Three additional de novo CTCF mutations in Chinese patients help to define an emerging neurodevelopmental disorder
Funding information: key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C, Grant/Award Number: 201610191; Major Research Plan of the Provincial Science and Technology Foundation of Guangxi, Grant/Award Number: AB16380219; National Natural Science Foundation of China, Grant/Award Number: 81873633; the “Eastern Scholar” Fund; the “Guangxi Bagui Scholar” fund; the GeneScience Pharmaceuticals Co., Ltd (Changchun, China); National key research and development program, Grant/Award Number: 2018YFC1002501
Abstract
CCCTC-binding factor (CTCF) is an important regulator for global genomic organization and gene expression. CTCF gene had been implicated in a novel disorder characterized by intellectual disability, feeding difficulty, developmental delay and microcephaly. So far, four patients have been reported with de novo CTCF mutations. We reported three additional Chinese patients with de novo variants in CTCF. The new evidence helped to establish the clinical validity between CTCF and the emerging disorder. We described the consistent phenotypes shared by all patients and revealed additional clinical features such as delayed or abnormal teeth development and a unique pattern of the eyebrow that may help to define a potential recognizable neurodevelopmental disorder. We also reported the first CTCF patient treated with recombinant human growth hormone. Follow-up and more case studies will further our understanding to the clinical presentations of this novel disorder and the prognosis of patients with this disorder.
1 INTRODUCTION
CTCF gene (OMIM:604167) encodes the CCCTC-binding factor (CTCF). CTCF has been implicated in the formation of topologically associated domains (TADs) by binding to tens of thousands of DNA sequence-specific sites (CTCF motifs) in the human genome (Barutcu, Lian, Stein, Stein, & Imbalzano, 2017; Barutcu, Maass, Lewandowski, Weiner, & Rinn, 2018; Nora et al., 2017). It functions as a transcriptional insulator by preventing interactions between promoter and nearby enhancers and silencers (Austin, Lu, Majumder, Ahmed, & Boss, 2014; MacPherson & Sadowski, 2010; Nora et al., 2017; Xie et al., 2007); acts as a chromatin barrier by preventing the spread of heterochromatin structures and epigenetic modification (Chang et al., 2015; Guerra-Calderas et al., 2018; Hou, Zhao, Tanimoto, & Dean, 2008; Miura, Miyazato, Satou, Tanaka, & Bangham, 2018; Satou et al., 2016; Yang et al., 2003); and causes the bound DNA to form loops which influence the higher-order chromatin structure by forming CTCF homodimers (Battistelli, Busanello, & Maione, 2014; de Wit et al., 2015; Weth et al., 2010). CTCF is a multivalent transcription factor with 11 highly conserved zinc finger (ZF) domains, functions both as a transcriptional activator or a transcriptional repressor depending in the context of the specific binding sites and binding partners in the complex (Filippova et al., 1996; Holwerda & de Laat, 2013; Kim, Yu, & Kaang, 2015; Liu, Wu, & Wang, 2018; Rubio et al., 2008). Overall, CTCF plays important roles in regulating global chromatin organization and gene expression (Franco, Prickett, & Oakey, 2014). The involvement of the CTCF gene in human disease pathogenesis is emerging.
In 2013, Gregor et al. (2013) first identified a de novo frameshift variant by trio exome sequencing in CTCF in a patient with intellectual disability. Two more de novo variants were detected in the subsequent screening among a cohort of patients with intellectual disability. In 2017, Bastaki et al. (2017) reported a de novo frameshift variant in an Arab female patient with syndromic intellectual disability. So far, only four patients have been reported worldwide. Consistent clinical features among the four patients included developmental delay/intellectual disability, hypotonia, early feeding difficulty, microcephaly and mild facial dysmorphism. Based on the current evidence, the gene-disease association is classified as “Moderate” by the ClinGen gene curation protocol (https://search.clinicalgenome.org/kb/gene-validity/10143). Thus, this is an emerging disorder that requires additional genetic or functional evidence to establish the clinical validity between CTCF and the disease. The disease is defined as “Intellectual disability-feeding difficulties-developmental delay-microcephaly syndrome” by Orphnet (ORPHA:363611) and “Mental retardation, autosomal dominant 21” by OMIM (OMIM:615502).
Here we reported three unrelated Chinese patients with de novo CTCF variants and similar clinical phenotypes. Addition of these new cases can elevate the level of gene-disease association in ClinGen to “Strong” (see Section 4), thus firmly establishing the causal relationship between CTCF and the novel neurodevelopmental disorder. This report also expanded the mutation spectrum of CTCF gene and revealed the consistent phenotypes across populations and novel features of this condition.
