What did Hadropithecus eat, and why should paleoanthropologists care?
Abstract
Over 40 years ago, Clifford Jolly noted different ways in which Hadropithecus stenognathus converged in its craniodental anatomy with basal hominins and with geladas. The Malagasy subfossil lemur Hadropithecus departs from its sister taxon, Archaeolemur, in that it displays comparatively large molars, reduced incisors and canines, a shortened rostrum, and thickened mandibular corpus. Its molars, however, look nothing like those of basal hominins; rather, they much more closely resemble molars of grazers such as Theropithecus. A number of tools have been used to interpret these traits, including dental microwear and texture analysis, molar internal and external morphology, and finite element analysis of crania. These tools, however, have failed to provide support for a simple dietary interpretation; whereas there is some consistency in the inferences they support, dietary inferences (e.g., that it was graminivorous, or that it specialized on hard objects) have been downright contradictory. Cranial shape may correlate poorly with diet. But a fundamental question remains unresolved: why do the various cranial and dental convergences exemplified by Hadropithecus, basal hominins, and Theropithecus exist? In this paper we review prior hypotheses regarding the diet of Hadropithecus. We then use stable carbon and nitrogen isotope data to elucidate this species' diet, summarizing earlier stable isotope analyses and presenting new data for lemurs from the central highlands of Madagascar, where Hadropithecus exhibits an isotopic signature strikingly different from that seen in other parts of the island. We offer a dietary explanation for these differences. Hadropithecus likely specialized neither on grasses nor hard objects; its staples were probably the succulent leaves of CAM plants. Nevertheless, aspects of prior hypotheses regarding the ecological significance of its morphology can be supported. Am. J. Primatol. 78:1098–1112, 2016. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Hadropithecus stenognathus, a recently extinct giant lemur (Primates, Archaeolemuridae) from Madagascar, bears remarkable resemblance in its cranial morphology and dental proportions to basal hominins (especially robust australopiths such as Paranthropus boisei and P. robustus) [Ryan et al., 2008] and in its molar occlusal morphology to grazing mammals such as geladas (Theropithecus), capybaras (Hydrochoerus), hippopotamuses (Hippopotamus), and kangaroos (Macropus) (Fig. 1). Like basal hominins, Hadropithecus has a short face, small and orthally implanted upper and lower incisors, small canines, early fusion of the mandibular symphysis, thick mandibular corpus, flaring zygoma, molariform posterior premolars and large and buccolingually expanded molars. Its mandibular ascending ramus is tall so that the temporomandibular joint is raised high above the mandibular occlusal plane. In contrast, its molar occlusal morphology looks nothing like that of a basal hominin. Its relatively tall cusps and crests form complex flat ribbons of enamel that retain sharp edges as they wear. Its enamel is only moderately thick (as in Theropithecus) and its enamel prisms are only weakly decussated [Godfrey et al., 2005]. Understanding how an animal with molars so similar to those of geladas and other grazers might also converge so strongly in cranial architecture, incisor and canine reduction, P4 molarization, and molar hypertrophication with basal hominins is clearly of paleoanthropological significance.
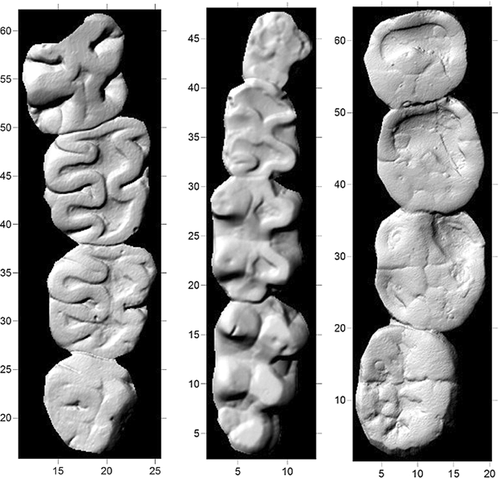
At least four hypotheses have been proposed to account for the morphological convergences of Hadropithecus with hominins, geladas, or both. Most have focused on diet, some more on the metric properties of food items, and others more on their material properties.
- H1: Hadropithecus was graminivorous. Shortly after Hadropithecus was first described [Lorenz von Liburnau, 1899], Forsyth-Major [1900] concluded on the basis of its molar morphology that it was a grazer. In his monograph of this extinct lemur, Lamberton [1938] drew the same conclusion, citing similarities in molar morphology to the molars of hippos, while also describing postcranial similarities to monkeys, and cranial similarities to fossil and living humans.
- H2: Hadropithecus was a small-object feeder. Jolly [1970] embraced the notion that Hadropithecus ate grasses, but he proposed a somewhat broader concept to simultaneously explain this animal's dental convergence with grazers such as Theropithecus gelada and cranial convergences with basal hominins [see also Tattersall, 1973]. In effect, Jolly identified an adaptive complex involving extreme terrestrialism and a diet of small, tough food objects, which, he argued, could account for the dental and cranial differences between Hadropithecus and its sister taxon, Archaeolemur, just as it could account for parallel differences between Theropithecus and Papio or Mandrillus, and between Australopithecus and Pan. Small food items, he argued, do not require heavy incisal preparation but may require heavy repetitive mastication. Specifically for Hadropithecus, Jolly [1970] argued, “By analogy with living forms, it seems likely that Archaeolemur fed mainly upon relatively large food-items, probably mostly fruit, requiring incisal preparation, while the diet of Hadropithecus was centred upon the stems, rhizomes, and probably also seeds of grasses, which, being a primate and lacking front teeth adapted for grazing, it presumably picked up by hand” (p. 622).
- H3: Hadropithecus was a hard-object feeder. Studies of dental microwear using scanning electron microscopy [Rafferty et al., 2002] and low magnification [Godfrey et al., 2004], as well as studies of microwear “texture” using confocal microscopy [Scott et al., 2009], all supported hard-object feeding for Hadropithecus. Stress-limited (hard) foods (such as nuts or certain seeds) can be large or small; they are simply foods that fail under the high loads required to fracture them. While the enamel of Hadropithecus was quite a bit thinner than that of its sister taxon, Archaeolemur (and thinner than fossil hominins) [Godfrey et al., 2005], its relative enamel thickness overlapped that of orangutans; thus, hard-object feeding could not be ruled out on this basis. Baab et al. [2014] found weak support for the hypothesis that diet accounts for variation in skull form in subfossil and living lemurs; however, they found that diet does a better job of accounting for such variation when Hadropithecus is scored as a hard object feeder than when this species is scored as a consumer of small, tough objects.
