Clinical and genetic characteristics of 10 Japanese patients with PROM1-associated retinal disorder: A report of the phenotype spectrum and a literature review in the Japanese population
Kaoru Fujinami and Akio Oishi are joint first authors.
Funding information: Fight for Sight UK, Early career investigator award; FOUNDATION FIGHTING BLINDNESS ALAN LATIES CAREER DEVELOPMENT PROGRAM, Grant/Award Number: CF-CL-0416-0696-UCL; Fuji Xerox Co., Ltd; Grant-in-Aid for Scientists to support international collaborative studies of the Ministry of Education, Culture, Sports, Science and Technology, Japan, Grant/Award Number: 16KK01930002; Grant-in-Aid for Young Scientists (A) of the Ministry of Education, Culture, Sports, Science and Technology, Japan, Grant/Award Number: 16H06269; Grant-in-Aid for Young Scientists of the Ministry of Education, Culture, Sports, Science and Technology, Japan, Grant/Award Number: 18K16943; Great Britain Sasakawa Foundation Butterfield Award; Health Labour Sciences Research Grant, The Ministry of Health Labour and Welfare, Grant/Award Number: 201711107A; Japan Agency for Medical Research and Development, Grant/Award Number: 18ek0109282h0002; Japan Society for the Promotion of Science, Grant/Award Numbers: 17H06820, 19K09929, H26-26462674; Kirin Company; Kowa Company, Ltd; National Hospital Organization Network Research Fund, Grant/Award Number: H30-NHO-Sensory Organs-03; NIHR-BRC at Great Ormond Street Hospital and UCL Institute of Child Health; NIHR-BRC at Moorfields Eye Hospital and the UCL Institute of Ophthalmology; Novartis Pharmaceuticals, Japan; Novartis Research Grant, Grant/Award Number: 2018; ROHTO Pharmaceutical Co.,Ltd.; Santen Pharmaceutical Co. Ltd; Tsubota Laboratory, Inc
Abstract
Variants in the PROM1 gene are associated with cone (−rod) dystrophy, macular dystrophy, and other phenotypes. We describe the clinical and genetic characteristics of 10 patients from eight Japanese families with PROM1-associated retinal disorder (PROM1-RD) in a nationwide cohort. A literature review of PROM1-RD in the Japanese population was also performed. The median age at onset/examination of 10 patients was 31.0 (range, 10–45)/44.5 (22–73) years. All 10 patients showed atrophic macular changes. Seven patients (70.0%) had spared fovea to various degrees, approximately half of whom had maintained visual acuity. Generalized cone (−rod) dysfunction was demonstrated in all nine subjects with available electrophysiological data. Three PROM1 variants were identified in this study: one recurrent disease-causing variant (p.Arg373Cys), one novel putative disease-causing variant (p.Cys112Arg), and one novel variant of uncertain significance (VUS; p.Gly53Asp). Characteristic features of macular atrophy with generalized cone-dominated retinal dysfunction were shared among all 10 subjects with PROM1-RD, and the presence of foveal sparing was crucial in maintaining visual acuity. Together with the three previously reported variants [p.R373C, c.1551+1G>A (pathogenic), p.Asn580His (likely benign)] in the literature of Japanese patients, one prevalent missense variant (p.Arg373Cys, 6/9 families, 66.7%) detected in multiple studies was determined in the Japanese population, which was also frequently detected in the European population.
1 INTRODUCTION
Inherited retinal disorder (IRD) is one of the major causes of blindness in developed countries, (Liew, Michaelides, & Bunce, 2014; Sohocki et al., 2001; Solebo, Teoh, & Rahi, 2017) including retinitis pigmentosa (RP), cone-rod dystrophy (CORD), cone dystrophy (COD), Stargardt disease (STGD), macular dystrophy (MD), Leber congenital amaurosis and others (Gill, Georgiou, Kalitzeos, Moore, & Michaelides, 2019; Hirji, Aboshiha, Georgiou, Bainbridge, & Michaelides, 2018; Kumaran, Moore, Weleber, & Michaelides, 2017; Michaelides, Hardcastle, Hunt, & Moore, 2006; Michaelides, Hunt, & Moore, 2003; Oishi et al., 2014, 2016; Rahman, Georgiou, Khan, & Michaelides, 2020; Tanna, Strauss, Fujinami, & Michaelides, 2017; Tee, Smith, Hardcastle, & Michaelides, 2016).
The clinical and genetic spectra overlap among IRDs. Pathogenic variants in the same gene may present different phenotypes. For example, relatively similar phenotypes of CORD, COD, STGD, and MD share causative genes such as ABCA4, BEST1, PRPH2, RPGR, CRX, GUCY2D, RS1, POC1B, PROM1, CNGA3, CNGB3, GUCA1A, KCNV2, and RIMS1 (Ba-Abbad, Robson, MacPhee, Webster, & Michaelides, 2019; Bouzia et al., 2020; Fujinami-Yokokawa et al., 2020; Fujinami, Lois, Davidson, et al., 2013; Fujinami, Lois, Mukherjee, et al., 2013; Fujinami et al., 2015; Georgiou et al., 2020; Gill et al., 2019; Hirji et al., 2018; Hunt, Buch, & Michaelides, 2010; Kameya et al., 2019; Kominami et al., 2018; Kondo et al., 2019; Liu et al., 2020; Mawatari et al., 2019; Michaelides et al., 2010; Mizobuchi et al., 2019; Nakanishi et al., 2016; Oishi et al., 2016; Rahman et al., 2020; Sisodiya et al., 2007; Strauss et al., 2016, 2018; Tanna et al., 2017; Tee et al., 2016, 2019). There are genes that are associated with different phenotypes of CORD/COD/MD and RP; EYS, CRX, PRPH2, GUCY2D, and RP1L1 (Ba-Abbad et al., 2019; Bouzia et al., 2020; Davidson et al., 2013; Fujinami-Yokokawa et al., 2019, 2020; Fujinami et al., 2019; Hull et al., 2014; Katagiri et al., 2018; Koyanagi et al., 2019; Liu et al., 2020; Nakamura et al., 2019; Oishi et al., 2014, 2016; L. Yang et al., 2020). Moreover, different inheritances such as autosomal dominant (AD) and autosomal recessive (AR) are associated with differences in phenotypes (Bouzia et al., 2020; Fujinami-Yokokawa et al., 2020; Gill et al., 2019; Hull et al., 2014; Liu et al., 2020; Rahman et al., 2020).