2 MATERIALS AND METHODS
2.1 Editorial policies and ethical considerations
Written informed consent for participation in this study was collected from the family members of all patients. The study was approved by the ethics committees of the following institutions: The Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region; The Maternal and Child Health Care Hospital of Dongguan city; Fuzhou Children's Hospital of Fujian Province.
2.2 Sample collection and DNA extraction
Genomic DNA was isolated from peripheral blood lymphocytes using Lab-Aid DNA kit (Zeesan Biotech Co., Ltd, Xiamen, China).
2.3 Whole-exome sequencing and Sanger sequencing
For whole-exome sequencing, genomic DNA samples were captured to create a sequencing library by Agilent SureSelect Human All Exon V5 Kit (Agilent Technologies, Santa Clara, CA) in accordance with the manufacturer's protocol. The prepared libraries were sequenced with a HiSeq2500 (Illumina, San Diego, CA). Sanger sequencing was used to verify the mutations and their origins. Paternity testing was performed with STR genotyping. All procedures followed the manufacturer's instructions.
2.4 Sequencing data analysis
The Genome Analysis Toolkit (GATK) was used for variant calling (GATK HaplotypeCaller; McKenna et al., 2010). The TGex software (LifeMap Sciences, Alameda, CA) was used to annotate the selected SNVs and indels. “Rare deleterious” mutations were defined as those that met the following criteria: (a) they led to a stop-gain, stop-loss, nonsynonymous, frameshift, or splice-site mutation; (b) their alternative allele frequencies were ≤0.5% in GnomAD database. RefSeq NM_006565.3 was used as the transcript for CTCF.
3 RESULTS
3.1 Case reports
All three Chinese patients were the first child of healthy parents without significant family history for genetic diseases. They all underwent uneventful full-term gestations. The main clinical features of the three Chinese patients have been listed in Table 1.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
Variants in CTCF (NM_006565.3) |
c.615_618delGAAA (p.Lys206Profs*15) | c.1699C > T (p.Arg567Trp) | c.329dupT (p.Gly111fs*29) |
| Gender | Female | Female | Male |
| Age at last examination | 1 year and 7 months | 8 years and 7 months | 7 years and 11 months |
| Gestation | Full-term | Full-term | 39 weeks |
| Birth weight | 2.8 kg (<−1 SD) | 2.65 kg (<−1 SD) | 1.9 kg (<−3 SD) |
| Birth length | 48 cm (<−1 SD) | 50 cm (normal) | 45 cm (<−3 SD) |
| Feeding difficulty | Yes | Yes | Uncertain |
| Muscular hypotonia | Yes | Yes, during childhood but improved afterward | No |
| Weight | 8.25 kg (<−2 SD) | 17.0 kg (<−2 SD) | 18 kg (<−2 SD) |
| Height | 76 cm (<−2 SD) | 119.8 cm (<−3 SD) | 120.3 cm (<−3 SD) |
| OFC | 44 cm (<−2 SD) | 48.2 cm (<−2 SD) | 47.5 cm (<−3 SD) |
| Developmental delay/intellectual disability/movement delay | Yes; cognitive impairment | Yes; IQ 71; memory deficient; unable to jump with both feet | Yes; memory deficit; cognitive impairment |
| Age of walking | 17 months | 19 months | 14–16 months |
| Age of first words | Language has yet to development | 12 months | 24 months |
| Behavior anomalies | Poor eye contact; irritability; | / | Sensitive; attention deficit; hyperactivity |
| Brain anomalies | / | MRI normal | CT normal |
| Recurrent infections | No | Yes | No |
| Abnormality of vision | Strabismus | Strabismus | Myopia |
| Hearing | Normal | Normal | Normal |
| Urogenital anomalies | Normal | Normal | Normal |
| Tooth development | No teeth development until 1 year and 7 months | Hypodontia; abnormal morphology of incisor; carious teeth | Hypodontia; caries |
| Facial dysmorphisms | Low set ears; cleft palate; upslanting palpebral fissures; unique eyebrows pattern; flat malar bone; micrognathia | Long eyelashes; unique eyebrows pattern; deep set eyes; upslanting palpebral fissures; strabismus; depressed nasal bridge; thin lips; flat malar bone; micrognathia | Widely spaced eye; depressed nasal bridge; upslanting palpebral fissures; flat malar bone; micrognathia |
| Ribs | 11 pairs | 12 pairs | 12 pairs |
| Other anomalies | Palmar creases | Bone age delay for 2 years | Persistent patent ductus arteriosus, repaired at 8-year-old |
Patient 1 was a 19-month-old girl who presented with developmental delay. At 22 weeks of gestation, an ultrasound examination showed that the fetus was 2 weeks smaller than gestation age. She did not present small for gestation age (SGA) at later gestation stages. She had hypotonia and feeding difficulty after birth. She developed postnatal growth retardation. She walked at 17 months and has yet to develop language. There was no dentition until 1 year and 7 months. She exhibited poor eye contact and strabismus. She also presented with irritability. Mild dysmorphic features included low set ears, high arched palate, as well as a unique eyebrow pattern (Figure 1a,d; see Section 4). In addition, the skeletal survey showed 11 pairs of ribs (Figure 2).