- H4: Hadropithecus was a bulk-food feeder, consuming large quantities of foods high in structural carbohydrates and poor in nutrients, requiring heavy repetitive trituration. In some ways, this hypothesis is similar to Jolly's [1970] except that it does not specify a preference for grass leaves, rhizomes, or seeds of grasses. The notion that Hadropithecus was, after all, a consumer of small, tough foods (that are displacement-limited rather than stress-limited) was championed by Dumont et al. [2011] on the basis of a finite element analysis (FEA) of the skulls of Hadropithecus and Archaeolemur, and by Godfrey et al. [2012] on the basis of Hadropithecus’ dental “complexity” (orientation patch count rotated, OPCR) and Dirichlet Normal Energy (DNE, a measure similar to occlusal relief). The only other lemur with similar values for OPCR and DNE is Prolemur simus, the greater bamboo lemur. By manipulating the scanned models of the skulls and jaws of Hadropithecus and Archaeolemur, Dumont et al. [2011] demonstrated that the former had a significantly smaller maximum gape. FEA demonstrated less structural strength in the skull of Hadropithecus than in Archaeolemur under specified loading conditions (P4 biting at maximum gape, M2 biting at maximum gape), whether or not scaled to equal body size. On the other hand, Hadropithecus had greater mechanical advantage in converting muscle force to bite force (especially for its molars). Dumont et al. [2011] therefore interpreted the craniofacial and dental features of Hadropithecus as adaptations for withstanding repetitive loads. They also interpreted the “hard-object” signal found in microwear and enamel surface texture analysis as possibly related instead to high grit in open environments. Constantino et al. [2012] effectively supported this idea. They estimated the maximum bite forces (critical failure loads) of Hadropithecus, Archaeolemur and other subfossil lemurs using dental fracture mechanics (taking into consideration tooth size and absolute enamel thickness). They found maximum bite force in Hadropithecus to be comparable to that of modern Homo sapiens, slightly lower than that of the largest-bodied Archaeolemur (A. edwardsi), and considerably lower than those of basal hominins (especially Paranthropus). They concluded on this basis that Hadropithecus was not a hard-object processor.
Testing the above hypotheses requires understanding more precisely what Hadropithecus ate. Stable isotope analysis can help us do exactly this. On the basis of its high stable carbon (δ13C) and nitrogen (δ15N) isotope values, Crowley & Godfrey [2013] posited a diet for Hadropithecus stenognathus rich in Didiereoideae (a subfamily of spiny succulent plants that is endemic to Madagascar). In southwestern Madagascar, these succulent plants, which rely on Crassulacean Acid Metabolism (CAM), have high δ13C values [Crowley & Godfrey, 2013; Kluge et al., 1991; 1995]. Additionally, Didiereoideae consumption fits the isotopic signal of Hadropithecus better than C4 plants in southern Madagascar. Whereas Hadropithecus and Alluaudia (a genus of Didiereoideae) both have high δ15N values, grasses have low δ15N values [Crowley & Godfrey, 2013]. However, in the Central Highlands of Madagascar (hereafter CH) where Didiereoideae do not exist, isotope values for Hadropithecus are strikingly different from those from sites in the dry south—the Spiny Thicket (ST) and Succulent Woodland (SW) ecoregions.
Here we expand our sample of isotope data for giant lemurs and other subfossil species from the CH, and we present new comparative analyses. We do so with two primary objectives. First, we seek to better understand how Hadropithecus differs from other species in its stable isotope values within and across ecoregions. We focus particularly on how individuals from the CH differ from individuals in the ST and SW. We then pool our samples for each genus across ecoregions, and compare Hadropithecus to (1) other extinct lemurs, and (2) extinct hippopotamuses and elephant birds. To better understand isotopic variability, we also compare Hadropithecus to Microcebus (mouse lemurs), which is a well-studied extant lemur genus with a large available stable isotope database [Crowley et al., 2011b, 2013, 2014]. Our second primary objective is to explain geographic variation in the δ13C values of Hadropithecus. We expect δ13C values in bone to be influenced by diet, and thus to be strongly correlated with the isotope values of consumed plants; if this is the case, we can use the pattern of correlation between plant and Hadropithecus δ13C values to develop a list of candidate plants for consumption by this species. We also expect plant carbon isotope values to be influenced by soil salinity, light, moisture, and temperature [Amundson et al., 2003; Crowley et al., 2011b; van der Merwe & Medina, 1991], all of which may be correlated with elevation and latitude. Our goal is to determine which environmental variables best explain geographical variation in the carbon isotope values for Hadropithecus, and to identify candidate food plants that are (1) eaten by living lemurs, and (2) have δ13C values that vary in a manner that is consistent with geographic isotopic variation for Hadropithecus.
METHODS
We compiled previously published collagen δ13C and δ15N isotope data for bones from giant lemurs and hippopotamuses, and collagen δ13C values for bones and eggshells from elephant birds from the ST and SW ecoregions [Berger et al., 1975; Burney, 1999; Burney et al., 2004; Clarke et al., 2006; Crowley et al., 2012; Crowley & Godfrey, 2013; MacPhee, 1986]. To these data we added new isotope data from subfossil lemurs, hippopotamuses (Hippopotamidae), and elephant birds (Aepyornithidae), concentrating on specimens from the CH (Table I). We also assembled published stable isotope data for extant Microcebus fur from the ST, SW, and CH [Crowley et al., 2011b, 2013, 2014].