PROM1 (OMIM: 604365), denoted as prominin 1, encodes a prominin pentaspan transmembrane glycoprotein selectively localized at the apical surface of murine neuroepithelial cells and is also known as CD133 and AC133 (Maw et al., 2000). The AC133 antigen was initially identified as a cell surface antigen and is expressed in hematopoietic stem cells, endothelial progenitor cells, and others (Maw et al., 2000; Miraglia et al., 1997; Yin et al., 1997). A later study by Maw et al. reported that prominin is concentrated in the base of the photoreceptor outer segments and that loss of prominin causes retinal degeneration with abnormal generation/conversion of the evaginations to disks (Maw et al., 2000). Recently, PROM1 has been associated with the regulation of photoreceptor autophagy in the retinal pigment epithelium (RPE) (Bhattacharya et al., 2017).
Variants in the PROM1 gene have been associated with CORD/COD, MD, STGD, and RP (Arai et al., 2015; Arrigoni et al., 2011; Beryozkin et al., 2014; Birtel et al., 2018; Boulanger-Scemama et al., 2015; Carss et al., 2017; Cehajic-Kapetanovic et al., 2019; Collison et al., 2019; Eidinger et al., 2015; Eisenberger et al., 2013; Imani et al., 2018; Jinda et al., 2014; Khan & Bolz, 2015; Kim et al., 2017, 2019; Kniazeva et al., 1999; Liang et al., 2019; Littink et al., 2010; Liu et al., 2016; Maw et al., 2000; Mayer et al., 2016; Michaelides et al., 2005, 2010; Michaelides, Johnson, et al., 2003; Permanyer et al., 2010; Pras et al., 2009; Ragi et al., 2019; Salles et al., 2017; Song et al., 2011; Strauss et al., 2018; Wawrocka et al., 2018; Yang et al., 2008; Zhang et al., 2007; Zhao et al., 2015). Over 90 disease-associated PROM1 variants have been identified in AD and AR manners (The Human Gene Mutation Database; http://www.hgmd.cf.ac.uk/ac/index.php) (Supporting Information 1). However, the clinical and molecular genetic characteristics remain uncertain due to the lack of large cohort studies, especially in the Asian population.
The purpose of this study was to characterize the clinical and molecular genetic features of PROM1-RD in a large Japanese cohort with IRD. A literature review of PROM1-RD was also performed to understand the genetic spectrum in the Japanese population.
2 METHODS
The protocol of this study adhered to the tenets of the Declaration of Helsinki and was approved by the ethics committee of the participating institutions from Japan (National Institute of Sensory Organs, National Hospital Organization, Tokyo Medical Center (Reference: R18-029), and Kyoto University Graduate School of Medicine (Reference: G0746)).
2.1 Participants
Patients in the Japan Eye Genetics Consortium (JEGC; http://www.jegc.org/) with a clinical diagnosis of IRD and available genetic data were studied between 2008 and 2018. A total of 1,294 subjects from 730 families were surveyed.
2.2 Clinical examinations
Medical and family history was obtained in all affected subjects and unaffected family members (where available), including ethnicity, chief complaints of visual symptoms, onset of disease, and duration of disease (defined as the term between the onset and the latest examination).
Comprehensive ophthalmological examinations were performed in all affected subjects and unaffected family members (where available), including measurements of best-corrected decimal visual acuity (BCVA) converted to the logarithm of the minimum angle of resolution (LogMAR), ophthalmoscopy, fundus photography, fundus autofluorescence (FAF) imaging, spectral-domain optical coherence tomography (SD-OCT), kinetic and static visual field testing, and electrophysiological assessments according to the international standards of the International Society for Clinical Electrophysiology of Vision (ISCEV) (Hood et al., 2012; McCulloch et al., 2015a, 2015b; Robson et al., 2018).
2.3 Genetic screening in the PROM1 gene
Genomic DNA was extracted from all affected subjects and unaffected family members (where available for co-segregation analysis). Whole-exome sequencing with target analysis of retinal disease-associated genes (RetNet https://sph.uth.edu/retnet/) was performed according to the previously published methods (Fujinami et al., 2016; Oishi et al., 2016; Pontikos et al., 2020; Yang et al., 2020). The identified variants were filtered using allele frequency (less than 1%) in the Human Genetic Variation Database (HGVD; http://www.hgvd.genome.med.kyoto-u.ac.jp/), which provides allele frequencies of the general Japanese population. Depth and coverage for the target exons were interrogated using the integrative Genomics Viewer (http://www.broadinstitute.org/igv/). Sanger direct sequencing was performed to confirm the detected variants in the PROM1 gene, and to conduct co-segregation analysis.
Together with the clinical features of affected subjects and the model of inheritance in the pedigree in consideration of the results of co-segregation analysis, disease-causing variants were determined from the called/detected variants in the retinal disease-associated genes.
2.4 In silico molecular genetic analysis
The allele frequency of all detected variants in the HGVD, Integrative Japanese Genome Variation (iJGVD 3.5 k, 4.7 k; https://jmorp.megabank.tohoku.ac.jp/ijgvd/), 1000 Genomes (http://www.internationalgenome.org/), and the Genome Aggregation Database (gnomAD) was established (http://gnomad.broadinstitute.org/). All variants were analyzed with four general prediction programs and three functional prediction programs: MutationTaster (http://www.mutationtaster.org), FATHMM (http://fathmm.biocompute.org.uk/9), Combined Annotation Dependent Depletion (CADD; https://cadd.gs.washington.edu/), REVEAL (https://labworm.com/tool/revel), SIFT (https://www.sift.co.uk/), PROVEAN (http://provean.jcvi.org/index.php), and Polyphen 2 (http://genetics.bwh.harvard.edu/pph2/). Prediction of splice site alteration was performed with Human Splicing Finder (http://www.umd.be/HSF3/). The evolutionary conservation score for each variant was calculated from the UCSC database (https://genome.ucsc.edu/index.html). Variant classification was performed for all detected variants according to the guidelines of the American College of Medical Genetics and Genomics (ACMG) (Richards et al., 2015).
2.5 Literature search
Peer-reviewed articles that report PROM1 variants in the Japanese population were searched using the public search engine (PubMed; https://www.ncbi.nlm.nih.gov/pubmed/). Phenotypes of reported cases were surveyed, and in silico molecular genetic analysis for previously reported variants was performed in the same manner applied for the detected variants in the current study.
