetal spleen, and GI tissues were carefully dissected using sterile surgical instruments. All samples were collected under stringent aseptic conditions to prevent environmental contamination. Before dissection, swabs of the workbench and operator hands were used as environmental controls, samples of phosphate-buffered saline (PBS) wash of the dissection instruments were used as PBS controls, and blank swabs and PBS solution were used as blank controls (Figure S1). Although we did not perform metagenomic sequencing on these control samples due to their low DNA content, we conducted library preparation for DNA quantification to verify that the potential contribution of environmental contamination to our sample data was minimal (mean ± SD ng/μL, controls: 0.48 ± 0.37).
Additionally, gut metagenomic data of seven healthy adult RMs were included for comparison with fetuses, two of which were downloaded from the China National GeneBank Database (CNGBdb) (accession number CNP0002963), and the remaining five were obtained from our laboratory and are part of unpublished research. We downloaded three metagenomic datasets of intestinal contents from a single human fetus, sourced from the Genome Sequence Archive for Human (accession number HRA003676). These samples were collected using aseptic techniques from elective pregnancy terminations during the 12–22th weeks.
This study was approved by the Ethics Committee of the College of Life Sciences, Sichuan University, China (SCU230810001). All sample collection and utility protocols fully complied with the guidelines of the Management Committee of Experimental Animals of Sichuan Province, China (SYXK-Sichuan, 2019-192). Our research complies with the American Society of Primatologists' Principles for the Ethical Treatment of Nonhuman Primates.
2.2 Metagenomic Sequencing and Quality Control
All DNA samples were extracted using a Tiangen DNA Stool Mini Kit (Tiangen Biotech Co. Ltd. China) and sent to Novogene Bioinformatics Technology Co. Ltd. (Beijing, China) for metagenomic sequencing. In total, 0.2 μg of DNA per sample was used as input for the DNA library preparations, and the sequencing library was generated using a NEBNext UltraTM DNA Library Prep Kit for Illumina (NEB, USA, Catalog #: E7370L). Index codes were added to each sample, and the genomic DNA was fragmented by sonication to a size of approximately 350 bp. The fragmented DNA was then end-polished, A-tailed, and ligated with full-length adapters for Illumina sequencing, followed by PCR amplification and purification using the AMPure XP system (Beverly, USA). After library quality assessment and quantification, the qualified libraries were sequenced using the Illumina 6000 platform with a paired-end length of 150 bp. Whole metagenome shotgun sequencing was performed to obtain comprehensive microbial community profiles, including bacterial, archaeal, and viral DNA. The Q30 and Q20 values of all samples were approximately 90% and 95%, respectively, indicating that the correct rate of base calling was relatively high (Table S1). Adapters and low-quality reads in the raw data were removed using Fastp (Chen et al. 2018a). Host contamination was removed using Bowtie2 (Langmead and Salzberg 2012) based on the RM reference genome (assembly Mmul_10).
2.3 Data Processing and Analyses
Metagenome de novo assembly was conducted using MEGAHIT (Li et al. 2015) with the option “–m 0.95 –min-contig-len 300.” The genes in the metagenomic data were obtained using Prodigal (Hyatt et al. 2010) with the option “-p meta.” Nonredundant genes were constructed using CD-HIT (Fu et al. 2012) with the option “-c 0.95 -aS 0.90” and quantified using Salmon (Patro et al. 2017) with the option “–meta.” The nonredundant amino acid sequences translated from the genes were used for subsequent functional prediction. Functional annotations of carbohydrate-active enzymes (CAZymes) and ARGs were performed using DIAMOND (Buchfink, Xie, and Huson 2015) with the option “–id 80% –query-cover 70% –evalue 1e-5” and RGI based on the Carbohydrate Active enZYmes Database (Lombard et al. 2014) and comprehensive antibiotic resistance database (Alcock et al. 2020). The total abundance of each functional gene type was the sum of the abundances of all genes mapped to the same type. The abundances of CAZyme genes and ARGs were normalized by transcripts per million (TPM). The microbial metabolic pathways and gene families were predicted using HUMANn3 (Franzosa et al. 2018) based on the ChocoPhlAn and UniRef90 EC filtered databases (Suzek et al. 2007), and abundances were normalized by counts per million (CPM). The taxonomic annotation of metagenomic sequences was performed using kraken2 (Wood and Salzberg 2014) with the option “–use-mpa-style”. The abundances of taxa were normalized to relative abundance.
Principal coordinate analysis (PCoA) and Bray-Curtis distance were calculated using the Adonis test in the vegan package of the R statistical environment (version 4.2.3) (R Core Team R 2013). Alpha-diversity analysis was done by the vegan package of R statistical software with Wilcoxon's rank-sum test. The statistical significance of differences in numbers was analyzed using Wilcoxon's rank-sum test with p < 0.05. Differentially abundant pathways were screened by STAMP (v2.1.3) based on Welch's t-test (FDR < 0.05; Benjamini–Hochberg method).
