The new era of Pompe disease: Advances in the detection, understanding of the phenotypic spectrum, pathophysiology, and management†
How to cite this article: Kishnani PS, Beckemeyer AA, Mendelsohn NJ. 2012. The new era of Pompe disease: Advances in the detection, understanding of the phenotypic spectrum, pathophysiology, and management. Am J Med Genet Part C Semin Med Genet 160C:1–7.
Abstract
Pompe disease is an autosomal recessive neuromuscular disorder marked by progressive muscle weakness due to lysosomal buildup of glycogen. Presentation is described as a spectrum, varying by age of onset, organ involvement, and degree of myopathy. Given the phenotypic variability, Pompe disease is broadly classified into an infantile form and a late onset (juvenile, childhood, adult onset) form. Prior to the advent of enzyme replacement therapy (ERT) with alglucosidase alfa and approval for human use in 2006, the natural history was limited due to death before age 2 years for infantile onset cases and significant morbidity and early mortality for late onset Pompe disease (LOPD). ERT with alglucosidase alfa redefined the once fatal outcome in infantile Pompe, establishing an emergent phenotype. Treatment in late onset patients resulted in improved outcomes, enhancing understanding of the phenotype, presentation, and extent of organ involvement. This Issue of the Seminars seeks to enumerate the recent advancements in the field of Pompe disease, including newborn screening, novel therapeutic targets, new insights in the pathophysiology including role of autophagy, and impacts of long-term disease burden and CNS glycogen accumulation on cognition in infantile survivors. It also addresses immunological challenges and the critical role of immunomodulation in ERT treatment outcome. Other topics discussed include the role of biomarkers in monitoring disease progression and treatment responses, the role of genotype in defining phenotype and treatment response, better insights into the clinical presentations in LOPD and finally the importance of a multidisciplinary approach to care with the role of physical therapy as an example. Many gaps in our scientific understanding of this disease still remain; however, we hope the next decade will bring new knowledge and therapies to the horizon. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Pompe disease, glycogen-storage disease type II, and acid maltase deficiency are alternative names for the same disorder. Pompe disease is caused by deficiency of the enzyme acid alpha glucosidase, which leads to lysosomal glycogen storage. Pompe disease falls into several disease categories: it is at once a neuromuscular disorder, a lysosomal storage disorder, and a glycogen storage disorder [Kishnani et al., 2006a; van der Ploeg and Reuser, 2008; Hirschhorn and Reuser, 2009]. Originally used to describe the infantile, most severe form of the disease described by Pompe [1932], Pompe disease is now used to describe the presentation of symptoms that can first occur in infants, children and adults. Presentation is currently described as a spectrum, varying by age of onset, level of organ involvement, and degree of myopathy. Classification of Pompe disease is also variable; broadly there are two categories—infantile and late onset [Kishnani et al., 2006b]. At this time, even within the infantile category, there exists a wide range of severity. The term infantile onset incorporates those patients who display symptoms before 1 year of age; however, those with cardiomyopathy are called classic infantile while those without are called atypical or muscular variant. The term late onset is utilized to describe those who develop symptoms any time after 1 year of age, and can be misleading as it includes the childhood, juvenile, and adult onset groups.
The approval of enzyme replacement therapy (ERT) with alglucosidase alfa for Pompe disease in 2006 was a significant landmark in the field. It represented many firsts: the first neuromuscular disorder with an FDA-approved treatment, the first condition where pivotal trials in infants led to broad label approval, and one of the first diseases where a natural history cohort was used as a control group in the conduct of the infantile pivotal trials [Kishnani et al., 2007]. More than a decade has passed since the first patients were treated with ERT, and since then there has been a dramatic increase in the understanding of the disease as well as a rise in the interest of clinicians and scientists. This is evidenced by the increasing number of publications since 2006: over 30% of PubMed entries about Pompe disease are dated from the last 5 years in comparison to the 70% published in the 50 years prior to 2006.
SOME HISTORICAL LANDMARKS IN POMPE DISEASE SINCE ITS DESCRIPTION
In 1932, Johannes C. Pompe, a Dutch pathologist, first documented Pompe disease when he recognized the accumulation of glycogen in the heart, liver and muscle in a 7-month-old baby girl who had presumably succumbed to pneumonia but whose autopsy portrayed cardiomyopathy, hypotonia and rapidly progressive muscle disease [Pompe, 1932] (for more details on history see OMMBID) [Hirschhorn and Reuser, 2009]. Since then and especially since the advent of a targeted therapy with alglucosidase alfa, many advances in the understanding and treatment of Pompe disease have been achieved (Figs. 1 and 2).