3 RESULTS
3.1 Participants
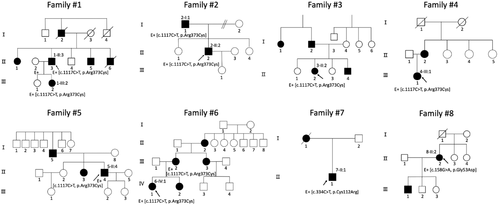
Ten affected subjects from eight families with a clinical diagnosis of IRD and who harbored heterozygous pathogenic PROM1 variants were ascertained in this study. Some data for one patient have been published elsewhere (Oishi et al., 2016). Eight out of 30 families (8/30, 26.7%) with AD COD/CORD/MD/STGD in the JEGC IRD cohort with available whole-exome sequencing results were associated with AD PROM1-RD. There were no families in the JEGC cohort with autosomal recessive PROM1-RD in the 529 families with AR or sporadic IRD.
The detailed demographic data are described in Table 1. All subjects were originally from Japan. There are five females and five males. The pedigrees of eight families are presented in Figure 1. AD family history was clearly reported in all eight families. The median age at the latest examination of 10 affected subjects was 44.5 years (range, 22–73).
| Family no | Patient no | Inheritance | Sex | Age (at latest examination) | Onset | Chief complaint | Best corrected visual acuity in the logMAR unit | |
|---|---|---|---|---|---|---|---|---|
| RE | LE | |||||||
| 1 | Patient 1(1-II:3) (TMC01-01) | AD | M | 73 | 45 | Reduced VA | 1.4 | 1 |
| 1 | Patient 2(1-III:2) (TMC01-02) | AD | F | 41 | 31 | Reduced VA | 0.7 | 0.15 |
| 2 | Patient 3(2-II:2) (JU01-01) | AD | M | 44 | 31 | Reduced VA | 0.7 | 0.1 |
| 2 | Patient 4(2-I:1) (JU01-02) | AD | M | 68 | 40 | Reduced VA | 1.05 | 1.3 |
| 3 | Patient 5(3-II:2) (TMC02-01) | AD | F | 40 | 10 | Photophobia | 1.3 | 1.22 |
| 4 | Patient 6(4-III:1) (TMC03-01) | AD | F | 38 | 30 | Reduced VA | 0.7 | 1 |
| 5 | Patient 7(5-II:4) (TU01-01) | AD | M | 45 | 34 | Reduced VA | 0.05 | 0.1 |
| 6 | Patient 8(6-IV:1) (KYU01-01) | AD | F | 22 | 10 | Reduced VA | 1 | 0.7 |
| 7 | Patient 9(7-II:1) (TMC04-01) | AD | M | 64 | 40 | Central visual field loss | 0.3 | 1 |
| 8 | Patient 10(8-II:2) (TMC05-01) | AD | F | 57 | 10 | Reduced VA | 0.7 | 0.82 |
- Note: Autosomal dominant family history (at least having two affected subjects in two consecutive generations) was clearly reported in all eight families. Age was defined the age when the latest examination was performed. The age of onset was defined as either the age at which visual loss was first noted by the patient or, in the “asymptomatic” patients, when an abnormal retinal finding was first detected.
- Abbreviations: AD, autosomal dominant; F, female; LE, left eye; LogMAR, logarithm of the minimum angle of resolution; M, male; No., number; RE, right eye.
3.2 Onset, chief complaint, and visual acuity
The median age of onset of 10 affected subjects was 31.0 years (range, 10–45). Three subjects had childhood onset of 10 years (3/10, 30%; Patients 5, 8, 10). Late onset of 45 years was reported in one subject (1/10, 10%; Patient 1). The other six subjects had intermediate onset between 15 and 45 years (6/10, 60%; Patients 2–4, 6, 7, 9). The median duration of disease of 10 affected subjects was 18.5 years (range, 8–47). Reduced visual acuity was reported as the chief complaint in eight subjects at the initial visit to the clinic (8/10, 80%; Patients 1–4, 6–8, 10).
The median values of BCVA in the right and left eyes of 10 affected subjects were 0.70 (range, 0.05–1.40, Snellen equivalent of 20/100) and 0.91 (0.1–1.22, Snellen equivalent of 20/160) in the LogMAR unit, respectively. Three subjects (3/10, 30.0%, Patients 2, 3, 7) had relatively favorable VA (LogMAR 0.22 or better in the better eye), four (4/10, 40.0%, Patients 6, 8–10) had moderate VA (between Log MAR 0.22 and 1.0 in the better eye), and three (3/10, 30.0%; Patients 1, 4, 5) had poor VA (LogMAR 1.0 or worse in the better eye). Asymmetric VA between the eyes (LogMAR 0.3 or more difference between the eyes) was observed in six subjects (6/10, 60%; Patients 1–3, 6, 8, 9).
3.3 Retinal imaging and morphological findings
Detailed findings of fundus, FAF, and SD-OCT images are presented in Table 2. Representative fundus and FAF images are shown in Figure 2. Central retinal atrophy was demonstrated in all 10 affected subjects. Pigmentation was noted to various degrees in the atrophic area. Central retinal atrophy was more evident in FAF. Mottled and patchy areas of decreased AF were present at the central retina corresponding to retinal atrophy in all subjects. Central atrophy was surrounded by a ring of increased AF in seven subjects (7/10, 70%; Patients 1–3, 7–10). A more diffuse area of increased AF with less evident increased AF surrounding the atrophy was observed in three subjects (3/10; Patients 4–6).
| Patient no | Fundus | FAF | SD-OCT | Visual field | Electrophysiological assessment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Central atrophy | Pigmentation | Mottled/patchy area of decreased AF | Ring of increased AF | Diffuse area of increased AF | FS (RE) | FS (LE) | Outer retinal disruption at the fovea | Outer retinal disruption at the parafovea | Increased signal of the choroid | EZ preservation at the fovea (RE) | EZ preservation at the fovea (LE) | Method | CS | Peripheral constriction | Responses in dark-adapted condition | Responses in light-adapted condition | |
| 1 | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | No | No | GP | Yes | Yes | Severely decreased | Mildly decreased |
| 2 | Yes | Yes | Yes | Yes | No | No | Yes (slight) | Yes | Yes | Yes | No | Yes | GP | Yes | No | Preserved | Mildly decreased |
| 3 | Yes | Yes | Yes | Yes | No | Yes (slight) | Yes (slight) | Yes | Yes | Yes | No | Yes | GP | Yes | No | Preserved | Moderately decreased |
| 4 | Yes | No | Yes | No | Yes | No | No | Yes | Yes | Yes | No | No | NA | NA | NA | Severely decreased | Severely decreased |
| 5 | Yes | No | Yes | No | Yes | Yes (slight) | Yes (slight) | No | Yes (extended) | Yes | Yes | No | GP/HFA | Yes | Yes | Severely decreased | Severely decreased |
| 6 | Yes | No | Yes | No | Yes | No | Yes (slight) | Yes | Yes | Yes | No | No | NA | NA | No | NA | NA |
| 7 | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | GP/HFA | Yes | No | Moderately decreased | Moderately decreased |
| 8 | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | No | No | GP | Yes | No | Preserved | Mildly decreased |
| 9 | Yes | No | Yes | Yes | No | Yes | Yes (slight) | No | Yes | Yes | Yes | No | GP/HFA | Yes | No | Preserved | Mildly decreased |
| 10 | Yes | No | Yes | Yes | No | No | No | No | Yes | No | Yes | No | GP | Yes | No | Preserved | Severely decreased |
- Note: Foveal sparing was defined as remaining foveal AF signal surrounded by the area of decreased AF.