| Ecoregion | Site name | Type | Genus and species | Specimen ID | Element | δ13C (‰) | δ15N (‰) | Atomic C:N |
|---|---|---|---|---|---|---|---|---|
| CH | Ampasambazimba | Lemur | Archaeolemur edwardsi | UA 1177 | Ulna | −22.4 | 8.2 | 3.3 |
| CH | Ampasambazimba | Lemur | Archaeolemur edwardsi | UA 1203 | Radius | −22.8 | 6.8 | 3.3 |
| CH | Ampasambazimba | Lemur | Archaeolemur edwardsi | UA 1153 | Femur | −22.9 | 6.2 | 3.4 |
| CH | Ampasambazimba | Lemur | Archaeolemur edwardsi | UA 1204 | Ulna | −15.5 | 6.2 | 3.5 |
| CH | Ampasambazimba | Lemur | Archaeolemur edwardsi | UA 1159 | Humerus | −21.5 | 7.2 | 3.3 |
| CH | Ampasambazimba | Lemur | Archaeolemur edwardsi | UA 1158 | Humerus | −20.8 | 7.5 | 3.3 |
| CH | Ampasambazimba | Lemur | Hadropithecus stenognathus | UA Uncat. | Tibia | −27.1 | 2.7 | 4.6 |
| CH | Ampasambazimba | Lemur | Hadropithecus stenognathus | UA 5164 | Femur | −21.3 | 7.8 | 3.4 |
| CH | Ampasambazimba | Lemur | Megaladapis grandidieri | UA 3984 | Ulna | −22.7 | 8.3 | 3.3 |
| CH | Ampasambazimba | Lemur | Megaladapis grandidieri | UA 8688 | femur | −21.7 | 8.0 | 3.2 |
| CH | Ampasambazimba | Lemur | Megaladapis grandidieri | UA7914 | Humerus | −21.8 | 8.7 | 3.3 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1667 | Humerus | −22.8 | 8.4 | 3.3 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1708 | Humerus | −22.6 | 4.9 | 3.2 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 2933 | Femur | −21.7 | 9.2 | 3.2 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1733 | Humerus | −23.0 | 7.3 | 3.2 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1709 | Humerus | −22.8 | 9.0 | 3.3 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1772 | Femur | −22.1 | 9.3 | 3.3 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1780 | Femur | −23.7 | 9.4 | 3.4 |
| CH | Ampasambazimba | Lemur | Pachylemur jullyi | UA 1675 | Femur | −22.0 | 6.1 | 3.4 |
| CH | Ampasambazimba | Lemur | Palaeopropithecus maximus | UA 3833 | Tibia | −22.3 | 9.3 | 4.2 |
| CH | Ampasambazimba | Lemur | Palaeopropithecus maximus | UA 3823 | Femur | −21.6 | 8.6 | 3.7 |
| CH | Ampasambazimba | Lemur | Palaeopropithecus maximus | UA 4444 | Mandible | −21.9 | 9.2 | 3.4 |
| CH | Ampasambazimba | Lemur | Palaeopropithecus maximus | UA 3825 | Femur | −22.1 | 9.4 | 3.3 |
| CH | Christmas river (Ilakaka) | Lemur | Archaeolemur majori | DPC 24153a | Humerus | −20.2 | 11.4 | 3.2 |
| CH | Christmas river (Ilakaka) | Lemur | Pachylemur insignis | DPC 24156 | Femur | −21.2 | 12.5 | 3.2 |
| CH | Antsirabe | Hippopotamus | Hexaprotodon guldbergi | NHMW Uncat. | Rib | −25.0 | 3.0 | 3.3 |
| CH | Antsirabe | Hippopotamus | Hexaprotodon guldbergi | NHMW Uncat. | Rib | −24.9 | 1.9 | 3.5 |
| CH | Antsirabe | Hippopotamus | Hexaprotodon guldbergi | NHMW Uncat. | Rib | −25.9 | 6.5 | 3.2 |
| CH | Antsirabe | Hippopotamus | Hexaprotodon guldbergi | NHMW Uncat. | Rib | −23.7 | 1.3 | 4.2 |
| CH | Antsirabe | Hippopotamus | Hexaprotodon guldbergi | NHMW Uncat. | Rib | −25.5 | 2.9 | 3.5 |
| CH | Masinandraina | Elephant bird | Aepyornis sp. | UUE–MAS-5 | Tibiotarsus | −14.0a | 6.7 | 3.4 |
| ST | Ankilitelo | Lemur | Archaeolemur majori | DPC 22008 | Innominate | −22.2 | 6.1 | 4.3 |
| ST | Ampehabe cave #12, West Mikoboka plateau | Lemur | Palaeopropithecus ingens | DPC 24136 | Femur | −20.8 | 10.7 | 3.2 |
| ST | Taolambiby | Hippopotamus | Hippopotamus lemerlei | UMASS Uncat. | Phalanx | −22.7 |
- CH, Central highlands, ST, Spiny thicket.
- Museum codes for specimen numbers: UA, University of Antananarivo; UMASS, University of Massachusetts, Amherst; DPC, Duke Primate Center (Division of Fossil Primates), NHMW, Naturhistoriches Museum Wien; UUE, Uppsala University Evolutionsmuseet.
- a Carbon isotope value for this specimen was previously published [Burney, 1999]. Nitrogen isotope data are new.
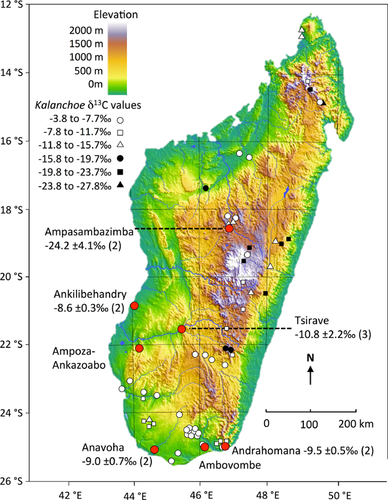
We included in our study all extinct lemur genera represented by 10 or more individuals as well as other extinct Madagascan megafauna with comparable samples. Our final dataset comprises δ13C and associated δ15N values (when available) for Hadropithecus (n = 11), Archaeolemur (n = 31), Megaladapis (n = 25), Pachylemur (n = 27), Palaeopropithecus (n = 35), hippopotamuses (n = 20), elephant birds (n = 10), and Microcebus (n = 463). We excluded Mesopropithecus spp., Daubentonia robusta, and Archaeoindris fontoynontii because of small sample sizes. Our Hadropithecus sample comes from five subfossil sites: Ampasambazimba (CH), Ankilibehandry (SW), Tsirave (SW), Anavoha (ST), and Andrahomana (ST) (see Fig. 2). Because the isotope data for Microcebus were collected from fur samples, we converted the fur values to look like subfossil collagen before comparing them to values of subfossil lemurs by (1) adding +0.9‰ to carbon and +0.8‰ to nitrogen isotope values (to allow for the average apparent enrichment between these two tissues in primates [Crowley et al., 2010]); and then (2) adding an additional +1.2‰ to fur carbon isotope values (to allow for the isotopic changes in atmospheric CO2 resulting from the burning of fossil fuels in the Southern Hemisphere [Keeling et al., 2010]).
