Oxygen isotope ratios in primate bone carbonate reflect amount of leaves and vertical stratification in the diet
Abstract
The stable isotopic biogeochemistry of free-ranging primates is a unique tool to assess dietary and ecological adaptions among sympatric populations. The present study tested the hypothesis that oxygen isotopes in the bone carbonate of five primate and four ungulate species that live in Kibale National Park, Uganda, would show minimal variability since the species obtain water from a single water source. Bones were analyzed for stable carbon (δ13C) and oxygen (δ18O) isotope ratios. Results for apatite δ13C are consistent with all species feeding in a closed forest habitat and do not exhibit niche partitioning. δ18O values, in contrast, cluster by species and correlate positively with the relative contribution of leaves to the whole diet are likely also modified by vertical niche partitioning between taxa within the forest canopy. These results show that biochemical markers from naturally deceased primate remains can aid our understanding of how living animals exploit available resources. Am. J. Primatol. 78:1086–1097, 2016 © 2015 Wiley Periodicals, Inc.
INTRODUCTION
The application of stable isotope analysis of primate tissues is now well established to better understand both the ecology of living species as well as nutritional adaptations in fossil primates [Crowley, 2012; Sandberg et al., 2012; Sponheimer et al., 2009]. The significance of oxygen isotope ratios from bone carbonate and enamel continues to be investigated as a means to evaluate local climatic and biogeochemical influences on body water composition and as a tool to differentiate herbivorous animals with dietary specializations [Levin et al., 2006]. This is particularly useful among sympatric forest-dwelling animals whose C3-dominated plant diets are relatively indistinguishable through comparison of their δ13C tissue values [Cerling et al., 2004; Crowley, 2014; Krigbaum et al., 2013; Nelson, 2013]. These recent studies demonstrate that relative stable isotope ratios in primate tissues can be analyzed directly to test and quantify assumptions made about dietary preferences and niche partitioning by primatologists through years of field observation. The accuracy of results and strength of the analysis can only be made robust by identifying similar trends in diverse species across habitats and continents.
Despite evidence that forest-floor and low-canopy plant tissues are depleted in 13C relative to those in upper canopy strata [Farquhar et al., 1989], carbon isotope ratios in bone collagen and carbonate have yet to distinguish niche separation between forest-dwelling animals that feed almost exclusively on vegetation that synthesizes its tissues using the C3-photosynthetic pathway [Carter, 2001; Cerling et al., 2004; Crowley et al., 2010; Krigbaum et al., 2013; Krigbaum, 2000; van der Merwe and Medina, 1991]. Based on similar environmental variables that alter isotope concentration in leaves, oxygen isotope ratios in animal tissues are proving to be a better measure of niche separation between rainforest taxa. The 18O/16O ratio in local water in any given climate is sensitive to mean annual temperature [Dansgaard, 1964]. Bone phosphate δ18O values from animals that obtain most of their ingested water from leaves reflect relative humidity of the local climate [Ayliffe and Chivas, 1990; Cormie et al., 1994]. The water in leaves that grow in dry habitats are positively enriched in 18O relative to leaves from moist climates [Gat, 1980]. In the study of prehistoric human diets, δ18O data from tooth enamel correlate with latitude [Fricke et al., 1995]. The 18O/16O ratio of animal tissues is ultimately influenced by that of drinking water (meteoric and surface water) as well as atmospheric O2, modulated by local temperature and humidity. To maintain balance, oxygen is excreted in the urine and feces, evaporated in sweat, and respired as carbon dioxide [Koch et al., 1989, 1994; Schmidt-Nielsen, 1964, 1997]. The δ18O signatures of bone phosphate and carbonate, both recovered from the hydroxyapatite or mineral phase of bone, are assumed to be in equilibrium with that of blood plasma and, therefore, is derived mostly from water in the diet [Kolodny et al., 1983; Longinelli, 1984; Luz et al., 1990, 1984; Luz and Kolodny, 1985]. Oxygen isotope ratios in tropical moist forest vegetation are further altered by evapotranspiration, so leaves have high δ18O relative to other parts of the same plant, including fruits [Barbour, 2007; Marshall et al., 2007; Sternberg et al., 1989]. There is a vertical stratification or “canopy effect” in increasing leaf δ18O values from forest floor to middle canopy to high canopy due to variations in humidity in local microhabitats [Sternberg et al., 1989]. In an individual plant, cellulose in the leaves has higher δ18O values than cellulose from its roots [Epstein et al., 1977; Sternberg et al., 1984, 1986; Sternberg, 1989; Yakir, 1992]. Krigbaum et al. [2013] propose that the proportion of leaves and fruit in the diet will influence the δ18O of consumer tissues of folivores and frugivores, respectively, because leaves have a high surface area/low volume ratio in contrast to the low surface area/high volume of fruits, resulting in an enrichment of 18O in leaves because of evapotranspiration. Actual δ18O values of leaves and fruit pulp from the same tree have not been published.