- Abbreviations: BE, both eyes; CS, central scotoma; EZ, ellipsoid zone; FAF, fundus autofluorescence; FS, foveal sparing; GP, Goldmann Perimetry; HFA, Humphrey visual field analyzer; LE, left eye; M, male; NA, not available; RE, right eye; SD-OCT, spectral domain optical coherence tomography.
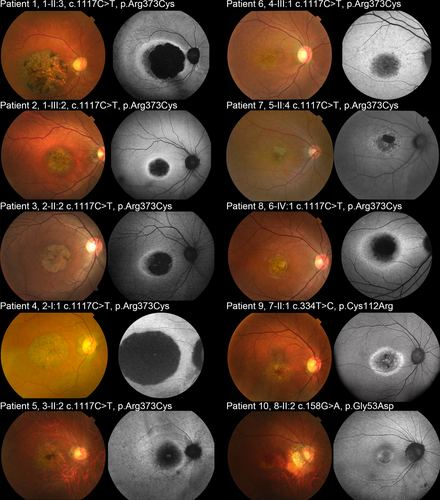
Foveal sparing (defined as remaining foveal AF signal surrounded by the area of decreased AF) was observed on FAF in three eyes of two subjects (3/20 eyes, 15.0%; Patients 7, 9). The median BCVA of the three eyes with foveal sparing was 0.10 (range, 0.05–0.3) in the LogMAR unit, respectively.
Representative SD-OCT images are shown in Figure 3. Outer retinal disruption was observed at the fovea and parafovea in six subjects (6/10, 60.0%; Patients 1–4, 6, 8). Outer retinal disruption at the parafovea was found in four subjects (4/10, 40.0%; Patients 5, 7 9, 10), and one of these subjects had outer retinal disruption from the parafovea to the periphery (1/10, 10.0%; Patient 5). Increased signal of the choroid, indicating RPE atrophy, was observed in nine subjects (9/10, 90.0%; Patients 1–9), and no evidence of hypertransmission was found in one subject (1/10, 10%; Patient 10).

The ellipsoid zone was preserved at the fovea in seven eyes of six subjects (7/20, 35.0%; Patients 2, 3, 5, 7, 9, 10). The median BCVA of seven eyes with preserved ellipsoid zones was 0.15 (range, 0.05–0.7) in the LogMAR unit.
3.4 Visual field and electrophysiological findings
Detailed findings of visual fields and electrophysiological assessment are presented in Table 2. Visual field testing was performed in all affected subjects except for two subjects (Patients 4, 6), with Goldmann perimetry (GP; 8 subjects) and Humphrey visual field analyzer (HFA; 3 subjects). Central scotoma was observed in all eight subjects (8/8, 100.0%, Patients 1–3, 5, 7–10), with peripheral constriction found in two subjects (2/8, 25.0%; Patients 1, 5).
Electrophysiological testing was performed in all affected subjects except for one subject (Patient 6). Mildly decreased generalized cone responses (amplitude reduction less than 50% of normal reference) were demonstrated in five subjects (5/9, 55.6%; Patients 1, 2, 8–10). Moderately decreased generalized cone responses (amplitude reduction between 25 and 50% of normal reference) were shown in two subjects (2/9, 22.2%; Patients 3, 7). Severely decreased generalized cone responses (amplitude reduction more than 75% of normal reference) with preserved rod responses were found in one subject (1/9, 11.1%; Patient 10). Severely decreased generalized cone and rod responses were identified in three subjects (3/9, 33.3%; Patients 1, 4, 5).
3.5 PROM1 variants
Variant data of 11 affected and two unaffected subjects of eight families are summarized in Table S1. Three heterozygous PROM1 variants were identified by whole-exome sequencing with target analysis of retinal disease-associated genes (Table S2): c.1117C>T, (p.Arg373Cys); c.334T>C, (p.Cys112Arg); c.158G>A, (p.Gly53Asp) (NM_006017.2). One variant (p.Arg373Cys) was previously reported, and two variants have never been reported (p.Cys112Arg, p.Gly53Asp) (Michaelides et al., 2005). These three PROM1 variants were confirmed with direct sequencing, and two variants were co-segregated within the families (p.Arg373Cys, p.Gly53Asp; Families #1, #2, #6, #8).
One variant was recurrent; p.Arg373Cys (6/8 families, 75.0%). Each of the other two variants was found in a single family (p.Cys112Arg for Family #7, p.Gly53Asp for Family #8). Called variants detected by whole-exome sequencing with targeted analysis in Family #7 were c.334T>C, (p.Cys112Arg) in the PROM1 gene; c.3404C>T, (p.Pro1135Leu) in the C2orf71 gene; c.1405G>T, (p.Val469Phe) in the MERTK gene; c.404T>C, (p.Ile135Thr) in the SLC7A14 gene; c.3489T>A, (p.Asn1163Lys) in the EYS gene; c.1282G>A, (p.Asp428Asn) in the CDH23 gene; and c.47G>A, (p.Gly16Asp) in the FZD gene. Two variants with AD inheritance (PROM1, FZD4) and low allele frequency (<0.5% of HGVD) were included. Called variants in Family #8 were c.158G>A, (p.Gly53Asp) in the PROM1 gene; c.1327C>T, (p.Arg443Trp) in the CYP4V2 gene; and c.8053G>A, (p.Val2685Ile) in the VCAN gene. Two variants with AD inheritance (PROM1, VCAN) and low allele frequency (<0.5% of HGVD) were included. Together with the clinical features of affected subjects and the model of inheritance in the pedigree, disease-causing variants in the PROM1 gene were determined.