3 Results
3.1 Microbial Composition of Maternal Intestine, Placenta, Umbilical Cord, and Fetal Organs
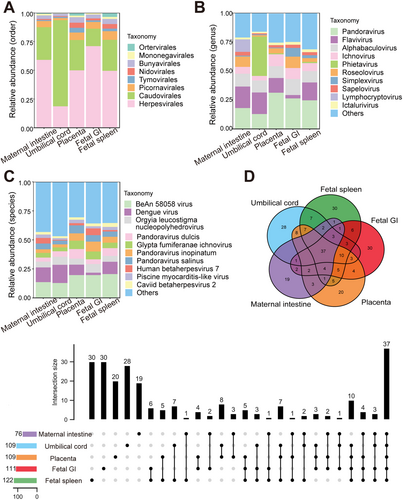
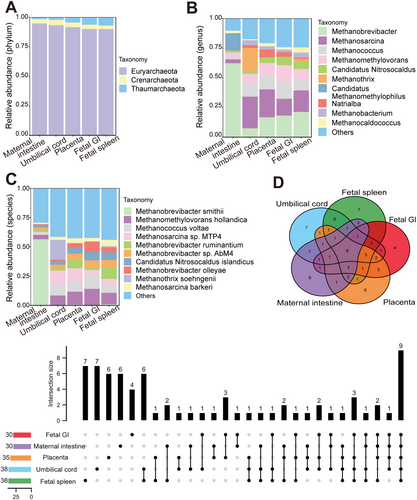
Microbial signals in the cesarean-delivered RM fetus were investigated. To minimize background contamination, environmental, PBS, and blank controls were utilized, with undetectable (below sequencing thresholds) microbial DNA indicating negligible impact on the results. Metagenomic sequencing of maternal intestinal content and placental, umbilical cord, fetal spleen, and fetal GI tissues identified 2 806 species of bacteria (belonging to 31 phyla and 891 genera, Figure 1A–C), 242 species of viruses (belonging to eight orders and 102 genera, Figure 2A–C), and 74 species of archaea (belonging to three phyla and 44 genera, Figure 3A–C). A PCoA plot, based on Bray-Curtis distances of microbial species-level relative abundance profiles, revealed that samples from the placenta, umbilical cord, fetal spleen, and fetal GI clustered closely together, distinct from the maternal intestinal sample (Figure S2A). For comparative analysis of microbial composition between fetal and maternal samples, samples from the placenta, umbilical cord, fetal spleen, and fetal GI were grouped as the “fetal group” for further analysis. Within this fetal group, a total of 2 554 species of bacteria (belonging to 28 phyla and 826 genera), 223 species of viruses (belonging to eight orders and 97 genera), and 68 species of archaea (belonging to three phyla and 142 genera) were identified.
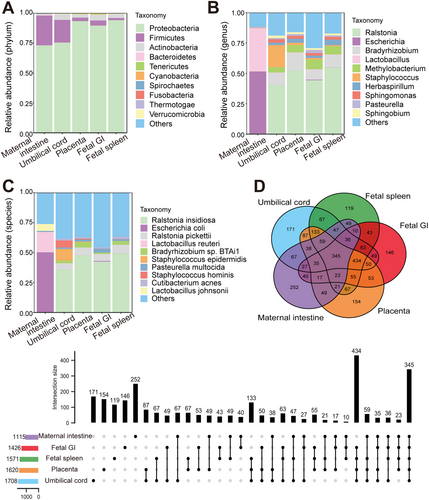
Among the 2 806 bacterial species identified, 345 were shared by the maternal and fetal groups, with the number of organ-unique bacteria varying from 119 (fetal spleen) to 252 (maternal intestine) (Figure 1D). In total, 779 out of 2 554 bacterial species were shared by the placenta, umbilical cord, fetal spleen, and fetal GI samples, each containing 203, 238, 168, and 186 unique bacteria species (Figure S2B). Notably, each fetal sample shared considerable bacterial species with the maternal intestinal sample, varying from 533 to 654 species (Table S2). Among the most dominant bacteria in the maternal and fetal samples (Table 1), Proteobacteria and Firmicutes were the dominant shared phyla in both groups. Interestingly, no dominant shared genera or species were found between the maternal and fetal groups. Within the fetal group, the dominant bacteria in the placenta, umbilical cord, fetal GI, and spleen samples were generally identical, although Staphylococcus was a dominant genus in the umbilical cord but not in the other fetal samples.