Research supports the assumption that trends in plant δ18O signatures will be preserved in diverse tissue of animals feeding on different types of vegetation. Early studies found that δ18O values in herbivore tooth enamel were more positive in browsers (70–95% C3 leaves, shoots, and young twigs) and mixed feeders (>30% C4 grass and >30% C3 browse) than in grazing herbivores (>70% C4 grass) [Cerling et al., 1997, 2003; Kohn et al., 1996]. Bocherens et al. [1996] had opposite results in that 18O was depleted in the enamel of a browser (black rhinoceroses) and mixed-feeder (African elephants), but this may be caused by either differences in body size and metabolic rate or aridity of their respective local habitats [Levin et al., 2006]. Studies on different species of well-studied forest-dwelling nonhuman primates present compelling evidence that δ18O data in bone and enamel reflect subsistence strategies adapted to different average feeding heights in the forest canopy [Crowley et al., 2014; Krigbaum et al., 2013; Nelson, 2013]. Cerling et al. [2004] suggest that the δ18O values from folivorous colobines are enriched relative to frugivores because their water source is predominantly leaves.
Kibale National Park (795 km2) is a mid-altitude tropical evergreen forest in southwestern Uganda (0°13́–0°41́ N and 30°19́–30°32́ E) where primate ecology and conservation have been studied for over 40 years [Bryer et al., 2013; Chapman and Lambert, 2000; Struhsaker, 1997]. Mean annual rainfall is 1712 mm (1990–2004) and occurs across two rainy seasons [Chapman et al., 2002a; Rode et al., 2006]. The primates and ungulates in the present study were collected from three ecologically distinct camps: Ngogo, Kanyawara, and Kanyanchu. Much of Kibale National Park is classified as Parinari forest [Skorupa, 1988]. Differences in altitude, however, result in differences in climate and vegetation. The climate at Ngogo is warmer and drier than at Kanyawara and the vegetation is quite different [Butynski, 1990]. The region of forest near Kanyanchu that has been documented is called the Dura River site, located roughly 15 km south of Kanyawara and is a combination of moist evergreen forest and lowland tropical forest [Chapman and Chapman, 1997]. At this time, small sample sizes of mammalian species from Kibale National Park preclude the investigation of the influence of local habitat on stable isotope ratios. Species presence and population density are known to vary for most primate and ungulate species at these study sites.
Bone carbonate δ18O and δ13C data from five well-studied East African primates are presented here. Data were generated from the analysis of skeletal remains of sympatric primates living in Kibale National Park prior to 1995 and represent a subset of data from a multi-isotopic study that tested various hypotheses derived from both stable isotopic biogeochemistry and primate ecology [Carter, 2001]. δ13C (bone collagen and apatite) and δ15N (bone collagen) data from these same specimens are published and discussed elsewhere [Crowley et al., 2010; Smith et al., 2010]. The present study tested the hypothesis that sympatric primates that obtain water from local sources will have similar δ18O values. Differences in δ18O values are considered meaningful and related to diet.
METHODS
Bone samples were collected from individuals representing five primate and four ungulate species from Kibale National Park (KNP), Uganda, in September, 1995 (Fig. 1). Permission to conduct research in Uganda was obtained from the Office of the President and related agencies upon agreement to adhere to legal requirements. Permission to export biological samples was obtained from the Uganda Wildlife Authority and Kenya Wildlife Authority and, following authorization from US Fish and Wildlife Service, samples were imported to the United States for analysis. The acquisition and handling of materials followed the Principles for the Ethical Treatment of Nonhuman Primates established by the American Society of Primatologists.