3.6 In silico molecular genetic analysis for detected variants in the current study
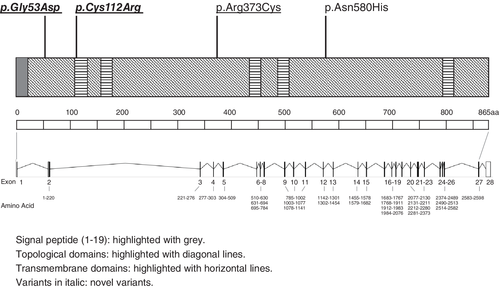
The detailed results of in silico molecular genetic analyses for the three detected PROM1 variants are presented in Table 3. The schematic genetic and protein structure of PROM1 and the location of the variants are shown in Figure 4, and multiple alignments of eight species of PROM1 is demonstrated in Figure 5.
| iJGVD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nucleotide change, amino acid change/effect | Study | Position | Coding impact | Location | dbSNP ID | HGVD | 3.5 k | 4.7 k | 1,000 genome |
| c.1738A>C,p.Asn580His | Arai et al. Journal of Ophthalmology 2015 | 15995639 | Missense | Exon 16 of 28 position 56 of 85 (coding) | rs199674847 | 0.0054 | 0.0075 | 0.0074 | NA |
| c.1578+1G>A, splice site alteration (denoted asc.1551+1G>A in the original report; NM_001145847.2) | Koyanagi et al. Journal of Medical Genetics 2018 | 16002118 | Splice site alteration | Intron 14 of 27 position 1 of 2007 (splicing, intronic) | rs1553901823 | NA | NA | NA | NA |
| c.1117C>T,p.Arg373Cys | This study/Koyanagi et al. Journal of Medical Genetics 2018 | 16014922 | Missense | Exon 11 of 28 position 40 of 64 (coding) | rs137853006 | NA | NA | NA | NA |
| c.334T>C, p.Cys112Arg | This study | 16035102 | Missense | Exon 5 of 28 position 31 of 206 (coding) | NA | NA | NA | NA | NA |
| c.158G>A, p.Gly53Asp | This study | 16077372 | Missense | Exon 2 of 28 position 370 of 432 (coding) | rs755064227 | 0.0021 | 0.0017 | 0.0019 | 4.82E-05 |
| Nucleotide change, amino acid change/effect | GnomAD | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele frequency (exome) | Allele frequency (genome) | Coverage in GnomAD Exomes samples | |||||||||||||||
| East Asian | South Asian | African | European (non-Finnish) | Total | Male | Female | East Asian | South Asian | African | European (non-Finnish) | Total | Male | Female | Mean coverage | Median coverage | % of samples over 20x coverage | |
| c.1738A>C,p.Asn580His | 0.00167 | NA | NA | NA | 0.00012 | 0.0000962 | 0.000149 | NA | NA | NA | NA | NA | NA | NA | 65.9 | 65 | 99.47 |
| c.1578+1G>A, splice site alteration (denoted asc.1551+1G>A in the original report; NM_001145847.2) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 26.4 | 23 | 67.96 |
| c.1117C>T,p.Arg373Cys | NA | NA | NA | NA | 0.00000401 | NA | 0.00000877 | NA | NA | NA | NA | NA | NA | NA | 63.8 | 59 | 99.23 |
| c.334T>C, p.Cys112Arg | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 33.4 | 31 | 75.29 |
| c.158G>A, p.Gly53Asp | 0.000612 | 0.0000328 | NA | NA | 0.0000482 | 0.0000519 | 0.0000439 | NA | NA | NA | NA | NA | NA | NA | 58.6 | 59 | 94.01 |
| Nucleotide change, amino acid change/effect | General prediction | Functional prediction | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation taster | FATHMM | CADD | REVEAL | SIFT | PROVEAN | Polyphen2 | ||||||||||||
| Prediction | Accuracy | Converted rank score | Prediction | Score | Converted rank score | Score | Prediction | Score | Rank score | Prediction | Score | Converted rank score | Prediction | Score | Converted rank score | Prediction | Score | |
| c.1738A>C,p.Asn580His | Polymorphism | 0.7083, 0.6447 | 0.3058 | Tolerated | 0.66 | 0.5242 | 18.87 | Benign | 0.3059 | 0.6309 | Tolerated | 0.052, 0.055 | 0.3909 | Damaging | −4.01, −3.91 | 0.7419 | Possibly damaging | 0.924 |
| c.1578+1G>A, splice site alteration (denoted as c.1551+1G>A in the original report; NM_001145847.2) | Disease causing | 1 | 0.81 | Damaging | 0.9845 | 0.8287 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| c.1117C>T,p.Arg373Cys | Disease causing automatic | 1.58E-10 | 0.08975 | Tolerated | 0.86 | 0.4678 | 6.971 | Benign | 0.2809 | 0.6024 | Damaging | 0.002 | 0.7215 | Damaging | −2.67 | 0.5711 | Possibly damaging | 0.936 |
| c.334T>C, p.Cys112Arg | Polymorphism | 0.9989, 0.9981 | 0.2234 | Tolerated | 0.77 | 0.4964 | 21.4 | Benign | 0.187 | 0.4672 | Damaging | 0.002 | 0.7215 | Damaging | −8.78, −8.98 | 0.9804 | Probably damaging | 0.996 |
| c.158G>A, p.Gly53Asp | Polymorphism | 1 | 0.08975 | Tolerated | 1.45, 1.06 | 0.3978 | 20.7 | Benign | 0.141 | 0.3826 | Tolerated | 0.083, 0.084, 0.253, 0.076, 0.08 | 0.3427 | Damaging | −2.52, −3.55, −2.54 | 0.6876 | Possibly damaging | 0.538 |
| Nucleotide change, amino acid change/effect | Human splice finder 3.0 | Conservation | Conservation | ACMG classification | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PhyloP46way | PhastCons46way | PhyloP100way | PhastCons100way | Verdict | Identified classification rules | |||||||||||
| Mammalian | Mammalian rank score | Mammalian | Mammalian rank score | Vertebrate | Vertebrate rank score | Vertebrate | Vertebrate rank score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | |||
| c.1738A>C,p.Asn580His | No impact | 1.068 | NA | 0.944 | NA | 4.2049 | 0.5827 | 1 | 0.7164 | Likely benign | BS1 | BP4 | ||||
| c.1578+1G>A, splice site alteration (denoted as c.1551+1G>A in the original report; NM_001145847.2) | NA | NA | NA | NA | NA | 7.662 | 0.8279 | 1 | 0.7164 | Pathogenic | PVS1 | PM2 | PP5 | |||
| c.1117C>T,p.Arg373Cys | Potential alteration | 0.336 | NA | 0.002 | NA | 0.303 | 0.1896 | 0.001 | 0.1379 | Pathogenic | PS3 | PM2 | PP4 | PP5 | BP4 | |
| c.334T>C, p.Cys112Arg | No impact | 1.356 | NA | 0.002 | NA | 1.445 | 0.3469 | 0.001 | 0.1379 | Likely pathogenic | PM2 | PP4 | PP5 | BP4 | ||
| c.158G>A, p.Gly53Asp | Potential alteration | 0.118 | NA | 0 | NA | −0.2119 | 0.09323 | 0 | 0.06391 | Uncertain significance | PM2 | PP4 | BP4 | |||