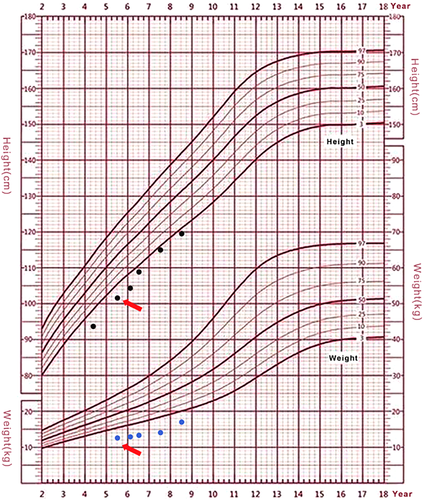
Patient 2 was an 8 years and 7 months girl who had a healthy younger brother. Parental heights were 170 cm for the father, and 160 cm for the mother. Her birth length (50 cm) and birth weight (2.65 kg) were within normal ranges. She exhibited hypotonia in early childhood but improved afterward. She started walking at 19 months. She still had difficulty jumping using both feet. She exhibited postnatal growth retardation (Figure 3) and delayed bone age (1 year and 4 months below chronological age). Growth hormone (GH) provocative test using arginine and l-dopa revealed a peak value of GH 3.58 ng/mL (1.95 ng/mL at 0 min, 3.58 ng/mL at 30 min, 2.79 ng/mL at 60 min, 0.71 ng/mL at 90 min, 1.88 ng/mL at 120 min, 1,96 ng/mL at 150 min, and 0.78 ng/mL at 180 min), suggesting a completely GH deficiency. Other hormonal measurements including luteinizing hormone (<0.1 IU/L), follicle stimulating hormone (0.53 IU/L), insulin-like growth factor 1(128 ng/mL), and insulin-like growth factor-binding protein 3 (2.68 mg/L) were also significantly lower than normal. From the age of 5 years and 7 months, she was started on recombinant human growth hormone (rhGH) treatment. She exhibited a good response to the treatment (Figure 3) during the first 2 years of the treatment. The growth velocity prior to treatment was 6.2 cm/year (from age 4 years 3 months to 5 years 7 months), her height at the start of treatment (age of 5 years 7 months) was 101.8 cm (−2.81 SD). The growth velocity of the first year treatment was 7.0 cm/year and the height increased 0.46 SD. The growth velocity of the second year treatment was 6.6 cm/year and the height increased 0.31 SD. By the end of the third year treatment (age 8 years 7 months), her height was 119.8 cm, the growth velocity dropped to 4.4 cm/year and the height decreased 0.12 SD (Figure 3). Her bone age was delayed for about 1 year at the end of the third year treatment. She underwent an intelligence test using the Chinese Wechsler Intelligence Scale for Children at the age of 8. Her Full-scale IQ was 71. She was particularly weak in numbering, math, and visual-spatial capability. Her verbal comprehension index was 81 and her processing speed index was 66. She had an amicable personality and good communication skills. She had an unusual interest in playing with crunchy and colorful papers. Her facial dysmorphic features included microcephaly, long eyelashes, and unique eyebrow patterns (Figure 1b,d), deep-set eyes, strabismus, depressed nasal bridge, thin lips and micrognathia. She had hypodontia and abnormal incisor. She had normal external genitalia.
Patient 3 was a 7 years and 11 months old boy at the time of the last examination. His mother had a history of spontaneous abortions. Oligohydramnios was noticed during pregnancy. His birth weight and birth length were below −3 SD. He was nursed in the incubator for 2 weeks after birth. It was not certain whether he had hypotonia and feeding difficulty after birth. He started walking at around 14 months of age and started talking at 2 years of age. He continued to exhibit growth retardation. He presented with attention deficit and hyperactivity, as well as irritability. He had poor memory and mild intellectual disability but had good social interactions. His facial dysmorphism included widely spaced eyes, depressed nasal bridge, severe tooth dysplasia, micrognathia, and an ear tag on the right side (Figure 1c,f).
3.2 Molecular analyses
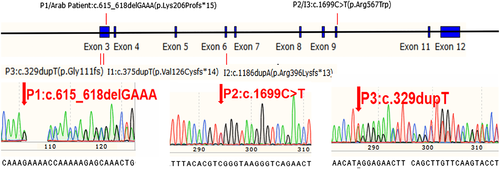
Exome sequencing detected the following heterozygous variants in CTCF gene in probands respectively (RefSeq NM_006565.3): c.615_618delGAAA (p.Lys206Profs*15) in Patient 1, c.1699C>T (p.Arg567Trp) in Patient 2 and c.329dupT (p.Gly111fs*29) in Patient 3 (Figure 4). Sanger sequencing of parental samples confirmed that they were all de novo. Paternity was confirmed for all three cases. All three variants were absent from population databases (e.g., GnomAD). The variants detected in Patients 1 and 2 were previously reported in affected individuals, and the variant in Patient 3 is novel. They all can be classified as pathogenic following the ACMG/AMP guidelines (Richards et al., 2015). The variants appeared to distribute in multiple locations across the whole gene (Figure 4).
4 DISCUSSION
CTCF is an important regulator for the global genomic organization and gene expression (Franco et al., 2014). Somatic mutations of the CTCF gene had been detected in numerous cancers (Bailey et al., 2018; Chen et al., 2016; Klenova, Morse, Ohlsson, & Lobanenkov, 2002; Soto-Reyes et al., 2012; Umer et al., 2016). Germline mutations had been implicated in human disease (Zhou, Werelius, & Lindblom, 2004). Prior to this publication, two reports described a total of four individuals with syndromic intellectual disability and de novo variants in the CTCF gene (Bastaki et al., 2017; Gregor et al., 2013). Based on the available evidence, the gene–disease relationship regarding clinical validity is at a moderate level per ClinGen gene curation classification (https://search.clinicalgenome.org/kb/gene-validity/10143). In order to reach an affirmatory level, additional cases or functional evidence is needed. The new cases reported in this study provided sufficient evidence to re-classify the gene–disease relationship as “Strong” (the genetic evidence at the case level can reach a maximal score of 12). Given the fact that detected variants are distributed in the different regions of the gene and most of the variants are loss of function variants (Figure 3), we agree with Gregor et al. that the disease mechanism of this condition is haploinsufficiency (the haploinsufficiency score for CTCF gene can reach 3 based on ClinGen gene dosage sensitivity scoring protocol). Similar phenotypes were observed in the few individuals with deletion CNV involving the CTCF gene, which further supports this notion (Gregor et al., 2013; Hori et al., 2017) .