The skeletal remains studied here were from naturally deceased animals that had been collected from various sites throughout the forest. Certain specimens had been previously identified [Struhsaker and Leakey, 1990]. A history of the Kibale faunal collection and means of recovery are discussed in Carter [2001]. Details of the acquisition of the chimpanzee remains is described elsewhere [Carter et al., 2008]. Between 2–10 grams of bone were collected from each specimen, primarily from long bones, ribs, or the cranial base (Table I). When available, long bone cortex was preferred to control for potential taphonomic contamination. Each of the following species was chosen for this study based on availability of remains, dietary specialization (e.g., folivory or frugivory), and vertical niche stratification (documented differences among average heights at which they forage for food within the forest canopy) (Table I): Procolobus rufomitratus spp. tephrosceles (red colobus; total sample numbers: N = 37; P. tephrosceles, N = 28; Colobus spp., N = 9), Lophocebus albigena johnstoni (grey-cheeked mangabey; N = 2), Cercopithecus ascanius schmidti (redtail guenon; N = 7), Papio hamadryas anubis (olive baboon; N = 6), and Pan troglodytes schweinfurthii (chimpanzee; N = 12). Bones in this study listed under “Procolobus” were predominantly identified as red colobus; specimens listed as “Colobus spp” or “Colobus?” could not be assigned to species with confidence and may represent Colobus guereza (black-and-white colobus). Both species are specialized folivores. For comparison to non-primate herbivores, bone samples were also collected from the following ungulates: bushbuck (Tragelaphus scriptus; N = 6), bush pig (Potomochoerus porcus; N = 5), duiker (two species: Cephalophus harveyi and C. monticola; N = 5), and African buffalo (Synerus caffer; N = 2). As with the primate remains, certain ungulate specimens could not be identified to species level with confidence. Other than chimpanzee remains, the age and sex of most animal remains could not be determined. Most specimens were adults, based on dental maturation and epiphyseal closure.
| Lab No. | Kibale ID | Species | Primary diet | Leaves in diet average % | Bone sampled | δ13Ccarbonate (PDB ‰) | δ18Ocarbonate (PDB ‰) |
|---|---|---|---|---|---|---|---|
| Primates | |||||||
| K40B | KFB 126 | Cercopithecus ascanius | frugivory | 21%a | multiple bones | −17.08 | −1.69 |
| K42B | KFB 127 | Cercopithecus ascanius | frugivory | 21%a | caudal vertebra | −17.18 | 1.77 |
| K57B | N6/TTSK 71 | Cercopithecus ascanius | frugivory | 21%a | caudal vertebra, ribs | −15.86 | −0.59 |
| K41B | KFB 56 | Cercopithecus ascanius? | frugivory | 21%a | parietal | −16.78 | −0.82 |
| K63B | N15/TTSK 50 | Cercopithecus ascanius? | frugivory | 21%a | cranial | −16.39 | −0.60 |
| Cercopithecus avg. ± SD | −16.66 ± 0.54 | −0.39 ± 1.29 | |||||
| K56B | N7/TTSK 55 | Lophocebus albigena | frugivory | — | ribs | −15.48 | −1.26 |
| K1B | KFB 107 | Pan troglodytes | frugivory | 10%b | rib | −15.64 | −1.16 |
| K2B | KFB 17 | Pan troglodytes | frugivory | 10%b | occipital | −12.09 | 7.32 |
| K3B | KFB 93 | Pan troglodytes | frugivory | 10%b | occipital | −12.65 | −1.82 |
| K4B | KFB 105 | Pan troglodytes | frugivory | 10%b | rib | −15.46 | −0.18 |
| K5B | KFB 106 | Pan troglodytes | frugivory | 10%b | rib | −15.99 | −0.72 |