| Level | Maternal intestine | Placenta | Umbilical cord | Fetal GI tissue | Fetal spleen |
|---|---|---|---|---|---|
| Phylum | Proteobacteria | Proteobacteria | Proteobacteria | Proteobacteria | Proteobacteria |
| Firmicutes | Firmicutes | Firmicutes | Actinobacteria | Actinobacteria | |
| Bacteroidetes | Actinobacteria | Actinobacteria | Firmicutes | Firmicutes | |
| Spirochaetes | Tenericutes | Bacteroidetes | Tenericutes | Tenericutes | |
| Actinobacteria | Bacteroidetes | Tenericutes | Bacteroidetes | Bacteroidetes | |
| Genus | Escherichia | Ralstonia | Ralstonia | Ralstonia | Ralstonia |
| Lactobacillus | Bradyrhizobium | Staphylococcus | Bradyrhizobium | Bradyrhizobium | |
| Campylobacter | Methylobacterium | Bradyrhizobium | Methylobacterium | Methylobacterium | |
| Streptococcus | Herbaspirillum | Methylobacterium | Enterobacter | Herbaspirillum | |
| Shigella | Sphingomonas | Sphingomonas | Pasteurella | Sphingomonas | |
| Species | Escherichia coli | Ralstonia insidiosa | Ralstonia insidiosa | Ralstonia insidiosa | Ralstonia insidiosa |
| Lactobacillus reuteri | Ralstonia pickettii | Staphylococcus epidermidis | Ralstonia pickettii | Ralstonia pickettii | |
| Lactobacillus johnsonii | Bradyrhizobium sp. BTAi1 | Staphylococcus hominis | Pasteurella multocida | Bradyrhizobium sp. BTAi1 | |
| Lactobacillus amylovorus | Pasteurella multocida | Ralstonia pickettii | Enterobacter cloacae | Herbaspirillum seropedicae | |
| Campylobacter hyointestinalis | Bradyrhizobium diazoefficiens | Bradyrhizobium sp. BTAi1 | Cutibacterium acnes | Cutibacterium acnes |
Compared with the identified bacteria, the proportion of identified viruses and archaea was notably small, with only 0.33%–1.85% viruses and 0.097%-0.18% archaea, respectively (Figure S2E). Among the 242 virus species identified, 37 were shared by the maternal intestine and fetal group, each sample harboring 19–30 organ-specific viruses (Figure 2D). The four fetal samples (placenta, umbilical cord, fetal spleen, and fetal GI) shared 47 virus species, each harboring 23, 28, 31, and 32 unique viruses, respectively (Figure S2C). There were 43-50 virus species shared by individual fetal samples and maternal intestinal samples (Table S2). Among the most abundant viruses, Caudovirales, Herpesvirales, and Picornavirales were the dominant shared orders between the maternal and fetal groups (Table 2), and Pandoravirus and Alphabaculovirus were the dominant shared genera. Most of the dominant virus genera were consistent across the four fetal samples, although the umbilical cord sample differed somewhat from the other fetal samples. Among the 74 archaea species identified, nine were common between the maternal and the fetal groups, with each harboring 4–7 unique species (Figure 3D). In total, 12 archaeal species were shared by the four fetal samples, with each fetal sample (Figure S2D). Furthermore, 15–18 archaeal species were shared by the maternal and four fetal samples (Table S2). Both groups contained the same dominant phyla (Table 3), although the umbilical cord sample differed somewhat in dominant genera.
| Level | Maternal intestine | Placenta | Umbilical cord | Fetal GI tissue | Fetal spleen |
|---|---|---|---|---|---|
| Order | Herpesvirales | Herpesvirales | Caudovirales | Herpesvirales | Herpesvirales |
| Caudovirales | Caudovirales | Herpesvirales | Caudovirales | Caudovirales | |
| Picornavirales | Picornavirales | Bunyavirales | Picornavirales | Picornavirales | |
| Bunyavirales | Bunyavirales | Picornavirales | Bunyavirales | Bunyavirales | |
| Mononegavirales | Nidovirales | Tymovirales | Nidovirales | Nidovirales | |
| Genus | Flavivirus | Pandoravirus | Phietavirus | Pandoravirus | Pandoravirus |
| Pandoravirus | Flavivirus | Flavivirus | Alphabaculovirus | Flavivirus | |
| Alphabaculovirus | Alphabaculovirus | Pandoravirus | Roseolovirus | Alphabaculovirus | |
| Lymphocryptovirus | Ichnovirus | Alphabaculovirus | Ichnovirus | Ichnovirus | |
| Roseolovirus | Roseolovirus | Ichnovirus | Simplexvirus | Roseolovirus | |
| Species | BeAn 58058 virus | BeAn 58058 virus | Dengue virus | BeAn 58058 virus | BeAn 58058 virus |
| Dengue virus | Dengue virus | BeAn 58058 virus | Pandoravirus inopinatum | Dengue virus | |
| Macacine gammaherpesvirus 4 | Pandoravirus dulcis | Orgyia leucostigma nucleopolyhedrovirus | Orgyia leucostigma nucleopolyhedrovirus | Pandoravirus dulcis | |
| Pandoravirus salinus | Glypta fumiferanae ichnovirus | Staphylococcus phage StB27 | Glypta fumiferanae ichnovirus | Pandoravirus inopinatum | |
| Orgyia leucostigma nucleopolyhedrovirus | Pandoravirus salinus | Staphylococcus virus PH15 | Human betaherpesvirus 7 | Orgyia leucostigma nucleopolyhedrovirus |