- Note: Reference: NM_006017.2, ENST00000510224.1, GRCh37.p13. Sequence variant nomenclature was obtained according to the guidelines of the Human Genome Variation Society (HGVS) by using Mutalyzer (https://mutalyzer.nl/). The allele frequency of all detected variants in the HGVD, Integrative Japanese Genome Variation (iJGVD 3.5 k, 4.7 k; https://jmorp.megabank.tohoku.ac.jp/ijgvd/), 1000 genome (http://www.internationalgenome.org/), and the genome Aggregation Database (gnomAD) was established (http://gnomad.broadinstitute.org/). All variants were analyzed with four general predictions programs and three functional prediction programs; MutationTaster (http://www.mutationtaster.org), FATHMM (http://fathmm.biocompute.org.uk/9), Combined Annotation Dependent Depletion (CADD; https://cadd.gs.washington.edu/), REVEAL (https://labworm.com/tool/revel), SIFT (https://www.sift.co.uk/), PROVEAN (http://provean.jcvi.org/index.php), Polyphen 2 (http://genetics.bwh.harvard.edu/pph2/). Prediction on splice site alteration was performed with Human splicing finder (http://www.umd.be/HSF3/). Evolutional conservation score for each variant was calculated from the UCSC database (https://genome.ucsc.edu/index.html). Variant classification was performed for all detected variants, according to the guidelines of the American College of Medical Genetics and Genomics (ACMG). Classification of predictions by the American College of Medical Genetics and Genomics (ACMG) was also applied for all detected variants; PVS1 (Null variant [nonsense, frameshift, canonical 1 or 2 splice sites, initiation codon, single or multiexon deletion] in a gene where loss of function is a known mechanism of disease); PS3 (Well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product); PM2 (pathogenicity moderate 2; absent from controls in Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium); PP4 (Patients phenotype or family history is highly specific for a disease with asingle genetic etiology); PP5 (pathogenicity supporting 5; reputable source recently reports variant as pathogenic, but the evidence is not available to the laboratory to perform an independent evaluation); BS1 (Allele frequency is greater than expected for disorder); BP4 (benign supporting 4; Multiple lines of computational evidence suggest no impact on gene or gene product).

Allele frequency for the three PROM1 variants (p.Arg373Cys, p.Cys112Arg, p.Gly53Asp) in the general population was 0.00041, 0.0, 0.0045%, respectively. The allele frequency of East Asian/South Asian/African/European (non-Finish) for these three PROM1 variants (p.Arg373Cys, p.Cys112Arg, p.Gly53Asp) was 0.0/0.0/0.0/0.0, 0.0/0.0/0.0/0.0%, and 0.058/0.0033/0.0/0.0%, respectively. There was one variant (p.Gly53Asp) which had a significantly higher allele frequency in the East Asian population than in other populations (p < .001, Fisher's exact test).
General prediction, functional prediction, and conservation were assessed for the three PROM1 variants, and pathogenicity classification according to the ACMG guidelines (Richards et al., 2015) was pathogenic for p.Arg373Cys, likely pathogenic for p.Cys112Arg, and uncertain significance for p.Gly53Asp, respectively. The specific evidence levels used in the ACMG classification for each PROM1 variant is presented in Table 3.
Overall, one disease-causing variant (p.Arg373Cys), one putative disease-causing variant (p.Cys112Arg) and one variant of uncertain significance (VUS) (p.Gly53Asp) in the PROM1 gene were ascertained in eight families with AD COD/CORD/MD.
3.7 Previously reported PROM1 variants in the Japanese population
There are two reports describing PROM1-RD in the Japanese population other than the cases reported here. Koyanagi et al. reported two patients in a cohort of 1,204 Japanese patients with RP (Koyanagi et al., 2019). One “solved” 69-year-old female with RP harbored a heterozygous PROM1 variant (c.1117C>T, (p.Arg373Cys)), as well as an ABCA4 variant (c.4462T>C, [p.Cys1488Arg]—likely pathogenic) and an SAG variant (c.926delA, p.Asn309ThrfsTer12—pathogenic). One “unsolved” 61-year-old female with RP had a heterozygous canonical splice variant [(c.1578+1G>A; denoted as c.1551+1G>A in the original report (NM_001145847.2)]. No further detailed phenotypic information is available in these two cases.
Arai et al. reported four cases of AD PROM1-RD (Arai et al., 2015). Three unrelated cases shared a heterozygous PROM1 variant (c.1738A>C,[p.Asn580His]). The other PROM1 variant was not described. One 37-year-old patient with this variant (p.Asn580His) showed a phenotype of cone dystrophy. One patient with the AD RP phenotype harboring the PROM1 variant (p.Asn580His) also harbored a heterozygous EYS variant (c.768A>G, p.Ile256Met –VUS) and a heterozygous CRB1 variant (c.2306G>A, p.Arg769His—benign). Another patient with AD RP phenotype harboring the PROM1 variant (p.Asn580His) had a heterozygous EYS variant (c.4957dupA, p.Ser1653LysfsTer2—pathogenic) and a heterozygous CRB1 variant (c.2306G>A, p.Arg769His—benign). Association of EYS, PROM1, and CRB1 in retinal dystrophies were proposed in the literature.
3.8 In silico molecular genetic analysis for previously reported variants
The detailed results of in silico molecular genetic analyses for the three PROM1 variants (c.1117C>T, p.Arg373Cys, c.1551+1G>A, c.1738A>C,p.Asn580His) described in previous reports are presented in Table 3. The first variant (p.Arg373Cys) was also identified in the current study and the latter two variants (c.1551+1G>A, c.1738A>C, (p.Asn580His)) were described only in the two Japanese reports. A schematic genetic and protein structure is shown in Figure 4, and multiple alignment of eight species is demonstrated in Figure 5.
Allele frequencies for the two previously reported PROM1 variants (p.Asn580His, c.1551+1G>A) in the general population were 0.012 and 0.0%, respectively. The allele frequencies of East Asian/South Asian/African/European (non-Finish) for these two PROM1 variants (p.Asn580His, c.1551+1G>A) were 0.167/0.0/0.0/0.0% and 0.0/0.0/0.0/0.0%, respectively. One variant (p.Asn580His) had a significantly higher allele frequency in the East Asian population than in other populations (p < .001, Fisher's exact test).