New subfossil isotope data (Table I) were generated following Crowley et al. [2011a]. We isolated collagen from the subfossil bone samples by decalcifying approximately 200 mg of each fragmented sample in 0.5 M EDTA for 10 days at 4 °C. We replaced the EDTA, sonicated the samples, and allowed them to sit an additional 10+ days at room temperature. We then rinsed the samples ten times using ultrapure water. We gelatinized the samples in 0.01 N HCl at 70 °C for 15 hr, filtered them using Whatman 1.5 μm glass fiber filters, and dried them under vacuum. Following sample treatment, we weighed 0.7 mg of collagenous residue into tin boats, combusted them, and analyzed their δ13C and δ15N values on a ThermoElectron (Finnigan) Delta-XP continuous flow system connected to an Elemental Analyzer at the University of California, Santa Cruz Stable Isotope Laboratory. Collagen preservation was evaluated using sample yield, isotope values, and elemental ratios [Ambrose, 1990].
We had previously compiled from the literature and from Google Earth a database that included latitude, longitude, and elevation for subfossil sites in Madagascar [Muldoon & Godfrey, 2013]. Using these data, we were able to extract estimated monthly site-specific rainfall and temperature data for the past 100 years from the online WorldClim database [Hijmans et al., 2005]. We selected January and July values for temperature and rainfall to represent austral summer and winter extremes, respectively. We also coded each site for soil salinity on the basis of its proximity to the coast (coastal sites are more saline than inland sites) using a simple binary code (coastal vs. inland). Finally, we compiled δ13C values from the literature for both C3 and CAM plants from localities in the vicinity of Hadropithecus subfossil sites (Table II).
| Site | Type | n | Mean Plant δ13C ± 1 SD (Min, Max) (‰) | Mean δ13C in subfossil lemur space (‰)a | Source |
|---|---|---|---|---|---|
| Tsinjoarivo continuous forest (Vatateza) | C3 | 61 | −29.1 ± 1.8 (−33.0, −24.2) | −22.9 | Crowley et al. [2013] |
| Central highlands | CAMb | 27 | −19.1 ± 5.4(−11.4, −27.3) | −12.9 | Kluge et al. [1991] |
| Kirindy forest | C3 | − | −29.1 ± 1.4c | −22.9 | Dammhahn [2008] |
| Dry deciduous forest and succulent woodland | CAMb | 7 | −14.5 ± 4.8(−11.2, −25.0) | −8.3 | Kluge et al. [1991] |
| Tsimanampetsotsa | C3 | 33 | −28.1d | −21.9 | Loudon et al. [2008] |
| Tsimanampetsotsa | CAM | 4 | −14.9d | −8.7 | Loudon et al. [2008] |
| Beza Mahafaly (Parcel 2) | C3 | 54 | −26.5 ± 2.0(−30.8, −22.8) | −20.3 | Crowley et al. [2011b] |
| Beza Mahafaly (Parcel 2) | CAM | 50 | −14.9 ± 1.3(−18.3, −11.3) | −8.7 | Crowley et al. [2011b] |
| Cap Sainte-Marie | C3 | 55 | −27.4 ± 1.5 (−31.0, −24.9) | −21.2 | Previously unpublished |
| Cap Sainte-Marie | CAM | 9 | −13.4 ± 0.5 (−14.2, −12.5) | −7.2 | Previously unpublished |
- a Mean δ13C values for plants have been corrected by +6.2‰ to account for fractionation between collagen and plants (5‰) and changes in atmospheric CO2.
- b CAM data from Kluge et al. [1991] are derived entirely from Kalanchoë spp.
- c Only summary data presented in graphical form. Values estimated.
- d Only mean isotope values provided.
Plant isotope data were derived from multiple plant parts (leaves, fruits, and seeds). All plant δ13C values were adjusted for fractionation between herbivore collagen and diet (+5‰) [Ambrose and Norr, 1993; Crowley et al., 2011b; Kellner & Schoeninger, 2007; Koch et al., 1991; Sullivan & Krueger, 1981; van der Merwe, 1989; Vogel, 1978] and for changes in atmospheric CO2 (+1.2‰) [Keeling et al., 2010]. Geographic distributions of candidate plants that may have been consumed by Hadropithecus were assessed for overlap with Hadropithecus sites using specimen geographic searches in www.tropicos.org.
To analyze data, we applied standard statistical tests (t-tests and ANOVAs with post hoc tests of honestly significant differences) using SPSS 22. We used correlation and regression analysis to examine the relationships between Hadropithecus bone δ13C values and the following characteristics of the specimen's geographic location: latitude, longitude, elevation, proximity to the coast, mean January and mean July rainfall and temperature, and regional δ13C values for C3 and CAM plants.
This research adhered to the American Society of Primatologists' principles for the ethical treatment of primates.
RESULTS
Table III compares δ13C and δ15N values among giant lemur taxa living in different ecoregions. We pooled isotope values for the SW and the ST because they are not significantly different; thus, we compare values for the CH to a larger sample from arid and semi-arid habitats. Both δ13C and δ15N values are lower in the CH than in the SW/ST, almost always significantly so. This applies to individual genera, taken alone, and to a pooled sample of all giant lemurs.
| Ecoregion | Isotope | n | Hadropithecus | Archaeolemur | Megaladapis | Pachylemur | Palaeopropithecus |
|---|---|---|---|---|---|---|---|
| Central highlands | δ13C | 25 | −24.2 ± 4.1 | −20.9 ± 2.6 | −22.1 ± 0.6 | −22.4 ± 0.8 | −22.0 ± 0.3 |
| SW/ST | δ13C | 104 | −9.6 ± 1.5 | −19.8 ± 1.4 | −20.6 ± 1.0 | −21.0 ± 1.4 | −20.5 ± 0.7 |
| t-tests comparing δ13C values in CH vs. SW/ST for each genus | t = −9.6, df = 9, P < 0.001 | t = −1.5, df = 29, NS | t = −2.4, df = 23, P = 0.023 | t = −2.9, df = 25, P = 0.008 | t = −4.0, df = 33, P < 0.001 | ||
| Central highlands | δ15N | 25 | 5.3 ± 3.6 | 7.6 ± 1.8 | 8.3 ± 0.4 | 8.5 ± 2.2 | 9.1 ± 0.4 |
| SW/ST | δ15N | 89 | 13.8 ± 3.2 | 11.1 ± 2.2 | 11.4 ± 1.7 | 11.5 ± 1.9 | 13.4 ± 2.0 |
| t-tests comparing δ15N values in CH vs. SW/ST for each genus | t = −3.2, df = 7, P = 0.01 | t = −3.7, df = 25, P = 0.001 | t = −3.0, df = 18, P = 0.008 | t = −3.6, df = 23, P = 0.001 | t = −4.2, df = 31, P < 0.001 | ||
| Summary data and t-test comparing δ13C values in CH vs. SW/ST for all genera | Mean for CH = −22.0‰, mean for SW/ST = −19.5‰, t = −3.7, df = 127, P < 0.001 | ||||||
| Summary data and t-test comparing δ15N values in CH vs. SW/ST for all genera | Mean for CH = 8.1‰, mean for SW/ST = 12.2‰, t = −8.1, df = 112, P < 0.001 | ||||||
| ANOVA comparing δ13C values among genera within the CH | F = 1.584, df = 4.20, NS | ||||||
| ANOVA comparing δ15N values among genera within the CH | F = 1.575, df = 4.20, NS | ||||||
| ANOVA comparing δ13C values among genera within the SW/ST | F = 179.607, df = 4.99, P < 0.001a | ||||||
| ANOVA comparing δ15N values among genera within the SW/ST | F = 6.040, df = 4.84, P < 0.001b | ||||||
- t-tests compare isotope data (Means ± SD) between ecoregions and ANOVAs compare isotope data among groups within each ecoregion.