| K6B | KFB 18 | Pan troglodytes | frugivory | 10%b | femur | −15.61 | −1.05 |
| K7B | KFB 20 | Pan troglodytes | frugivory | 10%b | occipital | −16.82 | −0.75 |
| K55B | KFB 1 | Pan troglodytes | frugivory | 10%b | tibia | −15.43 | −0.48 |
| K59B | KFB 4 | Pan troglodytes | frugivory | 10%b | femur | −15.72 | 0.51 |
| K60B | KFB 3 | Pan troglodytes | frugivory | 10%b | rib | −15.60 | −0.71 |
| Pan avg. ± SD | −15.10 ± 1.50 | 0.10 ± 2.61 | |||||
| K34B | KFB 80 | Papio anubis | omnivory | 7%c | mandible | −14.67 | −1.29 |
| K35B | KFB 63 | Papio anubis | omnivory | 7%c | occipital | −17.50 | −1.74 |
| K36B | KFB 62 | Papio anubis | omnivory | 7%c | occipital | −15.90 | −1.42 |
| K37B | KFB 16 | Papio anubis | omnivory | 7%c | occipital | −15.75 | −1.70 |
| K38B | KFB 64 | Papio anubis | omnivory | 7%c | occipital | −15.24 | −2.46 |
| K39B | KFB 29 | Papio anubis | omnivory | 7%c | occipital | −16.97 | −2.33 |
| Papio avg. ± SD | −16.00 ± 1.06 | −1.82 ± 0.48 | |||||
| K24B | No KFB # | Colobus? | folivory | 80%d | tibia | −15.45 | 2.16 |
| K27B | No KFB # | Colobus? | folivory | 80%d | fibula | −16.15 | 1.76 |
| K25B | KFB 125 | Colobus sp. | folivory | 80%d | mandibular ramus | −16.11 | 2.51 |
| K28B | KFB 124 | Colobus sp. | folivory | 80%d | tibia | −16.06 | 0.94 |
| K29B | No KFB # | Colobus sp. | folivory | 80%d | tibia | −16.46 | −1.06 |
| K30B | KFB 30 | Colobus sp. | folivory | 80%d | frontal | −15.78 | 2.22 |
| K62B | N18/TTSK 61 | Colobus sp. | folivory | 80%d | cranial | −15.88 | −0.69 |
| K14B | KFB 113 | Procolobus rufomitratus | folivory | 80%d | femur | −16.03 | 0.78 |
| K15B | KFB 111 | Procolobus rufomitratus | folivory | 80%d | radius | −17.06 | 0.46 |
| K16B | KFB 109 | Procolobus rufomitratus | folivory | 80%d | tibia | −18.13 | 0.49 |
| K17B | KFB 41 | Procolobus rufomitratus | folivory | 80%d | ribs | −16.20 | 0.06 |
| K18B | KFB 117 | Procolobus rufomitratus | folivory | 80%d | occipital | −17.19 | 1.04 |
| K19B | KFB 116 | Procolobus rufomitratus | folivory | 80%d | femur | −17.00 | 1.05 |
| K20B | KFB 112 | Procolobus rufomitratus | folivory | 80%d | caudal vertebra, ribs | −15.81 | 2.25 |
| K21B | KFB 61 | Procolobus rufomitratus | folivory | 80%d | occipital, mandible | −16.58 | 1.32 |
| K22B | KFB 19 | Procolobus rufomitratus | folivory | 80%d | ribs | −15.28 | 0.80 |
| K23B | KFB 115 | Procolobus rufomitratus | folivory | 80%d | ribs, metacarpal, phalanx | −17.03 | 1.15 |
| K26B | KFB 59 | Procolobus rufomitratus | folivory | 80%d | occipital | −16.47 | 0.76 |
| K31B | KFB 15 | Procolobus rufomitratus | folivory | 80%d | occipital, zygomatic | −16.13 | 0.37 |
| K32B | KFB 58 | Procolobus rufomitratus | folivory | 80%d | occipital, parietal | −17.08 | 0.85 |
| K33B | KFB 21 | Procolobus rufomitratus | folivory | 80%d | occipital | −16.56 | 0.19 |
| K58B | N12/TTSK 60 | Procolobus rufomitratus | folivory | 80%d | ulna | −15.86 | 0.74 |
| K64B | N20/TTSK 53 | Procolobus rufomitratus | folivory | 80%d | occipital | −15.46 | 0.43 |
| K65B | N9/TTSK 58 | Procolobus rufomitratus | folivory | 80%d | multiple bones | −16.05 | 1.26 |
| K66B | N27/TTSK 59 | Procolobus rufomitratus | folivory | 80%d | occipital | −15.81 | 0.44 |
| K67B | N21/TTSK 69 | Procolobus rufomitratus | folivory | 80%d | occipital | −16.11 | −2.11 |
| K68B | N11/TTSK 57 | Procolobus rufomitratus | folivory | 80%d | vertebrae | −15.44 | 1.50 |