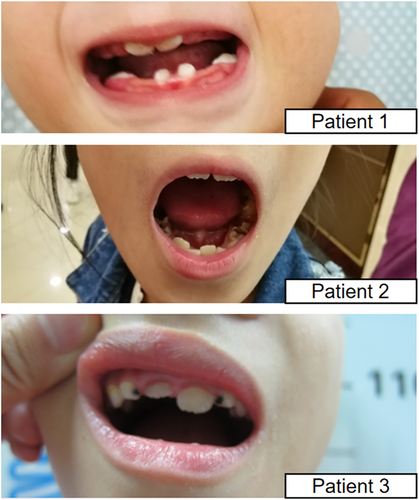
This condition has been named as “intellectual disability- feeding difficulties- developmental delay- microcephaly syndrome” by Orphanet (ORPHA:363611) based on the shared features of three patients described by Gregor et al. (2013). The consistent features now shared by the seven individuals are as follows: (a) neonatal hypotonia and feeding difficulties; (b) global developmental delay; (c) intellectual disability; (d) short stature; (e) microcephaly. In addition, we noticed that all our three patients have delayed or abnormal teeth development, presented with hypodontia and caries (Figure 5). This feature was also prominent in the Arab Patient [23]. The patient one reported by Gregor et al. (2013) also have abnormal teeth presentation, thus features related with teeth were observed in 5/7 patients. Strabismus and vision abnormalities were also recurrent (5/7). We noticed a unique pattern of eyebrow shared by most patients. As shown in Figure 1, the main characteristic of this unique eyebrow is that it spread out toward nasal root on the medial sides and the low edges of the eyebrow were positioned very close to the inner canthi. The eyebrow tapered off from middle-way to lateral two-third, so the overall morphology of the eyebrow is wider on the medial sides and thinner on the lateral sides. This eyebrow pattern is also visible from the patient photos published in the previous two papers. Long eyelashes were also a shared feature among our patients (Figure 1). Our patients also exhibited micrognathia, upslanting palpebral fissure, and flat malar bone (Figure 1). If these features can be validated in more patients, the condition can potentially be clinically recognized.
Yet the severity of the clinical presentation may differ significantly among patients, even between patients with the same mutation. Our Patient 1 carried the same mutation as the Arab patient (Bastaki et al., 2017). Our patient was born at full term without SGA whereas the Arab patient was delivered at 26-weeks of gestation with significant intra-uterine growth retardation and fetal stress. The Arab patient had a more severe motor delay, was unable to walk whereas our patient had normal motor function. The Arab patient needed the feeding tube whereas our patient had feeding difficulty but required no feeding tube. The congenital heart disease and external genitalia dysplasia observed in the Arab patient were absent from our patient. Our Patient 2 carried the same missense mutation as the Patient 3 reported by Gregor et al. (2013). Our patient had borderline intellectual disability whereas the latter had severe ID. Our patient has relatively preserved communication and language skills, while the latter had autistic features. Our patient showed more severe growth retardation.
CTCF affects genes and biological processes globally (Shen et al., 2015). The clinical consequence of CTCF gene mutations also seems to have a global effect on development and growth, both on neuronal (presented as microcephaly and intellectual disability; Hori et al., 2017; Lanni et al., 2013; Watson et al., 2014) and musculoskeletal (presented as short stature) tissues (Bailey et al., 2018; Lobanenkov & Zentner, 2018). Although variable, developmental delay and intellectual disability were mild among these Chinese patients. None of them exhibited autism presentations or significant behavioral issues, but they had attention problems, memory problems, and learning difficulties. The Patient 3 underwent rhGH treatment for 3 years, which showed a good response with no side effect. However, the effect did not last in the third year. With limited evidence, we do not have a good prediction regarding the prognosis of this condition, follow-up and more case studies are needed for better understanding this novel disorder.
In conclusion, we reported the clinical phenotypes of three unrelated Chinese patients with de novo heterozygous mutations in the CTCF gene. These new cases helped to establish the causal relationship and the clinical validity between the CTCF gene and this novel neurodevelopmental disorder. The presentations of developmental delay and growth retardation, as well as unique dysmorphism may help to define a clinically recognizable condition. rhGH treatment had some benefit but the long term effect is not known and the overall prognosis is uncertain.
ACKNOWLEDGMENTS
The authors appreciate the participating patients and their family. This work is partially funded by the “Eastern Scholar” Fund; the “Guangxi Bagui Scholar” fund; the Natural Science Foundation of China [grant number 81873633]; Major Research Plan of the Provincial Science and Technology Foundation of Guangxi [grant number AB16380219]; key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C (201610191); National key research and development program (2018YFC1002501) and the GeneScience Pharmaceuticals Co., Ltd (Changchun, China).
Biographies