General prediction, functional prediction, and conservation were assessed for the two PROM1 variants, and pathogenicity classification according to the ACMG guidelines was likely benign for p.Asn580His and pathogenic for c.1551+1G>A.
4 DISCUSSION
Detailed clinical and genetic characteristics of a Japanese cohort of 10 affected subjects from eight families with AD PROM1-RD are illustrated. Characteristic features of macular atrophy and generalized cone-dominated retinal dysfunction were identified in all available cases. Foveal sparing was frequently found in AD PROM1-RD: 15.0% on FAF and 35.0% on SD-OCT. One common missense variant (p.Arg373Cys, 6/9 families, 66.7%) found both in the current and previous studies was determined out of five PROM1 variants, in keeping with the common phenotype of MD/COD/CORD in the Japanese population.
The detailed data of a large cohort of Japanese patients with AD PROM1-RD are first described in the present study, while there are two large cohort studies of the detailed phenotype of European families with AD or AD/AR PROM1-RD (Cehajic-Kapetanovic et al., 2019; Michaelides et al., 2010). PROM1-RD accounts for 26.7% of AD COD/CORD/MD/STGD in the JEGC IRD cohort, in contrast with a lower prevalence of PROM1-RD in the other population (11.3% in United Kingdom) (Gill et al., 2019). There were no families with AR PROM1-RD in the current study, although a higher prevalence (approximately twice) of AR PROM1-RD than AD PROM1-RD was reported in another U.K. report (Cehajic-Kapetanovic et al., 2019). These facts indicate the relatively high prevalence of PROM1-RD in AD IRD predominantly affecting the macula/cone system in the Japanese population.
The age of onset of AD PROM1-RD in our cohort was variable, ranging from childhood to the forties. The early onset and long duration of the disease were not necessarily associated with poor vision; the duration of disease was similar in Patients 6 and 7 (8 vs. 11 years), but much better vision was found in Patient 6. In addition, the duration of disease was 47 years in Patient 10, but this patient still had a similar level of vision compared to a patient with 8 years of disease (Patient 6). These findings suggest that age of onset and duration of disease are not clearly related to the severity of visual acuity in PROM1-RD, unlike other macular dystrophies (Fujinami, Sergouniotis, Davidson, Wright, et al., 2013; Fujinami, Sergouniotis, Davidson, Mackay, et al., 2013; Fujinami et al., 2014, 2015, 2019; Nakamura et al., 2019). These are probably because of the frequent presence of foveal sparing (bull's eye maculopathy) in PROM1-RD.5
In our cohort, all 10 affected subjects showed concentrated macular atrophy in fundus photographs, and the atrophic or mottled areas were clearly demonstrated on FAF images. The very similar macular findings on fundus and FAF images found in our cohort were identified in previous studies of a specific variant (p.Arg373Cys) (Cehajic-Kapetanovic et al., 2019; Kim et al., 2017; Michaelides et al., 2010). It is of note, however, the peripheral atrophic changes were also found in a previous study (Michaelides et al., 2010).
All nine subjects with available data in our cohort demonstrated generalized cone or cone rod dysfunction. Five of nine subjects (55.6%) had preserved rod function. Four subjects (44.4%) with good VA showed slight or moderate reduction in cone responses; thus, the maintained generalized cone function could be an indicator for good vision. As shown in the previous study, the cone-dominated reduction of generalized retinal function is a key feature of AD PROM1-RD (Michaelides et al., 2010).
One disease-causing heterozygous variant, one putative disease-causing heterozygous variant, and one VUS were identified in our cohort (p.Arg373Cys, p.Cys112Arg, and p.Gly53Asp). Three variants (c.1117C>T, (p.Arg373Cys); c.1551+1G>A; c.1738A>C, (p.Asn580His)) were previously reported in the Japanese population. The recurrent variant (p.Arg373Cys) was reported in several publications of IRD in European/South Asian/African populations and our six AD Japanese families demonstrated very similar clinical findings, although the phenotypic information of a previously reported family was limited. Due to the limited genetic data source for haplotype analysis in this study to determine a single or multiple founder events, additional investigations are necessary to discern these. This variant introduces an additional cysteine residue in the extracellular loop of PROM1 (O43490; UniProt; https://www.uniprot.org/; Figure 4), which can cause disulfide bridge network disruption and abnormal homophilic protein interactions (Yang et al., 2008). The Arg373Cys mutant protein is mislocalized and causes mislocalization of the wild-type PROM1 protein and wild-type PCDH21 protein, which eventually leads to retinal degeneration (i.e., dominant-negative effect) (Yang et al., 2008).
One putative disease-causing variant (p.Cys112Arg) and a VUS (p.Gly53Asp) are located in a transmembrane domain and an extracellular domain of the PROM1 protein (Figure 4). Perfect evolutionary conservation was shown in the locations for both variants (p.Cys112Arg and p.Gly53Asp) (Figure 5). Given the extremely low allele frequency and the in silico prediction of the former variant (p.Cys112Arg; none in the general population detected by gnomAD whole exome sequence), this variant may represent a cause of AD PROM1-RD, although co-segregation analysis of family members was unavailable in this family (Family #7). The latter variant (p.Gly53Asp) with a relatively high allele frequency (0.0000482 detected by gnomAD whole exome sequence) in the general population suggests little supporting evidence for causation. The clinical effect of these variants is still uncertain, and function analysis is therefore needed to further understand the disease mechanism caused by these two variants.
Four of five PROM1 variants (p.Gly53Asp; p.Cys112Arg; c.1551+1G>A; p.Asn580His) identified in the Japanese population have never been found in other populations. This finding suggests the distinct genetic background in the Japanese population with regard to the PROM1 gene. Significantly high allele frequencies of two variants (p.Gly53Asp; p.Asn580His) in the general East Asian population could support this finding; although the provided information for the two unique variants (c.1551+1G>A—unsolved; p.Asn580His—a modifier) reported in previous Japanese reports was limited; (Arai et al., 2015; Koyanagi et al., 2019) thus, further genomic and functional analyses would help to interpret how these two variants have clinical effects as well as determine an inheritance manner.
There are limitations in this study. The selection bias related to the disease severity cannot be excluded, since it is unusual for subjects genetically at risk who show foveal sparing and no impaired visual acuity to visit clinics/hospitals. Moreover, this study is a cross-sectional retrospective study; thus, longitudinal natural history studies in a larger cohort could provide more accurate information for the phenotypic variation and the disease progression of PROM1-RD. The molecular mechanisms of most autosomal dominant PROM1 variants are not yet known, and further functional investigation is required to conclude the disease-causation. The allele frequency provided by the public databases was applied for assessment of pathogenicity for each variant, however, it would be expected for individuals with the late-onset disease to be potentially included in the general population databases. The number of studies on PROM1-RD in the Japanese population obtained by a literature search was still limited, and further data accumulation should improve the achievements obtained by reviewing articles.