| Colobine avg. ± SD | −16.27 ± 0.66 | 0.80 ± 1.02 | |||||
| Ungulates | |||||||
| K43B | No KFB # | Cephalophus harveyi | herbivore/browser | — | rib | −16.45 | −2.42 |
| K50B | KFB 130 | Cephalophus monticola | herbivore/browser | — | cranial | −14.53 | −1.72 |
| K49B | KFB 25 | Cephalophus harveyi | herbivore/browser | — | ulna, ribs | −16.44 | −2.15 |
| K44B | KFB 44 | Cephalophus sp. | herbivore/browser | — | occipital | −15.34 | −1.78 |
| K12B | No KFB # | Cephalophus sp. | herbivore/browser | — | phalanx, calcaneus | −15.85 | −2.17 |
| K61B | KFB 6 | Cephalophus sp. | herbivore/browser | — | scapula | −15.84 | −2.88 |
| Cephalophus avg. ± SD | −15.74 ± 0.73 | −2.19 ± 0.43 | |||||
| K8B | KFB 78 | Potamochoerus porcus | omnivory | — | mandible | −14.40 | −2.87 |
| K10B | KFB 75 | Potamochoerus porcus | omnivory | — | mandible | −9.63 | −3.29 |
| K11B | KFB 76 | Potamochoerus porcus | omnivory | — | mandible | −14.81 | −3.08 |
| K52B | KFB 77 | Potamochoerus porcus | omnivory | — | mandibular ramus | −13.76 | −1.68 |
| K54B | No KFB # | Potamochoerus porcus | omnivory | — | rib | −16.91 | −3.25 |
| Potamochoerus avg. ± SD | −13.90 ± 2.66 | −2.83 ± 0.67 | |||||
| K13B | No KFB # | Syncerus caffer | herbivore/grazer | — | occipital | −0.56 | −1.22 |
| K9B | KFB 82 | Syncerus caffer? | herbivore/grazer | — | mandible | −3.54 | −1.62 |
| Syncerus avg. ± SD | −2.05 ± 2.11 | −1.42 ± 0.28 | |||||
| K46B | No KFB # | Tragelaphus scriptus | herbivore/browser | — | tibia | −19.65 | −1.60 |
| K47B | KFB 46 | Tragelaphus scriptus | herbivore/browser | — | ribs | −19.23 | −2.09 |
| K48B | KFB 129 | Tragelaphus scriptus | herbivore/browser | — | calcaneus | −20.62 | −3.32 |
| K51B | KFB 45 | Tragelaphus scriptus | herbivore/browser | — | rib | −16.72 | −1.29 |
| K53B | KFB 128 | Tragelaphus scriptus | herbivore/browser | — | rib, occipital, C1 | −19.60 | −1.62 |
| K45B | KFB 50 | Tragelaphus scriptus? | herbivore/browser | — | cranial | −19.47 | −1.09 |
| Tragelaphus avg. ± SD | −19.21 ± 1.31 | −1.84 ± 0.80 |
Leaves comprise at least 73–87% of diet of red colobus and 77–88% of that of black-and-white colobus, categorizing the colobines as the most folivorous of the Kibale primates [Chapman and Chapman, 1999; Chapman et al., 2002b; Struhsaker, 1975; Struhsaker and Oates, 1975] (Table I). Colobinae have a multi-chambered stomach that allows for a ruminant digestive physiology [Baranga, 1982; McKey et al., 1981; Ohwaki et al., 1974]. Frugivorous primates from Kibale include grey-cheeked mangabeys and redtail guenons, though both species supplement their diets substantially with arthropods [Struhsaker, 1978, 1980; Waser, 1975, 1977]. Kibale baboons were thought to be omnivorous and opportunistic predators [Rowell, 1966; Struhsaker, 1997], though recent study documents significant frugivory and limited predation [Johnson et al., 2012]. Kibale chimpanzees are well studied and are frugivorous omnivores, with fruit comprising as much as 80% of their annual diet [Potts et al., 2009, 2011; Wrangham et al., 1992, 1993a, 1993b]. While research on acquisition and metabolism of dietary water in primates is limited, the assumption is that they obtain water indirectly by consuming succulent plant foods (that contain meteoric water) instead of drinking directly from surface waters [Kempf, 2009; Rothman et al., 2012].