- a Hadropithecus is significantly different from all other genera.
- b Hadropithecus is significantly different from all other genera except Palaeopropithecus.
Looking only at extinct lemurs from the CH, neither average δ13C values nor average δ15N values differ significantly among genera (Table III). Hadropithecus lies at the low end of the spectra for both δ13C and δ15N. In contrast, in the SW/ST, there are strongly significant differences in both δ13C values and δ15N values among lemur genera, and Hadropithecus has the highest values for both isotopes. Its δ13C values differ significantly from those of each of the other giant lemurs, and its δ15N values differ significantly from all other lemurs except Palaeopropithecus (Table III). The variance for δ13C values for Hadropithecus is high in the CH (standard deviation, or SD = 4.1‰) but unexceptional in the SW/ST (SD = 1.5‰).
Table IV compares carbon and nitrogen isotope values for lemur genera without regard to ecoregion. ANOVAs demonstrate significant differences among genera for both δ13C and δ15N values. Once again, Hadropithecus is unusual not merely for its high mean δ13C value, but also for its strikingly high δ13C variance (SD = 6.2‰). This exceptionally high variance is a consequence of dramatic inter-regional differences, coupled with high variance within the CH itself. Variance for δ15N is also high for Hadropithecus (SD = 4.8‰), though to a lesser degree.
| Genus | n | δ13C mean ± SD (‰)a | n | δ15N mean ± SD (‰)b |
|---|---|---|---|---|
| Hadropithecus | 11 | −12.3 ± 6.2 | 9 | 11.9 ± 4.8 |
| Archaeolemur | 31 | −20.0 ± 1.8 | 27 | 10.2 ± 2.6 |
| Megaladapis | 25 | −20.8 ± 1.1 | 20 | 10.9 ± 1.9 |
| Pachylemur | 27 | −21.5 ± 1.4 | 25 | 10.4 ± 2.4 |
| Palaeopropithecus | 35 | −20.7 ± 0.8 | 33 | 12.9 ± 2.4 |
| ANOVA | 128 | F = 40.13, df = 4, 124, P < 0.001 | 113 | F = 5.20, df = 4, 109, P = 0.001 |
- Summary data for each group are derived from three ecoregions (ST, SW, and CH).
- a Hadropithecus is significantly different from all other genera; there are no significant differences among other genera.
- b Hadropithecus does not differ significantly from any other genus; Palaeopropithecus differs significantly from Archaeolemur and Pachylemur.
Isotopic variance is exceptional in Hadropithecus despite the fact that our samples for each of the other giant lemur genera comprise multiple recognized species, while only one species is recognized for Hadropithecus. Data derived from a well-studied extant lemur genus, Microcebus, underscore the unusual nature of isotopic variation in Hadropithecus. In contrast to Hadropithecus, standard deviations of mouse lemur δ13C and δ15N from the CH, ST, and SW are much closer to those of other extinct lemurs (1.5‰ and 2.3‰ respectively).
Table V compares Hadropithecus to hippopotamuses and elephant birds from the ST, SW and CH. Mean δ13C and δ15N values for Hadropithecus are significantly higher than for either of the other two taxa. Elevated δ13C values for Hadropithecus suggest that this species' diet differed not merely from those of other giant lemurs but also from those of hippopotamuses and elephant birds. Higher δ15N values for Hadropithecus suggest that this lemur tended to occupy drier habitats than hippopotamuses (i.e., feeding further from bodies of water); nitrogen isotope data for elephant birds are insufficient to allow any conclusions about the habitats they occupied.
| Genus | n | δ13C mean ± SD (‰)a | n | δ15N mean ± SD (‰)b |
|---|---|---|---|---|
| Hadropithecus | 11 | −12.3 ± 6.2 | 9 | 11.9 ± 4.8 |
| Hippopotami | 20 | −21.3 ± 2.8 | 19 | 7.5 ± 3.2 |
| Elephant birds | 10 | −20.4 ± 5.0 | 1 | 6.7 |
| ANOVA | 40 | F = 15.613, df = 2, 38, P < 0.001 | 28 | F = 4.275, df = 2. 26, P = 0.025 |
- Summary data for each group are derived from three ecoregions.
- a Hadropithecus is significantly different from hippopotami and from elephant birds.
- b Hadropithecus is significantly different from hippopotami (sample size is too small to compare Hadropithecus to elephant birds).
Combined, our results suggest that Hadropithecus had a highly unusual diet, and that in the CH it consumed plants that differed dramatically in their isotopic composition from those eaten in the ST or SW. Its isotopic signal can be summarized simply: Hadropithecus displays elevated δ13C and δ15N in arid areas (but not in the much wetter CH) and highly variable isotope values in the CH.
Several (but not all) of the environmental variables that we compiled to explore factors potentially influencing this pattern of variation (i.e., latitude, longitude, elevation, proximity to the coast, January and July mean rainfall and temperature, and regional δ13C values for C3 and CAM plants) are themselves strongly collinear. Across Hadropithecus sites, localities at higher elevation are also significantly closer to the equator. Rainfall is highest during the rainy season (in January, austral summer) and at high elevations. Temperature also changes with season, but is not significantly correlated with elevation, likely because Hadropithecus sites that are closer to the equator are also higher in elevation. Temperature is significantly correlated with longitude (warmer on the west coast than further east, in the CH), both for January and July. The δ13C values of CAM plants are negatively correlated with elevation (CAM plants are more enriched in 13C at low elevations); there is no relationship between δ13C values of C3 plants and elevation.