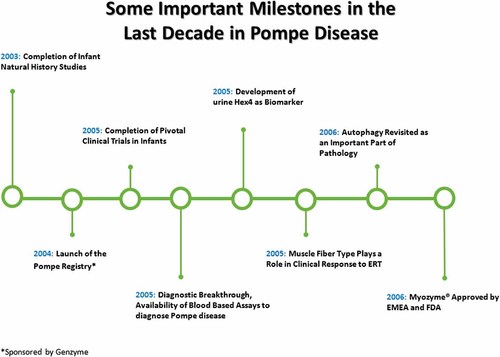
Some important milestones in Pompe disease leading up to FDA approval of ERT.
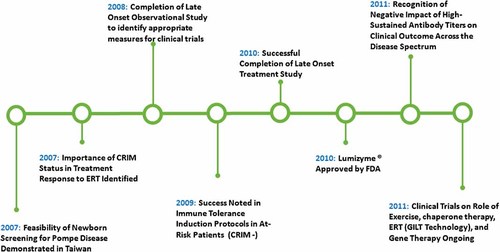
Some important milestones following FDA approval of ERT in 2006.
- (1)
To provide in a succinct manner useful information to clinicians caring for individuals with Pompe disease.
- (2)
To stimulate research in areas where there are current gaps.
- (3)
To highlight how a rare disease can shed light on challenges noted in more common conditions.
The chosen nine topics represented in the Issue here reflect the most burning questions regarding Pompe disease at this time.
NEWBORN SCREENING FOR POMPE DISEASE: AN UPDATE 2011
The paper by Burton provides a comprehensive overview of the importance of NBS for Pompe disease. Given the rapid progression of the disease, especially in the infantile form, and the poor outcome noted across the spectrum when there is a delayed diagnosis, the author emphasizes the need for Pompe disease to be considered for universal NBS. The paper discusses the various technology platforms available for enzyme testing for Pompe disease. Burton also elaborates on other benefits of NBS, including genetic counseling for the parents and other family members, avoidance of the diagnostic odyssey that often accompanies rare disorders, and a better understanding of the disease. With NBS, determination of the true frequency of Pompe disease will be possible.
AUTOPHAGY AND MITOCHONDRIA IN POMPE DISEASE: NOTHING IS SO NEW AS WHAT HAS LONG BEEN FORGOTTEN
In this paper, Raben et al. highlight the role of autophagy and its improper function in Pompe disease as an area of clinical concern. The focus of the last decade has been on development of a therapy, yet the pathology of Pompe disease remains unclear. Increased understanding of the pathology will illuminate the complex nature of this disease that expands beyond simple lysosomal glycogen storage. Furthermore, the importance of muscle biopsy should not be understated as this method enables monitoring of treatment response. It is known that autophagic abnormalities are present in many muscle cells, and in many fibers autophagic accumulation is the overwhelming (and in some fibers the only) pathology. There are differences in accumulation in type I versus type II fibers, as well as accumulation in infantile versus late onset patients. Autophagic buildup and mitchondrial abnormalities are being noted despite ERT, raising the issue that various other processes exist or are downstream effects of glycogen accumulation. This underscores need for new therapeutic targets for Pompe disease, encourages better understanding of these pathological mechanisms and further highlights that ERT alone may not be sufficient for treatment success.
COGNITIVE AND ADAPTIVE FUNCTIONING OF CHILDREN WITH INFANTILE POMPE DISEASE TREATED WITH ENZYME REPLACEMENT THERAPY: LONG-TERM FOLLOW-UP
Spiridigliozzi and coauthors discuss the cognitive and adaptive functions in long-term infantile survivors treated with ERT. For the first time, we have an opportunity to examine if there is an unmasking of CNS disease manifestations in infantile survivors. Glycogen accumulation in the cerebral cortex, brainstem and anterior horn cells in the spinal cord has been described at autopsy in infantile-onset Pompe disease patients [Gambetti et al., 1971; Sakurai et al., 1974]. Brain abnormalities such as delays in myelination and white matter changes in CRIM negative survivors have been observed on imaging studies [Chien et al., 2006; Rohrbach et al., 2010]. The clinical significance of these findings has not been clear due to the short survival of these patients at the time of the studies and the lack of detailed evaluations to assess cognitive abilities. With longer-term survival in ERT-treated infantile-onset patients, now there is an opportunity to evaluate cognitive development in this cohort of patients. Previously uncharacterized CNS manifestations such as hypernasal speech, with a flaccid dysarthria and aspiration risk suggestive of bulbar involvement and sensorineural hearing loss have been noted in infantile survivors [Jones et al., 2010; van Capelle et al., 2010]. Much more work and systematic assessment via neuroimaging is needed to establish correlation. This paper seeks to introduce the importance of further studies to better understand if there is unmasking of CNS disease in infantile survivors. It also raises the question of whether there are differences between long-term CRIM positive and CRIM negative infantile survivors.