In conclusion, this large cohort study in the Japanese population determined the clinical and genetic characteristics of AD PROM1-RD. Concentrated macular atrophy on retinal imaging is a striking feature, with generalized cone-dominated retinal dysfunction. Disease onset and natural course was not clearly associated with severe visual impairment, and preservation of foveal structure and function is crucial for maintaining visual acuity. One prevalent missense variant (c.1117C>T, p.Arg373Cys) was determined in the Japanese population, which was also frequently detected in the European population. This information helps to monitor and counsel patients, as well as in designing future therapeutic trials.
ACKNOWLEDGMENTS
Kaoru Fujinami is supported by grants from Grant-in-Aid for Young Scientists (A) of the Ministry of Education, Culture, Sports, Science and Technology, Japan (16H06269), grants from Grant-in-Aid for Scientists to support international collaborative studies of the Ministry of Education, Culture, Sports, Science and Technology, Japan (16KK01930002), grants from National Hospital Organization Network Research Fund (H30-NHO-Sensory Organs-03), grants from FOUNDATION FIGHTING BLINDNESS ALAN LATIES CAREER DEVELOPMENT PROGRAM (CF-CL-0416-0696-UCL), grants from Health Labour Sciences Research Grant, The Ministry of Health Labour and Welfare (201711107A), and grants from Great Britain Sasakawa Foundation Butterfield Awards. Akio Oishi is supported by grants from, Japan Society for the Promotion of Science, Tokyo, Japan (17H06820 and 19K09929). Yu Fujinami-Yokokawa is supported by grants from Grant-in-Aid for Young Scientists of the Ministry of Education, Culture, Sports, Science and Technology, Japan (18K16943). Gavin Arno is supported by a Fight for Sight (UK) Early career investigator award, NIHR-BRC at Moorfields Eye Hospital and the UCL Institute of Ophthalmology, NIHR-BRC at Great Ormond Street Hospital and UCL Institute of Child Health, and Great Britain Sasakawa Foundation Butterfield Award, UK. Nikolas Pontikos is funded by a Moorfields Eye Charity Career Development Award (R190031A), the NIHR-BRC at Moorfields Eye Hospital and the UCL Institute of Ophthalmology. Toshihide Kurihara is supported by Tsubota Laboratory, Inc, Fuji Xerox Co., Ltd, Kirin Company, Ltd, Kowa Company, Ltd, Novartis Pharmaceuticals Japan, Santen Pharmaceutical Co. Ltd, and ROHTO Pharmaceutical Co., Ltd. Takeshi Iwata is supported by Japan Agency for Medical Research and Development (AMED) (18ek0109282h0002). Kazushige Tsunoda is supported by AMED, the Ministry of Health, Labor and Welfare, Japan (18ek0109282h0002), Grants-in-Aid for Scientific Research, Japan Society for the Promotion of Science, Japan (H26-26462674), grants from National Hospital Organization Network Research Fund, Japan (H30-NHO-Sensory Organs-03) and Novartis Research Grant (2018).
CONFLICT OF INTEREST
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s). Kaoru Fujinami is a paid consultant of Astellas Pharma Inc, Kubota Pharmaceutical Holdings Co., Ltd, and Acucela Inc., Novartis AG., Janssen Pharmaceutical K.K., Sanofy Genzyme, NightStart. Kaoru Fujinami reports personal fees from Astellas Pharma Inc, personal fees from Kubota Pharmaceutical Holdings Co., Ltd., personal fees from Acucela Inc., personal fees from NightStar, personal fees from SANTEN Company Limited, personal fees from Foundation Fighting Blindness, personal fees from Foundation Fighting Blindness Clinical Research Institute, personal fees from Japanese Ophthalmology Society, personal fees from Japan Retinitis Pigmentosa Society. Laboratory of Visual Physiology, Division of Vision Research, National Institute of Sensory Organs, National Hospital Organization Tokyo Medical Center, Tokyo, Japan is supported by grants from Astellas Pharma Inc (NCT03281005), outside the submitted work. Akio Oishi receives grant from Novartis AG and Alcon. Akio Oishi reports personal fees from Bayer, Novartis, Alcon, Santen, and HOYA outside the submitted work. Akitaka Tsujikawa receives research grant by Novartis, Bayer, Santen, Canon, Alcon, Bayer, HOYA, Kowa, Pfizer, AMO, Senju, Wakamoto, Japan Medical Industry Association, Tomey, JFC salesplan, Atsuzawa Prosthesis. Akitaka Tsujikawa reports personal fees from Novartis, Alcon, Bayer, Pfizer, Santen, Senju, Nidek, AMO, Kowa, Chugai, Sanwa chemical, Kyoto Soyaku, HOYA, Otsuka, Daiichi Sankyo, Wakamoto, Johnson and Johnson, and Yansen pharma.
AUTHOR CONTRIBUTIONS
Kaoru Fujinami and Akio Oishi: Have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Kaoru Fujinami, Akio Oishi, Akitaka Tujikawa, Kazushige Tsunoda: Research design. Kaoru Fujinami, Akio Oishi, Lizhu Yang, Gavin Arno, Nikolas Pontikos, Kazutoshi Yoshitake, Yu Fujinami-Yokokawa, Xiao Liu, Takaaki Hayashi, Satoshi Katagiri, Kei Mizobuchi, Atsushi Mizota, Kei Shinoda, Natsuko Nakamura, Toshihide Kurihara, Kazuo Tsubota, Yozo Miyake, Takeshi Iwata, Akitaka Tsujikawa, Kazushige Tsunoda; Japan Eye Genetics Consortium study group: Data acquisition and/or research execution. Kaoru Fujinami, Akio Oishi, Lizhu Yang, Gavin Arno, Nikolas Pontikos, Kazutoshi Yoshitake, Yu Fujinami-Yokokawa, Xiao Liu, Takaaki Hayashi, Satoshi Katagiri, Kei Mizobuchi, Atsushi Mizota, Kei Shinoda, Natsuko Nakamura, Toshihide Kurihara, Kazuo Tsubota, Yozo Miyake, Takeshi Iwata, Akitaka Tsujikawa, Kazushige Tsunoda; Japan Eye Genetics Consortium study group: Data analysis and/or interpretation. Kaoru Fujinami, Akio Oishi, Lizhu Yang, Yu Yokokawa-Fujinami, Xiao Liu, Kei Shinoda, Akitaka Tujikawa, Kazushige Tsunoda: Manuscript preparation.