Two of our environmental variables are excellent predictors of δ13C values for Hadropithecus bone: elevation (r = −0.957, P < 0.001, n = 11) and δ13C values for CAM plants (r = 0.923, P < 0.001, n = 11). Two additional variables are significantly (but more weakly) correlated with δ13C values for Hadropithecus bone: January rainfall (r = −0.733, P = 0.01, n = 11) and latitude (r = −0.668, P = 0.025, n = 11). No other variables, including δ13C values for C3 plants, are significantly correlated with δ13C values for this extinct lemur. The regression with the highest adjusted R2 value (0.90) uses elevation and δ13C values for CAM plants to predict δ13C values for Hadropithecus bone. Its multiple R value is 0.961.
CAM plants that were consumed by Hadropithecus should (1) have δ13C values that vary in a manner consistent with geographic isotopic variation for Hadropithecus, (2) exist in the regions formerly occupied by Hadropithecus, and (3) be eaten, albeit perhaps in small quantities, by living lemurs. Table VI lists plant genera that appear to satisfy the first two criteria. Figure 1 demonstrates how dominant consumption of facultative CAM plants such as Kalanchoë spp. could account for the isotopic variation exhibited by Hadropithecus, even in the CH. The δ13C values for Kalanchoë in the CH show tremendous variation. Table VII provides observations that support the third criterion. In most cases, the parts eaten are leaves.
| Genus | Family | Plant δ13C values (‰) | Sources for isotope data | Distribution |
|---|---|---|---|---|
| Aloe | Xanthorrhoeaceae | −12.9 to −14.2 (N = 4) | Unpublished dataa, Cap Sainte-Marie, Spiny thicket | Common in the Spiny thicket and central highlands. Rarer in the succulent woodland |
| Alluaudia | Didiereaceae | −12.7 to −16.0(N = 9) | Kluge et al. [1995] Parc National, Berenty, spiny thicket | Common in the spiny thicket. Rarer in the succulent woodland. Only present in an isolated portion of southern central highlands |
| −12.0 to −15.3 (N = 10) | Winter [1979] Near Fort Dauphin | |||
| −13.1 to −17.1 (N = 12) | Crowley et al. [2011b] BMSR July 2007 (dry season, spiny thicket) | |||
| −14.0 to −15.0 (N = 5) | Crowley & Godfrey [2013] BMSR January 2009 (wet season, spiny thicket) | |||
| Cissus | Vitaceae | −15.4 (N = 1) | Kluge et al. [1995] Parc National Berenty, spiny thicket | Present throughout spiny thicket and central highlands. Only a few localities in the succulent woodland |
| −15.0 (N = 1) | Ziegler et al. [1981] Saudi Arabia | |||
| Cynanchum | Apocynaceae | −14.5, −14.7, −26.6 (3 species, N = 1 each) | Kluge et al. [1995] Parc National Berenty, spiny thicket | Abundant throughout the spiny thicket and the central highlands. Rare in the succulent woodland |
| −13.7 to −15.7 (N = 4) | Crowley et al. [2011b] BMSR July 2007 | |||
| Didierea | Didiereaceae | −20.1 (N = 1) | Kluge et al. [1995] Parc National Berenty, spiny thicket | |
| −16.1 to -17.7 (N = 2) | Winter [1979] Near Fort Dauphin | |||
| Euphorbia | Euphorbiaceae | −13.0 to −17.0 (N = 7) | Kluge et al. [1995] Parc National Berenty, spiny thicket | Present throughout the spiny thicket, the eastern central highlands, and southern succulent woodland |
| −12.6 to −17.8(N = 5) | Winter [1979] Near Fort Dauphin | |||
| −12.3 to −17 (N = 45) | Crowley et al. [2011b] BMSR July 2007 | |||
| −13.8 to −16.3 (N = 10) | Crowley & Godfrey [2013] BMSR January 2009 | |||
| Kalanchoë | Crassulaceae | −12.8 to −14.2 (N = 9) | Kluge et al. [1995] Parc National Berenty, spiny thicket | Present throughout the spiny thicket, central highlands, and central succulent woodland |
| −20.2 to −27 (N = 2) | Kluge et al. [1995] Parc Analamazoatra, humid forest, central highlands | |||
| −15.9 to −22.5 (N = 2) | Kluge et al. [1995] Station forestière Manankazo, Central Highlands | |||
| −10.2 to −13.5 (N = 2) | Winter [1979] Near Fort Dauphin | |||
| −13.8 (N = 1) | Unpublished dataa, Cap Sainte-Marie, Spiny Thicket | |||
| Seyrigia | Cucurbitaceae | −16.8 to −17.3 (N = 2) | Crowley et al. [2011b] BMSR July 2007 | Present throughout the spiny thicket. Rare in the southern central highlands. Absent from the succulent woodland? |
| Xerosicyos | Cucurbitaceae | −13.5 to −16.0 (N = 4) | Kluge et al. [1995] Parc National Berenty, spiny thicket | Present throughout the spiny thicket. Rare in the succulent woodlands and the southern central highlands |
| −15.0 (N = 1) | Winter [1979] Near Fort Dauphin | |||
| −11.3 to −14.2 (N = 4) | Crowley et al. [2011b] BMSR July 2007 | |||
| −14.7 to −18.3 (N = 5) | Crowley & Godfrey [2013] BMSR January 2009 |
- BMSR, Beza Mahafaly Special Reserve.