THE ROLE OF IMMUNE TOLERANCE INDUCTION IN RESTORATION OF THE EFFICACY OF ERT IN POMPE DISEASE
Rosenberg et al.'s paper is an overview of the current understanding of the immune response to ERT in Pompe disease. The authors discuss ways in which the immune response can be minimized through immune modulation. There is increasing evidence of the impact of immune response on the long-term efficacy of alglucosidase alfa. It is now clear that those patients with high titer, sustained antibody responses to rhGAA administration have poor clinical outcomes relative to patients who do not mount such responses or have only transient immune responses [Amalfitano et al., 2001; Banugaria et al., 2011]. The rapid progression of infantile Pompe disease allows it to serve as an excellent model to study the impact of antibodies and immune tolerance induction strategies for ERT. Lessons learned from Pompe disease can be applied to other conditions treated with a therapeutic protein. However, better insights are needed on the immunological mechanisms and challenges faced when utilizing ERT.
PREDICTING CROSS REACTIVE IMMUNOLOGICAL MATERIAL (CRIM) STATUS IN POMPE DISEASE USING GAA MUTATIONS: LESSONS LEARNED FROM 10 YEARS OF CLINICAL LABORATORY TESTING EXPERIENCE
The immunological challenges when ERT is administered to Pompe patients underscores the need to develop systems and models to predict patients at risk for an immune response. It is key to identify such patients prior to an established immune response since immune tolerance is more feasible in the naïve setting [Messinger et al., 2012]. CRIM-negative status has been recognized as a poor prognostic factor. CRIM-negative patients make no GAA protein and develop sustained high antibody titers to ERT that render the treatment ineffective. While some CRIM-positive patients also develop high antibody titers, antibody titers are low for most patients and better clinical outcome has been shown [Banugaria et al., 2011]. High, sustained antibody titers are also noted in some late onset Pompe patients. The reason for these differing responses can be attributed to underlying mutations: CRIM-negative patients possess two GAA alleles that completely disrupt protein production. In contrast, most CRIM-positive patients carry at least one missense or splice site mutation, consistent with the production of some residual protein. This paper by Bali et al. describes the laboratory experience and provides detailed information from over 200 infantile Pompe cases where CRIM status can be predicted from GAA mutations. A better prediction modeling system is needed to predict immunological responses to ERT across the disease spectrum.
ASSESSING DISEASE SEVERITY IN POMPE DISEASE: THE ROLES OF A URINARY GLUCOSE TETRASACCHARIDE BIOMARKER AND IMAGING TECHNIQUES
This paper by Young et al. explicates the importance of biomarkers as non-invasive monitoring tools in this multidisciplinary disease. Urinary Glc4 or Hex4 is noted as an important marker of overall disease burden; however, it falls short in determining the location and extent of accumulated glycogen [Young et al., 2009]. To account for these shortcomings, the authors propose the use of magnetic resonance spectroscopy and other imaging techniques as non-invasive methods [Carlier et al., 2011]. These techniques must be further investigated as they can quantify glycogen accumulation and help monitor disease progression as well as clinical response to ERT.
THE GENOTYPE–PHENOTYPE CORRELATION IN POMPE DISEASE
In this paper, Reuser et al. outline the correlation between genotype and phenotype in Pompe disease. This paper highlights the fact that modifying factors have a sizeable effect on the clinical course of Pompe disease due to the variability seen among a large cohort of patients with the same GAA genotype [Kroos et al., 2007]. The future rests in discovering these modifying factors and investigating their potential in novel therapeutic avenues.
INFANTILE POMPE DISEASE ON ERT—UPDATE ON CLINICAL PRESENTATION, MUSCULOSKELETAL MANAGEMENT, AND EXERCISE CONSIDERATIONS
Case et al. describe the emerging clinical/musculoskeletal phenotype in long-term infantile survivors. The new phenotype appears to be distinct from the late-onset phenotype, rather than a shift from infantile to late-onset phenotype that might be expected from a simple diminution of symptoms with ERT. Case and colleagues provide updated recommendations regarding musculoskeletal management in Pompe disease, particularly in those children who are now surviving longer with ERT. The potential impact and role of exercise in infantile Pompe survivors treated with ERT is discussed.
TOWARDS DECONSTRUCTING THE PHENOTYPE OF LATE-ONSET POMPE DISEASE
As discussed, the advent of ERT has also impacted the prognosis of late onset Pompe disease (LOPD). Schoser et al. provide a literature overview as well as a study of the group's cohort of 44 adults from their clinic in Munich, delineating the various symptom patterns and groupings seen in the studied cases. The authors highlight the variable clinical presentations such as rigid spine syndrome and vascular complications as presenting features of LOPD [Laforet et al., 2008, 2010; El-Gharbawy et al., 2011]. Recognition of these symptom patterns in LOPD will allow for early diagnosis, proper management and early treatment intervention.