As for the nonprimates in this study, the bushbuck is a forest bovid that browses on shrubs and leguminous herbs [Haschick and Kerley, 1997]. Bush pigs have an omnivorous diet, similar to that of Kibale baboons, that includes low-canopy/forest-floor items such as fruits, berries, insects, and small animals [Ghiglieri et al., 1982; Harris and Cerling, 2002; Kingdon, 1979; Nummelin, 1990]. A suid, the bush pig is a water-dependent browser and the only non-ruminant among the ungulates in this study [Harris and Cerling, 2002]. Red (Cephalophus harveyi) and blue (Cephalophus moniticola) duikers, both found in Kibale National Park, mainly eat fruits that drop from forest trees and shrubs, but may supplement with floral parts and leaves [Lwanga, 2006; Nummelin, 1990]. The African buffalo is a strict grazer that is less water-dependent than the other Kibale ungulates and is seen foraging only in Kibale grasslands, located predominantly in central and southern regions of the park [Levin et al., 2006; Struhsaker, 1997; Wanyama et al., 2010]. They may consume low-canopy browse in forest margins [Field, 1976].
Bone samples were cleaned by removing the outer cortex with a rotary sander. Large pieces were fragmented and sonicated in distilled water with multiple rinses. When dry, bone fragments were powdered using a Wiley mill. The smallest fraction (<0.25 mm) from each sample was treated with a 50% Clorox bleach solution and stirred on a Vortex Genie 2. Each sample was refreshed and stirred twice a day until production of bubbles ceased. Samples were buffered with acetic acid for 24 hours, then rinsed and frozen to await analysis. Carbonate oxygen isotope data were generated using the VG Isogas PRISM Series II mass spectrometer in the Department of Geology at the University of Florida, Gainesville. Carbonate isotope values are reported relative to PeeDee Belemnite (PDB). Precision was 0.07 ‰ for δ13C and 0.10 ‰ for δ18O.
Data were analyzed using nonparametric two-tailed Mann-Whitney U tests to identify significant differences between primate taxa. Regression analysis was used to identify correlation between stable isotope ratios and amount of leaves in the diet of each species. Significance was set at P < 0.05.
RESULTS
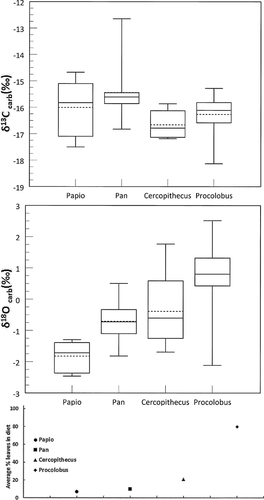
Isotope data for individual samples and summary statistics are itemized in Table I. Each individual was represented by analysis of the single bone listed. Figure 2 illustrates taxon-specific means for δ18OPDB and δ13CPDB values. As expected, the only significantly different δ13CPDB species was the African buffalo, a C4-grazer that had the most positive mean δ13CPDB value (N = 2, −2.05 ± 2.11‰). The remaining animals are forest-dwelling species and have individual carbonate δ13CPDB values that range between −20.62‰ (bushbuck) and −9.63‰ (bush pig). Carbonate δ13CPDB data show no differences between primate species, and individual values range from −18.13‰ (Procolobus) to −12.09‰ (Pan).