- a Previously unpublished data.
| Genus | Extant lemur consumers | Sources for lemur consumer data |
|---|---|---|
| Aloe | Lemur catta | Yamashita [1996]; Goodman & Langrand [1996]; Simmen et al. [2003]; Soma [2006]; Gemmill & Gould [2008]; Gould et al. [2009]; Ellwanger & Gould [2011]; Kelley [2011]; LaFleur [2012] |
| Alluaudia | Lemur catta | Simmen et al. [2003, 2006a]; Gould et al. [2009, 2011]; Kelley [2011]; LaFleur [2012] |
| Lepilemur leucopus | Charles-Dominique & Hladik [1971]; Hladik & Charles-Dominique [1974]; Russell [1977] | |
| Propithecus verreauxi | Richard [1977] | |
| Didierea | Lemur catta | Simmen et al. [2003, 2006a]; LaFleur [2012] |
| Cissus | Lemur catta | Sussman [1977] |
| Propithecus verreauxi | Simmen et al. [2003] | |
| Cynanchum | Lemur catta | Sauther [1992]; Simmen et al. [2003]; Kelley [2011] |
| Lepilemur leucopus | Russell [1977] | |
| Microcebus griseorufus | Rasoazanabary [2011] | |
| Propithecus verreauxi | Charrier et al. [2007] | |
| Euphorbia | Lemur catta | Simmen et al. [2003, 2006b]; Ellwanger & Gould [2011]; LaFleur [2012] |
| Lepilemur leucopus | Russell [1977] | |
| Lepilemur petteri | Nash [1998] | |
| Microcebus griseorufus | Rasoazanabary [2011] | |
| Microcebus murinus | Martin [1973] | |
| Propithecus verreauxi | Jolly [1966]; Richard [1974, 1977, 1978]; Yamashita [1996, 2002]; Simmen et al. [2003] | |
| Kalanchoë | Lemur catta | Rakotoarisoa [1999]; Gould et al. [2003]; Simmen et al. [2003, 2006b]; Kelley [2011] |
| Seyrigia | Lemur catta | Gould et al. [2009, 2011] |
| Propithecus verreauxi | Charrier et al. [2007] | |
| Xerosicyos | Lemur catta | Pinkus et al. [2006]; Gould et al. [2009]; Kelley [2011] |
| Lepilemur leucopus | Charles-Dominique & Hladik [1971]; Hladik & Charles-Dominique [1974] | |
| Microcebus griseorufus | Rasoazanabary [2011] | |
| Propithecus verreauxi | Yamashita [1996]; Simmen et al. [2003] |
DISCUSSION
Isotope data provide strong support for the inference that Hadropithecus specialized on CAM (and not C3) plants. The difference between CAM and C3 plants in accounting for variation in the carbon isotope signature of Hadropithecus is impressive, and comprises strong evidence that Hadropithecus preferred CAM plants, even in the CH. CAM plants typically have elevated δ13C values in arid regions and can exhibit highly variable δ13C values in humid areas [Kluge et al., 1991, 1995]. The variability of δ13C values of Hadropithecus is truly unusual, not merely in comparison to other fossil lemurs, but in comparison to a very well measured living lemur, Microcebus. No other lemur, living or extinct, shows the combination of exceptionally high δ13C values in arid regions and extreme variability of δ13C values in humid regions, as is consistent with dedicated CAM plant consumption.
While our stable isotope values for Hadropithecus do not reveal plant part or parts eaten, there is good reason to believe that the succulent leaves of CAM plants were the dietary staples for Hadropithecus in all ecoregions. Leaves account for the vast majority of CAM consumption by extant lemurs (Table VII). Endemic CAM plants of Madagascar tend to use wind or water to disperse their seeds. Alluaudia and Kalanchoë are prime examples of this, with very tiny seeds adapted for wind dispersal and not animal consumption. Some endemic CAM plants, including all members of the Didiereoidea, have spines to protect their leaves, which strongly suggests that these plants are (or were) subjected to significant leaf predation.
There are also reasons to exclude a predominantly C4 diet for Hadropithecus. Unlike C4 plants, succulent CAM plants are abundant in the southern parts of the island and can provide food and water during the prolonged dry season, when grass leaves become desiccated and of little nutritional value. In arid places where Hadropithecus was abundant, isotope values in C4 plants are less affected by aridity than are C3 or CAM plants. Consequently, C4 plants typically have lower δ15N values than C3 or CAM plants in arid settings, including southern Madagascar [Crowley & Godfrey, 2013; Koch et al., 1991; Swap et al., 2004]. Therefore, grass consumption cannot explain the very high δ15N values for Hadropithecus in the arid south.
Minimal reliance by other endemic taxa on C4 plants in Madagascar is also supported by their δ13C values. Neither Madagascan hippopotamuses nor elephant birds have isotope values indicative of a diet rich in C4 grasses [Clarke et al., 2006; Crowley & Godfrey, 2013]. Bond & Silander [2007] note the existence (albeit scant) of enriched 13C for some hippopotamuses reported by Burney et al. [2004]. However, the only hippo δ13C values that match dedicated C4 grass consumption derive from a single individual Hippopotamus whose skull and mandible may have been brought from continental Africa to the Académie Malgache in the 1800s for comparative analysis.
A final argument against C4 grass consumption being critical to Hadropithecus is that C4 grasses likely arrived too late to explain the derived suite of traits that distinguish Hadropithecus from its sister taxon, Archaeolemur. Recent phylogenetic research based on complete or nearly complete mitochondrial genome sequences for extinct and extant lemurs suggests that the archaeolemurids diverged from a palaeopropithecid/indriid clade roughly 24 Ma [Kistler et al., 2015]. This establishes an upper limit for the divergence timing of the two archaeolemurid genera. Closely related lemur genera for which divergence estimates are available fall between 10 and 15 million years (∼10.9 Ma for Pachylemur and Varecia, a bit more for Eulemur-Lemur and for Propithecus-Avahi) [Kistler et al., 2015]. A divergence date for Archaeolemur and Hadropithecus between 10 and 20 million years seems reasonable. This is exactly when the common ancestor of the Didiereoideae likely arrived on Madagascar. Estimates for the timing of the origin of the family to which the Didiereoideae belongs (i.e., the Didiereaceae, including the continental African Calyptrothecoideae and Portulacarioideae as well as the Madagascan Didiereoideae; see Bruyns et al., 2014]) vary from 15 to ∼30 Ma [Arakaki et al., 2011; Ocampo & Columbus, 2010]. Arakaki et al. [2011] place the initial diversification of the Madagascan subclade at ∼17 Ma. Ocampo & Columbus [2010] place the divergence of African and Madagascan clades at 12.1 Ma. But even that late estimate substantially predates the global radiation of C4 grasses [Cerling et al., 1997] and likely post-Miocene spread of C4 grasses to Madagascar [Bond et al., 2008], which is believed to be related to a decrease in concentrations of atmospheric CO2 below a threshold that favored C3 photosynthesis [Cerling et al., 1997].
There is substantial evidence that C4 grasses expanded into tropical and subtropical latitudes of continental Africa during the Late Neogene [Strömberg, 2011; Uno et al., 2011]. Furthermore, that evidence supports a latitudinal gradient, with C4 grasses expanding 4–5 million years later in southern Africa than in eastern and central Africa [Segalen et al., 2007; Strömberg, 2011]. If the same latitudinal gradient applies beyond continental Africa, one can expect a relatively late spread of C4 grasses to Madagascar.