| Level | Maternal intestine | Placenta | Umbilical cord | Fetal GI tissue | Fetal spleen |
|---|---|---|---|---|---|
| Phylum | Euryarchaeota | Euryarchaeota | Euryarchaeota | Euryarchaeota | Euryarchaeota |
| Crenarchaeota | Thaumarchaeota | Crenarchaeota | Thaumarchaeota | Thaumarchaeota | |
| Thaumarchaeota | Crenarchaeota | Thaumarchaeota | Crenarchaeota | Crenarchaeota | |
| Genus | Methanobrevibacter | Methanosarcina | Methanosarcina | Methanobrevibacter | Methanobrevibacter |
| Candidatus Methanomethylophilus | Methanobrevibacter | Methanothrix | Methanococcus | Methanosarcina | |
| Methanomethylovorans | Methanococcus | Methanococcus | Methanomethylovorans | Methanomethylovorans | |
| Methanosarcina | Methanomethylovorans | Methanomethylovorans | Methanosarcina | Methanococcus | |
| Methanosphaera | Methanothermococcus | Methanobrevibacter | Candidatus Nitrosocaldus | Candidatus Nitrosocaldus | |
| Species | Methanobrevibacter smithii | Methanomethylovorans hollandica | Methanothrix soehngenii | Methanomethylovorans hollandica | Methanobrevibacter ruminantium |
| Candidatus Methanomethylophilus alvus | Methanosarcina sp. MTP4 | Methanosarcina sp. MTP4 | Methanococcus voltae | Methanomethylovorans hollandica | |
| Methanomethylovorans hollandica | Methanococcus voltae | Methanococcus voltae | Methanobrevibacter olleyae | Methanobrevibacter sp. AbM4 | |
| Methanosphaera stadtmanae | Methanothermococcus okinawensis | Methanomethylovorans hollandica | Candidatus Nitrosocaldus islandicus | Methanococcus voltae | |
| Methanobrevibacter ruminantium | Methanobrevibacter ruminantium | Methanosarcina siciliae | Methanobrevibacter sp. AbM4 | Candidatus Nitrosocaldus islandicus |
3.2 Comparison of Microbial Composition and Function Between Fetal and Adult Rms
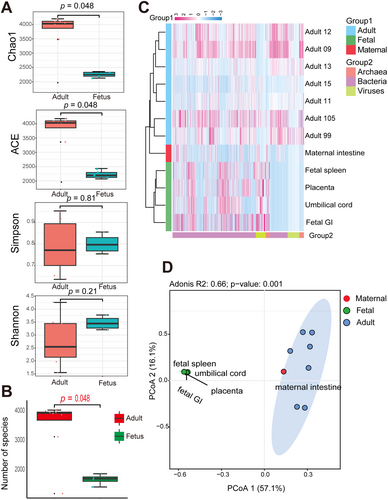
To assess microbial differences between fetal and adult RMs, seven gut metagenomic datasets obtained from adult RMs were included for analysis. Comparing α-diversity between the adult and fetal groups revealed significant differences in the ACE and Chao1 indices (richness) (p < 0.05; Figure 4A), but no significant differences in the Shannon and Simpson indices (richness and heterogeneity). Notably, the number of microbial species was significantly higher in adult samples compared to the fetal samples (p < 0.05; Figure 4B). The heatmap of microbes at the genus level indicated that placental, umbilical cord, fetal spleen, and fetal GI samples clustered separately from maternal intestinal and other adult RM samples, highlighting the distinct microbial composition between the adult and fetal groups (Figure 4C). Abundant microbes exhibited differences between the adult and fetal groups. Specifically, the predominant bacteria in the adult microbiome were Bacteroidetes and Firmicutes, whereas Proteobacteria was dominant in the fetal microbiome (Table S3). It is evident that while the maternal samples clustered with other adult RM samples, their microbial composition closely aligned with that of the fetal group (Figure 4C). The PCoA results based on microbial species-level abundance further corroborated the heatmap results (Figure 4D). Furthermore, the PCoA results for viruses, bacteria, and archaea (Figure S3) showed that viral composition between the fetal and maternal samples was more similar (Figure S3A) than their bacterial or archaeal compositions (Figure S3B, C).
Functional differences between the fetal and adult groups were also studied. The PCoA analysis demonstrated a distinct separation between fetal and adult microbial gene families, with maternal samples displaying a closer similarity to those of the fetal microbiomes (Figure S3D). Building on these genetic findings, we next explored the associated metabolic pathways. The microbiota of the adult group was primarily enriched in protein, ribonucleotide, and peptidoglycan synthesis pathways (Figure S4A), while the fetal group was primarily enriched in pathways related to nucleotide and amino acid synthesis, fatty acid synthesis and oxidation, and energy metabolism (Figure S4B–E). A total of 39 significantly distinct metabolic pathways were identified between the groups (Figure 5A). Of these, 28 pathways were enriched in the adult group, which were mainly related to carbohydrate metabolism essential for energy conversion, metabolic regulation, and energy balance. Conversely, of the 11 pathways enriched in the fetal group, the most significant were associated with energy synthesis and metabolism, including molybdopterin biosynthesis, aerobic respiration I (cytochrome c), and heme b biosynthesis II (oxygen-independent).