In contrast, there were significant differences using pair-wise comparisons between mean δ18OPDB of certain primate species, thus the hypothesis is rejected. Procolobus (N = 27) has the most positive mean δ18OPDB (+0.80 ± 1.02‰) of the primates and is significantly higher than the means of Pan (N = 10, P = 0.0053) and Papio (N = 6; −1.82 ± 0.48‰; P = 0.0004). Cercopithecus (N = 5) is only significantly higher than Papio (P = 0.0173). Papio has the most negative δ18OPDB and is significantly lower than the other primate species (vs. Pan, P=0.003; vs. Cercopithecus, P = 0.0173). The single value for Lophocebus (−1.26 ‰) is closest to the mean of Papio. The mean of Pan is higher than mean of Cercopithecus, but less difference is found if one extremely high outlier was removed from Pan data.
Among the ungulates, the African buffalo has the most positive average δ18OPDB value (−1.42‰ ± 0.28) (Fig. 2). The omnivorous bush pig has the most negative value (−2.83‰). The δ18OPDB signatures of the bushbuck and duiker are intermediate, with the former species, a browser, is more positive. Thus, bone carbonate of a forest animal that eats leaves, like the bushbuck, is more enriched in 18O than a forest omnivore, the bush pig. The bushbuck obtains water mostly from leaves, while the bush pig gets water from forest-floor roots, corms, and tubers and occasional animal flesh [Apio and Wronski, 2005; Ghiglieri et al., 1982; Nummelin, 1990].
Current theory on oxygen isotope fractionation is that differences reflect environmental alterations of consumed foods and dietary water if major variations in metabolism, such as internal body temperature and metabolic rate, are controlled [Crowley, 2012; Kirsanow and Tuross, 2011; Koch et al., 2007; Krigbaum et al., 2013; O'Brien and Wooller, 2007]. To examine if the δ18OPDB values in primate bone were related to the amount of leaves in the diet, mean values for each species were plotted against current estimates of average percentage leaves in their respective diets (Fig. 3). Regression analysis shows a positive correlation (R2 = 0.79). No such linear relationship is noted between percentages leaves in the diet and mean δ13CPDB. This trend is also illustrated in Figure 4.
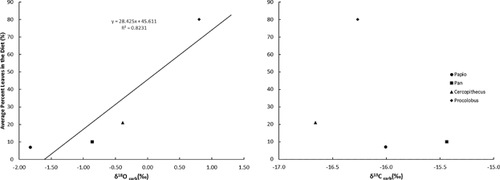
DISCUSSION
These results show that δ18OPDB data from bone carbonate are not similar across primate species that obtain water from a single meteoric supply in a tropical moist forest. Folivorous colobines have the highest δ18Ocarbonate signature, baboons have the lowest, and chimpanzees, redtails, and mangabeys have intermediate values. This trend is also noted in ungulates; bushbuck that rely on forest browse have the most positive δ18OPDB values; omnivorous bush pigs have the most negative, and frugivorous duikers are intermediate. Sponheimer and Lee-Thorp [2001] propose that tissue from omnivores may be more depleted in 18O than herbivores because of the consumption of animal flesh in the diet. This trend is upheld by data from both Kibale baboons and bush pigs, but not by the chimpanzees that are known to supplement the diet with small animals including other primates. Buffaloes, the only Kibale mammal in this study that is known to feed in open, more arid grasslands, do not have remarkably more positive δ18OPDB values than bushbucks, but sample sizes are small and the 18O enrichment may also be explained by this species being an obligate drinker. Based on results presented here, Kibale baboons forage mostly in the closed forest, not open grasslands, and they obtain most dietary water from sources other than leaves. Both baboons and chimpanzees likely get water from eating succulant piths of terrestrial herbaceous vegetation [Conklin-Brittain et al., 1998]. Colobines likely get the water they need from leaves, while chimpanzees and the other frugivores get water mostly from fruits. Chimpanzees are also known to construct leaf wadges which they use as sponges to soak up water that has pooled in nooks of trees and will also lap water from terrestrial pools [Tuttle, 1986].