Fei Chen MD, is an assistant researcher, medical technologist and genetic counselor of Metabolic Central Laboratory, Birth Defect Prevention Research Institute, Maternal and Child Health Hospital, Children's Hospital of Guangxi Zhuang Autonomous Region. He received his bachelor and master degree on biomedical science and genetics in XiangYa School of Medicine, Central South University in 2014 and 2018, respectively. His main research direction is genetic disease screening, preimplantation genetic diagnosis and clinical application of induced pluripotent stem cells.

Haiming Yuan PhD, is a director of Genetic Molecular Diagnostics Laboratory, Dongguan Maternal and Child Health Hospital; a visiting scholar of Harvard University Boston Children's Hospital; a specially-invited expert of Maternal and Child Health Hospital Genetic Center of Hubei Province; a member of the National Physician Association Genetics Committee; a permanent member of Preventive Medicine Association of Hebei Province. He earned his PhD in genetic from Fudan University, Shanghai. He specializes in data interpretation and genetic counseling for chromosome microarray and whole exon sequencing.

Wenyong Wu MD, he is a master degree candidate currently studying at Fujian Medical University. In 2018, he entered Fujian Medical University to obtain a master's degree in clinical medicine with excellent performance. He is now studying under professor Ruimin Chen and mainly engaged in the research of pediatric endocrine genetics and metabolism. He is a resident physician in Fuzhou Children's Hospital of Fujian Province and is currently in the stage of national standardized training for medical practitioners.