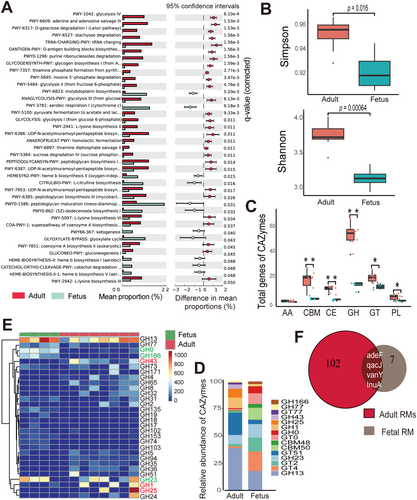
We next identified microbial CAZymes, including glycoside hydrolase (GH), glycosyl transferase (GT), carbohydrate-binding module (CBM), carbohydrate esterase (CE), polysaccharide lyase (PL), and auxiliary activity (AA) between the two groups. The Shannon and Simpson indices indicated that CAZyme diversity was significantly higher in the adult group than in the fetal group (p < 0.05; Figure 5B). The adult microbiome contained a significantly greater number of CAZyme genes than the fetal microbiome (Figure S4F). Notably, the adult samples displayed significantly more GHs, GTs, CBMs, CEs, and PLs than the fetal samples (Figure 5C). In both groups, GH was the most abundant family, followed by GT. The top five prevalent CAZyme families in the adult group were GH13, GT51, GH1, GH25, and GH23, and in the fetal group were GT4, GH13, GT2, GH23, and CBM50 (Figure 5D). Given the critical role of the GH family in carbohydrate degradation, we compared the abundances of different GH family members between the adult and fetus groups. GH13 was the most abundant GH family member, while GH1, GH25, and GH43 were more abundant in the adult group, and GH23, GH0, and GH166 were more abundant in the fetal group (Figure 5E and S4G). Many GH family genes abundant in the adult group were less so in the fetal group. Notably, seven ARGs were identified in the fetal samples (six ARGs in the umbilical cord, one ARG in the placenta, one ARG in the fetal spleen, and two ARGs in fetal GI) and 102 ARGs were identified in the adult group, including the mother. Among them, four ARGs (adeF, qacJ, vanY, and InuA) were shared by the adult and fetal groups (Figure 5F).
3.3 Comparison of Fetal Microbial Composition Between RM and Human
Metagenomic datasets of intestinal contents from a single human fetus were downloaded for comparison with the RM fetus data. A total of 96 species of viruses (belonging to 52 genera), 32 species of archaea (belonging to three phyla and 19 genera), and 982 species of bacteria (belonging to 20 phyla and 431 genera) were identified in the human fetal samples. Comparison of α-diversity between the two species showed no significant differences in Shannon and Simpson indices (Figure 6A). However, at microbial species-level abundance, PCoA distinctly separated the human and RM fetal samples (p < 0.05; Figure 6B). A similar distinction emerged following PCoA of gene family abundance of microbiota (p < 0.05, Figure 6C).
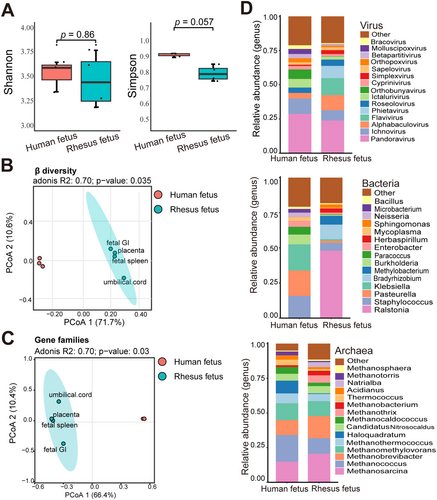
Comparing microbial composition between the RM and human fetal samples, bacteria exhibited more pronounced differences at the genus levels than viruses and archaea (Figure 6D). Despite the differences in microbiota between the two species, several microbes were shared. For instance, both the RM and human fetal samples contained methanogens, being the dominant archaea (Table S4). The dominant bacterial phyla in the fetal microbiota of both species displayed a high degree of similarity (Figure S5 and Table S5).