CONCLUSION
Pompe disease serves as a beacon of hope in the realm of (formerly) untreatable neuromuscular diseases and lysosomal storage diseases. In the last decade alone, several important advances in the field of Pompe disease have been achieved that not only have increased survival but also provided further understanding of the pathophysiology of Pompe disease. The importance of earlier detection in infantile cases as well as earlier treatment in late onset cases are developing observations, each facilitating understanding about the importance of changing the natural history course by earlier treatment. The availability of ERT has increased general awareness and demand for rapid and more sophisticated diagnosis at both the enzymatic and molecular level. A principal development has been the ability to make a diagnosis of Pompe disease via blood-based assays. This has shortened the diagnostic odyssey and simplified diagnosis delivery, which traditionally depended on demonstration of enzyme deficiency in skin fibroblasts or muscle biopsy. Alglucosidase administration has served as a life-prolonging therapy in infantile patients treated with Myozyme®, Genzyme Corporation, Cambridge, MA [Kishnani et al., 2007], and has resulted in stabilization of disease in the late onset forms [van der Ploeg et al., 2010]. ERT has clearly changed the outcome of patients with Pompe disease, however a lot of work remains ahead and new second generation therapies are needed. ERT with Myozyme has allowed infants and adults with Pompe disease to have a better quality of life, enabling some to even walk and breathe without assistance. This treatment has given hope to patients and their families that a diagnosis of Pompe disease is no longer as limiting as it once was. However, along with progress always comes new challenges. A lack of complete efficacy from ERT has been observed in long-term infantile survivors and patients with LOPD. Even in patients with a good response to ERT, a residual motor weakness (neck flexor weakness, dorsiflexor weakness, mypathic facies, ptosis, and strabismus) has been observed. Respiratory insufficiency is observed especially in those started late. Findings of sensorineural hearing loss, hypernasal speech, with a flaccid dysarthria and aspiration risk suggestive of bulbar involvement is commonly observed in long-term survivors. It is becoming increasingly clear that ERT does not provide a cure, therefore, further research into novel therapies is essential [Nicolino et al., 2009]. The advent of NBS for Pompe disease may change the course on ERT, and result in better outcomes, and long-term data from the Taiwan NBS program will shed light on this.
Targeting muscle in Pompe disease remains a formidable challenge. A significantly higher dose of alglucosidase alfa (30- to 100-fold greater) than other LSDs is required [Desnick, 2004]. There are several reasons for this: (1) muscle comprises approximately 40% of body weight, (2) there is a low abundance of cation independent—mannose 6 phosphate receptor (CI-MPR) on skeletal muscle, and (3) high doses of ERT are needed to surpass a specific threshold in blood in order to reach skeletal muscle and elicit a therapeutic response. Given these obstacles, second generation ERT's with more efficient targeting to skeletal muscle, and other therapies such as chaperone-mediated therapy, substrate reduction therapy, and gene replacement therapies are needed. The application of chaperones in Pompe disease to promote transport of mutant, misfolded, endogenous acid α-glucosidase from the endoplasmic reticulum to the lysosomes is in clinical trials at this time. A GILT-(glycosylation independent lysosomal targeting) tagged GAA is currently being studied in a phase 1/2 clinical trial (NCT01230801, 2011). ERT using synthetic oligosaccharide chains containing bis-M6-P linked to GAA (neo-GAA) are currently being considered for clinical trials [Zhu et al., 2004, 2005]. Gene therapy as part of immunomodulatory therapy for Pompe disease has shown success in a GAA knock out mouse model [Sun et al., 2010]. Another approach includes a broader application of gene therapy, whereby specific treatment to target tissues is provided and is currently in a Phase I/II clinical trial. Adjunctive therapies such as B2 agonists with ERT that can increase CIMPR expression through enhanced receptor-mediated uptake of ERT could reduce the dosage requirements for ERT and allow for better delivery to skeletal muscle and also to the CNS may prove beneficial [Koeberl et al., 2011a, b].
The role of a high protein diet and exercise is also being increasingly recognized [Slonim et al., 1983; Padberg et al., 1989; Bodamer et al., 1997; Terzis et al., 2011].
Given the progress in the field, updated guidelines for the diagnosis and management of Pompe disease are needed.
The Pompe disease experience is illustrative of many of the challenges faced in the development of targeted treatments for rare diseases, but also of approaches that can result in successful product development. The complexity of Pompe disease in presentation and therapeutic response highlights the importance of interdisciplinary collaboration in developing our ever-evolving understanding of this condition. Partnership between industry, academia, patients, and overall international cooperation has been and will continue to be pivotal in the advancement of understanding Pompe disease. Most importantly, these advancements are bettering the lives of patients and promising brighter futures not only to those living with the disease today, but also to those of future generations.