While others state that primate δ18O values show a “canopy effect” in 18O enrichment in the leaves consumed based on vertical stratification of niches [Cerling et al., 2004; Krigbaum et al., 2013; Nelson, 2013], a conservative interpretation is that δ18O values show a positive correlation based on the amount of leaves in the diet, supported by the data presented here. Since the relative amount of 18O is higher in leaves than other plant parts and increases in leaves located in higher levels of the canopy [Marshall et al., 2007; Sternberg et al., 1989], chances are that both percentage leaves in the diet and vertical niche are influences on the ultimate δ18O of primate tissues. In Kibale National Park, red colobus was once thought to feed mostly on young leaves throughout high canopy strata [Struhsaker, 1975, 1978], but with habituation of groups the vertical niche of red colobus is now known to overlap significantly with chimpanzees (C. Chapman, personal communication). The higher δ18O of colobine bone carbonate than that of Pan might have been interpreted to reflect vertical niche stratification in the past, while current knowledge of niche overlap directs interpretation towards average percentage of leaves in the diet of both species.
δ13C values cannot be used to distinguish between forest primates that rely predominantly on parts of plants that have a C3 photosynthetic pathway. Though chimpanzees have the most positive δ13C mean among Kibale primates, this value is more negative than that of the omnivorous, forest-dwelling bush pig. An omnivorous diet in a rain forest can include local C4 cultivated plants, such as corn, but known crop-raiding primate species in Kibale National Park were not isotopically distinct [Carter, 2001]. Perhaps bush pigs routinely raid C4 crops, prey on primary consumers that feed heavily on C4 grasses, or include both in their diet. Buffalo are clearly the only species in this Kibale assemblage that consistently feed on C4 vegetation from gap habitats.
The main limitation of this study of Kibale faunal remains is the small sample size for most species. Despite the small population sample, the trend in δ18O values in Kibale primates supports such trends observed in other studies of rain-forest primates [Cerling et al., 2004; Crowley, 2014; Krigbaum et al., 2013; Nelson, 2013; Smith et al., 2010]. The outlier value noted in Pan is more than two standard deviations beyond the mean and may be due to laboratory error, such as sample preparation [Crowley and Wheatley, 2014].
Primate stable isotope ecology can make a valuable contribution to primate conservation if efforts are increased to amass large, well curated collections of faunal remains from discrete habitats and populations. In addition to confident identification of taxa to the species level, collections will benefit from proper recording of age, sex, and date/season of death. For example, comparing the body chemistry of a collection of animals that died prior to 1995 to that of a collection obtained after that time could be evaluated to identify markers of significant climate change, such as the increase in wetlands from 1995 to 2003 around Kibale National Park [Hartter and Southworth, 2009]. Access to multiple tissue types with known developmental timing and rates from single individuals, such as teeth, hair, and bones, will allow for fluctuations in stable isotope ratios throughout life as well as seasonal modifications to diet and body chemistry [Koch et al., 1989].
ACKNOWLEDGMENTS
Permission to conduct research in Uganda was granted by the Office of the President, Uganda, and the National Council for Science and Technology, and the research protocol was approved by the Makerere University Research Committee and Biological Field Station. With CITES permits from both the Uganda Wildlife Authority (No. unknown) and Kenya Wildlife Authority (No. 007319) and authorization from US Fish and Wildlife Service, samples were imported to the United States for analysis. We thank many people who made this study possible: Jane Buikstra, Russell Tuttle, Julian Kerbis Peterhans, Margaret Schoeninger, Stanley Ambrose, Richard Wrangham, Colin and Lauren Chapman, Lynette Norr, Larry Tieszen, Gilbert Isabirye-Basuta, John Kasenene, Richard Malenky, and Maeve Leakey. MLC is especially grateful to her field assistant Pascal Baguma who helped with sample collection and cleaning. Technical assistance in preparation of the manuscript was provided by Larissa Collier and Jean Carr. Travel to collect samples was funded by the University of Chicago and the Sigma Xi Research Society. Laboratory analyses were funded by the Wenner-Gren Foundation for Anthropological Research. We sincerely thank Brooke Crowley and Matt Sponheimer for allowing us to participate in this special issue. We are also grateful to an anonymous reviewer for insightful recommendations.