4 Discussion
Previous studies indicate that the development of the human immune system begins early in fetal life (Rackaityte and Halkias 2020; Mishra et al. 2021), with exposure to maternal antigens and microbial entities that traverse the placental barrier into fetal tissues (Jain 2020; Aagaard et al. 2014). This microbial and antigenic exposure is believed to prime the fetal immune system, establishing a foundation for lifelong immunity and tolerance (Mishra et al. 2021). As a widely used animal model, RMs offer a valuable opportunity to investigate the fetal microbiota (Carlsson et al. 2004; Carter 2007; Buse et al. 2014; Estes, Wong, and Brenchley 2018). In the present study, we performed metagenomic analysis on fetal organs obtained in a sterile manner by cesarean section, and further collected samples of placenta, umbilical cord, and maternal intestine content to study microbial signal in fetus. Following rigorous experimental design, including the exclusion of background contamination and removal of low-quality and host sequences, we detected diverse microbes (including 223 virus species, 2 554 bacteria species, and 68 archaea species) in the placental, umbilical cord, and fetal organ samples. A considerable number of shared microbes were found in the placenta, umbilical cord, and fetal organs, with certain microbes exhibiting significant similarity to those in the maternal intestine. Our findings suggest that in RMs, the fetus is exposed to maternal microbes before birth due to vertical transmission. These findings are consistent with previous studies in humans, NHPs, and mice that show the presence of microbes in the intrauterine environment (Younge et al. 2019; Mishra et al. 2021; Leon et al. 2018; Chu et al. 2018; Prince et al. 2019). However, we detected microorganisms present in fetal samples but absent in the maternal intestine. Several studies suggested that the fetal microbiome may be attributed to multiple potential maternal sources, including oral, endometrial, and urogenital microbiotas (Koleva et al. 2015; Schoenmakers, Steegers-Theunissen, and Faas 2019; Stout et al. 2013). Such a diverse range of microbiota sources could account for the difference in microorganisms observed between the maternal intestine and fetal samples. Together, our analysis provides significant insights into the microbiota and immune development of rhesus monkey fetuses.
However, the presence of fetal microbiota remains controversial, with the long-held view positing the fetus develops in a sterile intrauterine environment until birth (Walter and Hornef 2021). Moreover, a recent study by Theis et al. isolated Cutibacterium acnes from one colony out of 96 fetal and placental samples of RMs, and with 16S rRNA sequencing, they found the bacterium in the fetal samples, particularly in the maternal decidua with relatively high abundance. However, given that this bacterium was also detected in nearly half of the background technical controls and is commonly present on human skin (Omer, McDowell, and Alexeyev 2017) there is a possibility of contamination (Theis et al. 2020). This study obtained an RM fetus in a sterile manner by cesarean section without contamination from the birth canal, and we applied stringent experimental conditions and rigorous control settings to avoid contamination. We also detected Cutibacterium acnes with a relatively high abundance in the fetal samples. Consistent with our result, Cutibacterium acnes was reported to be a significant portion of bacterial isolates from the human placenta and amniotic fluid in normal-term pregnancies (Collado et al. 2016). Unfortunately, due to the limited sample size, whether Cutibacterium acnes exists in the intrauterine environment of RM or not, still needs further verification with advanced analysis methods and more fetal samples in the future. Different from the case of Cutibacterium acnes, other bacteria such as Acinetobacter and Ottowia, identified in our fetal samples, were also detected in uterine wall samples of RMs but were seldom found in controls by Theis et al's study (Theis et al. 2020). These bacteria have also been detected in the human endometrium (Leoni et al. 2019). Additionally, Ralstonia insidiosa, found in high abundance in our fetal samples, was a resident bacterium at the maternal-fetal interface (Parnell et al. 2019). This bacterium was detected in the human placental basal plate and villus through 16S rRNA sequencing and validated by quantitative real-time PCR and fluorescent in situ hybridization (Parnell et al. 2017, 2019). Parnell et al. further demonstrated that Ralstonia insidiosa can enter the placenta via the intrauterine route in a pregnant mouse model, suggesting a mechanism for its presence at the maternal-fetal interface (Parnell et al. 2019). This evidence supports their relevance to the uterine environment and reinforces our hypothesis regarding their presence in the RM uterine environment. The existence of non-pathogenic bacteria in the uterine contributes to fetal tolerance, inducing the establishment of antigen-specific tolerance during fetus development and preparing the fetus for exposure to a microbially rich environment after birth (Jain 2020; Mishra et al. 2021; Huang, Chi, and Qiao 2020). In particular, the abundant Staphylococcus found in the fetal samples of macaque might be involved in the development of the immune system. Previous study indicates that these microbes mediate T cell immune responses, facilitating the fetus's recognition and response to microbial antigens (Mishra et al. 2021; McGovern et al. 2017). However, the detailed biological significance of microbes in the uterine and their influence on the fetal immune system need further investigation in the future.
Recent studies have highlighted the functional involvement of archaea, single-celled prokaryotes, in health and disease (Lewis et al. 2015). Our results indicated that the most abundant archaea at the phylum level, Euryarchaeota, was shared by maternal and fetal samples. Euryarchaeota contains the greatest number and diversity of archaea (Baker et al. 2020), including methanogens. The methanogens were also the dominant archaeal component in fetal samples in our study. At present, however, the functional and pathogenic impacts of archaea in animals remain poorly understood (Cavicchioli et al. 2003). Additionally, the viral profiles in both maternal and fetal samples were similar, indicating the possibility of vertical transmission of viruses from the mother to the fetus within the uterus.