Shaoke Chen , is a pediatric chief physician of Maternal and Child Health Hospital, Children's Hospital of Guangxi Zhuang Autonomous Region, a member of the Genetic Diseases Group of the Committee on Birth Defects and Control of Chinese Preventive Medicine Association, a member of the Genetics Advisory Branch of the Chinese Genetic Society, a member of the Genetics Group of the Chinese Preventive Medicine Association, a member of the National Neonatal Disease Screening Group of the Chinese Preventive Medicine Association, a member of the Pediatric Endocrine Genetic Metabolism Group of the Chinese Medical Association.

Qi Yang , is an assistant researcher and medical technologist of Metabolic Central Laboratory, Birth Defect Prevention Research Institute, Maternal and Child Health Hospital, Children's Hospital of Guangxi Zhuang Autonomous Region. He received his master degree in genetics at Southern Medical University. He is mainly engaged in Mendelian genetic disease testing. He participated in 10 national, provincial and ministerial projects, published 17 SCI papers.

Qiang Zhang , is a technologist-in-charge of Metabolic Central Laboratory, Birth Defect Prevention Research Institute, Maternal and Child Health Hospital, Children's Hospital of Guangxi Zhuang Autonomous Region. He received his master degree in genetics at Southern Medical University. He is mainly engaged in screening and diagnosis of thalassemia and childhood genetic diseases. He participated in 3 national, provincial and ministerial projects, published 13 SCI papers.

Baoheng Gui PhD, is a research associate in the Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, and is a postdoctoral researcher at Shenzhen Research Institute, The Chinese University of Hong Kong. Dr. Gui received his bachelor and doctorate degree on genetics at the State Key Laboratory of Medical Genetics of China, Central South University in 2010 and 2015, respectively. He has been in the USA for one year as a visiting scholar at Human genetics division, Cincinnati Children's Hospital Medical Center. His research interests focus on molecular genetics and cytogenetics, preimplantation genetic testing (PGT), next-generation sequencing (NGS), chromosome microarray (CMA), embryo assessment and selection in assisted reproductive technologies (ART), especially in embryo mosaicism. He has obtained several funding including National Natural Science Foundation of China, China Postdoctoral Science Foundation, National Major Research Plan, and Major Research Plan of the Provincial Science and Technology Foundation of Guangxi, etc.

Xin Fan , is a pediatric deputy chief physician and deputy director of Metabolic Central Laboratory, Birth Defect Prevention Research Institute, Maternal and Child Health Hospital, Children's Hospital of Guangxi Zhuang Autonomous Region. She is mainly engaged in the diagnosis and treatment of pediatric endocrine and hereditary metabolic diseases. She has been a visiting scholar at the Cincinnati Children's Hospital and Taipei City Union Hospital.

Ruimin Chen , is a director, professor, chief physician of Department of Endocrinology, Children's Hospital of Fujian Province. She is a member of the Endocrinology and Metabolism Group of the Pediatrics Branch of the Chinese Medical Association, a standing member of the Committee of Youth Health and Medicine of the Chinese Medical Doctor Association, a deputy director of the Pediatrics Branch of the Fujian Medical Association and leader of the Endocrine and Metabolic Diseases Group, a chairman of the Pediatric Branch of Fuzhou Medical Association, etc.

Yiping Shen PhD, is an assistant professor at the Department of Neurology, Harvard Medical School. He is currently at the Department of Genetics and Genomics, Boston Children's Hospital, Harvard Medical School, as a coDirector of Harvard Medical School Genetic Training Program course. he is a fellow of American College of Medical Genetics (ACMG), Certified by American Board of Medical Genetics and Genomics (ABMGG) in 2005, a member of Chinese Medical Doctor's Association Medical Genetics subcommittee, a member of Chinese Medical Genetics Society Genetic Counseling subcommittee, an expert committee member of the March of Dimes foundation China, and an adjunct professor at the Shanghai Jiaotong University School of Medicine. He is a distinguished professor and director of Medical Genetics Department of Shanghai Children's Medical Center and director of the Genetic diagnostic laboratory at Guangxi Maternal and Child Health Hospital.