The timing of the diversification of the Madagascan Didiereoideae is critical because spines protecting the leaves from predation likely evolved in the ancestral lineage soon after initial colonization. Spines occur on all extant species of Didiereoideae but not their continental African relatives. Only lemurs and elephant birds were present on Madagascar when the ancestral didiereoid arrived on the island; hippopotamuses would have arrived later. Bond & Silander [2007] ruled out elephant birds on the basis of their feeding anatomy, which makes climbing lemurs the likely candidates. Exploitation by climbing lemurs would explain the evolution of spines on the tallest branches of didiereoid trees such as Alluaudia [Crowley & Godfrey, 2013], and the fact that spine length matches or slightly exceeds the leaves beneath them. Differential exploitation of this new resource might also help to explain the divergence of Archaeolemur and Hadropithecus.
Recent discoveries of some previously unknown postcranial bones of Hadropithecus are fascinating in this light [Godfrey et al., 1997, 2006; Lemelin et al., 2008], as they suggest that Hadropithecus, despite being highly terrestrial, was maladapted for speed but adept at climbing. The femur was slightly shorter than the humerus, but very robust. Its shaft was anteroposteriorly compressed as in slow climbers; its femoral condyles show an asymmetry similar to that of chimpanzees or gorillas, likely reflecting differential weight bearing during femoral rotation on the tibia under abducted femoral excursion. The patellar groove was shallow and wide. This animal was neither a leaper nor suspensory; it was also not cursorial. Polk et al. [2010] measured the density of subchrondral bone in the distal femur to reconstruct knee postures. They found a broad range, from highly flexed to highly extended. That Hadropithecus was slower and more deliberate than Archaeolemur is corroborated by data on the radius of curvature of its semicircular canals [Walker et al., 2008].
Curiously, Hadropithecus also had an unusual manus [Lemelin et al., 2008]. Its short digits suggest cercopithecoid-like terrestriality but differences from terrestrial cercopithecoids are also striking. Papionins in general, and Theropithecus in particular, have a long and mobile pollex and a very short index finger, which help them in securing small objects such as the seeds of grasses, as well as underground storage organs such as rhizomes of grasses or corms of sedges, which become essential resources when grasses are dry [Etter, 1973; Jablonski et al., 2002]. Hadropithecus, in contrast, had an exceptionally short thumb, which suggests poor manipulative skills. The few hand bones known for Hadropithecus include the fifth metacarpal and the wrist bone that articulates with it, the hamate. When fitted together, they form a peculiar angle; this carpometacarpal joint displays a hyperextended set [Lemelin et al., 2008]. This is a very odd adaptation with no known analogue in the world of primates or other mammals. One might imagine that Hadropithecus would have benefited from being able to remove leaves manually if it indeed specialized on the succulent leaves protected externally by long spines. At present we do not know how it would have done this. More discoveries of bones of its hand may help us to understand whether or not Hadropithecus' manual peculiarities relate to a unique feeding adaptation.
With our new interpretation of the diet of Hadropithecus, we can now better evaluate the hypotheses that have been proposed to explain this animal's dental convergences with geladas and largely cranial convergences with basal hominins. Grass blades, rhizomes, and small seeds of grasses are unlikely, despite being mechanically challenging, because the isotope signature does not fit, and because Hadropithecus and Archaeolemur likely diverged before C4 grasses became widespread in Madagascar [H1 and, in part, H2]. There is no evidence that hard foods or large seeds were significant components of the diet of Hadropithecus [H3]. Large seeds or fruits with hard protective coats cannot have been staples, because they derive virtually exclusively from C3 plants. Heavy microwear pitting on the molars of Hadropithecus is likely to reflect high grit levels on the foods rather than the consumption of hard objects, as many endemic CAM plants of Madagascar have tiny seeds that are wind dispersed. In arid or relatively open habits, grit levels on leaves are likely to be high, whether the leaves are cropped from plants on the ground or from trees [Green & Kalthoff, 2015; Ungar et al., 1995].
Elements of Jolly's [1970] small object hypothesis [H2] can be defended in that succulent leaves may require little incisal preparation and not more than a small gape, and may have been consumed in large quantities. Repetitive chewing may have been required. Furthermore, succulent leaves may well have been conveyed by hand to the mouth. It is not clear that the leaves of these succulents are mechanically challenging (they have yet to be systematically tested), but having molar teeth that function in the manner of a grazer is not surprising for an animal masticating large quantities of succulent leaves. High-bulk feeding [H4] cannot be discounted; this is, in effect, a component of Jolly's small object hypothesis [H2]. However, while some of our candidate plants do not grow tall, others are trees. Hadropithecus likely accessed its foods via climbing as well as on the ground. Here, Jolly's [1970] gelada analogy fails.
Finally, we stress that determining the diet of Hadropithecus is only the first step of several that must be taken if we are to understand its hominin and papionin likenesses. While our research here contributes importantly to this task, we recognize that more research is needed to trim our long list of candidate plants and eliminate some of them. Nitrogen isotope data, in particular, are scant.
Ultimately, we would like to be able to address the question: What ecological or dietary shift in the ancestral archaeolemurid could have produced the evolutionary shift in gnathic and dental structure that we observe in the Hadropithecus lineage? And why do some (but not all) of its features parallel apomorphies of basal hominins and others of Theropithecus? To address these questions, we must know the diets of the fossils, but that is only the beginning. We must then collect appropriate data on the mechanical properties of likely resources, the mechanical efficiency of the molars, and processes by which foods break down during mastication. In our view, the cranial and dental convergences of Hadropithecus to basal hominins and to grass-eating papionins are too striking to ignore, and understanding their significance could help us to better understand the adaptive significance of the craniodental anatomy of basal hominins and of grass-eating papionins themselves.
ACKNOWLEDGMENTS
We thank Paul Garber and two anonymous reviewers for their comments on an earlier version of this manuscript, which helped us to substantially improve this article. We thank Dyke Andreasen for assisting with isotopic analysis and David Burney for supplying previously unpublished nitrogen isotope data. All new sample collection adhered to the legal requirements of the organizations that oversee collection or institutions at which collections are held. Support for this research was provided by startup funding to B.E.C. and University of California Lab Fees Research Program Grant No. 115818 (to B.E.C and N.J. Dominy).