4.1 Microbes in the Placenta and Umbilical Cord
Identification of microbes in the placenta and the umbilical cord is crucial, as the placenta serves as the primary barrier between the fetus and mother, and the umbilical cord supplies essential nutrients for fetal development and survival (Koleva et al. 2015; Millen and Woollam 1960). However, the mechanism by which microbes traverse the placental barrier and reach the fetus remains unresolved, despite the potential impact of microbes on fetal health (Megli and Coyne 2022). Studies on the presence of microorganisms in the placenta and umbilical cord are pivotal for developing effective strategies to prevent fetal microbial infections.
In the present study, diverse microbes were identified in the placenta, umbilical cord, and two fetal organs, while the umbilical cord exhibited a lower microbial DNA content relative to other samples. The microbial profiles of the placenta and umbilical cord more closely resembled those of the fetal spleen and fetal GI than the maternal intestine. The placenta, umbilical cord, and fetal organs shared various dominant microbes and showed similar microbial diversity and composition, strongly suggesting that placental microbes may be the primary contributors to the fetal microbiome (Younge et al. 2019; Chu et al. 2017; Schoenmakers, Steegers-Theunissen, and Faas 2019). Proteobacteria was the most abundant bacteria in the placenta, consistent with findings from human studies (Collado et al. 2016). The umbilical cord microbiota differed somewhat from that of other fetal samples, possibly due to its transportation role, relatively low microbial DNA content, or increased host contamination. Staphylococcus was notably abundant in the umbilical cord samples. This bacterium has been detected in fetal and placental samples from Japanese macaques (Chu et al. 2017), as well as in human amniotic fluid, placental, and cord blood samples (Collado et al. 2016; Jiménez et al. 2005). While prevalent in the human placenta and various fetal organs through clinical culturing (Mishra et al. 2021), some sequence-based studies suggest their presence may be due to background contamination (Glassing et al. 2016).
4.2 Microbial Differences Between Adult RMs and Fetus
Physiological and nutritional changes from the fetal stage to adulthood induce persistent changes in the microbial composition and enrichment of genes associated with carbohydrate metabolism (Koenig et al. 2011; Wardman et al. 2022). In contrast to fetal nutrition, which relies on glucose provided by the mother (Kalhan and Parimi 2000), adult RMs encounter a much more complex environment and diet. Our results showed pronounced differences in microbial compositions and functions between adult RMs and fetuses. Regarding microbial composition, the abundant microbial taxa differed between adult and fetal samples, with adults exhibiting significantly higher microbial richness. In addition, the different bacteria possess carbohydrates targeting distinct polysaccharides for degradation and facilitate their colonization in the gut through the evolution of carbohydrates (Martens et al. 2014; Kaoutari et al. 2013). Consequently, adult RMs may exhibit a more pronounced carbohydrate metabolism than fetuses, as reflected by our results showing the greater diversity and abundance of CAZyme genes detected in the adult microbiome. The dominant CAZymes in the adult and fetal microbiomes were different. In the adult microbiome, GHs were the predominant CAZymes, while in fetuses, both GHs and GTs were prevalent. These enzyme families are primarily responsible for carbohydrate degradation and synthesis, respectively (Comtet-Marre et al. 2018; Kuntothom et al. 2009). GH family genes were significantly enriched in the adult microbiome than fetal microbiome, especially GH1 and GH43, which are associated with hemicellulose and cellulose degradation (Comtet-Marre et al. 2018; Kuntothom et al. 2009) and support the dietary demands of adult RMs to metabolize fiber-intensive foods. In both the adult and fetal microbiomes, GH13 was the dominant GH family. Notably, GH13, a significant family of glycoside hydrolases, is involved in the degradation of glycogen, oligosaccharides, polysaccharides, and starch (Stam et al. 2006).
Maternal antibiotic treatment during pregnancy has long-lasting effects on the fetal microbiota and health (Chen, Chou, and Yang 2021). Maternal ARGs can be transmitted to the fetus and influence the fetal microbiome and resistome profiles to induce resistance (Patangia et al. 2022; Bi et al. 2021). As the fetal microbiota is a critical determinant of early immunity development, overall health, and antibiotic treatment efficacy (Mishra et al. 2021), a comprehensive understanding of fetal ARGs is crucial. In this study, even without direct antibiotic exposure to the fetus, we identified four ARGs in the fetal samples that aligned with those in maternal and adult RMs, suggesting a vertical transmission of ARG-containing microbes during pregnancy. Subsequent examinations of bacteria harboring ARGs, and the impact of specific antibiotics may aid in the development of preventive and treatment strategies for healthy pregnancy outcomes (Klassert et al. 2020).
4.3 Microbial Differences Between Human and RM Fetuses
The microbial components of NHPs tend to resemble those of humans more closely than those of other animals (Nagpal et al. 2018). Our analysis revealed a convergence in certain dominant microbiota between human and RM fetuses. Specifically, Proteobacteria was the most abundant phylum in both human fetal intestines and RM fetal organs. Other research has highlighted the dominance of Proteobacteria in the human neonatal gut during the first week of life (Guaraldi and Salvatori 2012), which facilitates colonization by strict anaerobes and underscores the susceptibility of bacterial communities at this stage (Chow and Lee 2006; Wilson 2005). Proteobacteria constitutes a major community in the human placenta and umbilical cord blood (Aagaard et al. 2014; Miko et al. 2022), with overlaps noted between placental communities and those in amniotic fluid and meconium (Ardissone et al. 2014; Madan et al. 2012). Additionally, the intestinal abundance of Proteobacteria in both macaques and humans significantly increases during pregnancy (Koren et al. 2012; Sun et al. 2020). Based on published studies and the high abundance of Proteobacteria observed in maternal RM in our study, it is reasonable to speculate that, similar to humans (Shin, Whon, and Bae 2015), Proteobacteria in RM fetuses may also be transmitted from the mother in utero. Firmicutes and Bacteroidetes are reported to be the primary phyla in both adult humans and RMs (Arumugam et al. 2011; Chen et al. 2020; Adriansjach et al. 2020), suggesting that, during the transition from fetus to adulthood in both species, Firmicutes and Bacteroidetes displace Proteobacteria as the prevailing bacteria.
Moreover, we found Staphylococcus abundant in both human and RM fetal samples. The bacteria have been reported to induce in vitro activation of memory T cells in fetal mesenteric lymph nodes to play a role in fetal immune priming (Mishra et al. 2021). These observations support the use of macaques as good models for studying human microbial composition and transmission. However, there were evident disparities in the microbiota between RM and human fetuses, which likely stem from significant differences in genetic, physiological, dietary, and environmental factors, among others (Ley et al. 2008; Chen et al. 2018b). Notably, Klebsiella and Enterobacter were abundant in the human fetus, whereas their abundances in the RM fetus were low (Figure 6). Both genera belong to the Enterobacteriaceae family, constituting a major portion of the meconium microbiota and neonatal gut microbiome (Gosalbes et al. 2013; Matsuki et al. 2016). Bacteria in this family offer protective effects by resisting colonization by exogenous pathogens in the human gut, potentially interacting with the immune system (Moreira de Gouveia, Bernalier-Donadille, and Jubelin 2024). Enterobacteriaceae also contributes to gut health by maintaining an anaerobic environment and producing essential vitamins, supporting overall immune function (Moreira de Gouveia, Bernalier-Donadille, and Jubelin 2024). Our study highlights the differences in microbial exposure between human and RM fetuses, which may lead to disparities in fetal immune development between the two primates. Consequently, caution is warranted when extrapolating these findings to human biology due to the distinct microbial profiles observed in RM and human fetuses.
In conclusion, our research reported a detailed case analysis of the microbial composition and function within an RM fetus, meticulously obtained via cesarean section to avoid contamination from the birth canal. By preparing DNA libraries from environmental and blank controls, we confirmed low environmental contamination in our samples, reinforcing the validity of our findings. We observed a diverse array of microorganisms in the womb and fetus. As crucial connections between mother and fetus, the placenta and umbilical cord exhibit a microbial composition that more closely resembles that of the fetus rather than the mother. These observations suggest a potential maternal-fetal microbial transfer, possibly through these structures, as supported by substantial microbial sharing and clustered microbial profiles between the mother and fetus compared to other adult RMs. Importantly, the discovery of seven ARGs in the RM fetus further suggests potential vertical transmission, highlighting the possible critical role these microbes may play in fetal health and immune system development. In terms of the unique fetal microbial profile, our findings reveal substantial differences in microbial composition and functional pathways between the fetus and adults. Notably, the fetus exhibited a less developed capacity for carbohydrate metabolism, characterized by fewer diverse genes and less complex pathways compared to adults. Our findings not only emphasize maternal-fetal microbial transfer but also help in formulating strategies to tackle microbial-induced challenges in fetal health and development. However, it is noteworthy that obtaining placental and fetal samples from NHPs is known to be difficult due to ethical and welfare considerations. Future research, enriched with more microbiome data from macaque fetal samples and innovative methodologies, will help elucidate the impact of fetal microbiota on the developing immune system, thereby advancing our understanding of these complex biological processes.
Author Contributions
Qiao Du: writing–original draft, writing–review and editing, formal analysis, visualization. Xu Liu: writing–review and editing, methodology, formal analysis. Rusong Zhang: writing–review and editing, methodology. Gang Hu: writing–review and editing, data curation, resources. Qinghua Liu: writing–review and editing, data curation, resources. Rui Wang: writing–review and editing, data curation, resources. Wen Ma: visualization. Ying Hu: visualization. Zhenxin Fan: writing–review and editing, project administration. Jing Li: supervision, writing–review and editing, funding acquisition.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32171607).
Conflicts of Interest
Authors Gang Hu, Qinghua Liu and Rui Wang are employed by SCU-SGHB Joint Laboratory on nonhuman Primates Research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the National Genomics Data Center (GSA: CRA014938 and CRA014939) that are accessible at https://ngdc.cncb.ac.cn/gsa.




