Assessing disease severity in Pompe disease: The roles of a urinary glucose tetrasaccharide biomarker and imaging techniques†
How to cite this article: Young SP, Piraud M, Goldstein JL, Zhang H, Rehder C, Laforet P, Kishnani PS, Millington DS, Bashir M, Bali D. 2012. Assessing disease severity in Pompe disease: The roles of a urinary glucose tetrasaccharide biomarker and imaging techniques. Am J Med Genet Part C Semin Med Genet 160C:50–58.
Abstract
Defining disease severity in patients with Pompe disease is important for prognosis and monitoring the response to therapies. Current approaches include qualitative and quantitative assessments of the disease burden, and clinical measures of the impact of the disease on affected systems. The aims of this manuscript were to review a noninvasive urinary glucose tetrasaccharide biomarker of glycogen storage, and to discuss advances in imaging techniques for determining the disease burden in Pompe disease. The glucose tetrasaccharide, Glcα1-6Glcα1-4Glcα1-4Glc (Glc4), is a glycogen-derived limit dextrin that correlates with the extent of glycogen accumulation in skeletal muscle. As such, it is more useful than traditional biomarkers of tissue damage, such as CK and AST, for monitoring the response to enzyme replacement therapy in patients with Pompe disease. Glc4 is also useful as an adjunctive diagnostic test for Pompe disease when performed in conjunction with acid alpha-glucosidase activity measurements. Review of clinical records of 208 patients evaluated for Pompe disease by this approach showed Glc4 had 94% sensitivity and 84% specificity for Pompe disease. We propose Glc4 is useful as an overall measure of disease burden, but does not provide information on the location and distribution of excess glycogen accumulation. In this manuscript we also review magnetic resonance spectroscopy and imaging techniques as alternative, noninvasive tools for quantifying glycogen and detailing changes, such as fibrofatty muscle degeneration, in specific muscle groups in Pompe disease. These techniques show promise as a means of monitoring disease progression and the response to treatment in Pompe disease. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Biomarkers are molecules that are used to detect and monitor disease processes. In combination with other laboratory and clinical assessments, biomarkers contribute to the assessment of disease severity, disease progression, and treatment response. In Pompe disease, the disease severity is primarily related to the extent of glycogen deposition and the subsequent damage to affected tissues. Serum enzyme biomarkers of tissue damage such as creatine kinase (CK), CK-MB, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) are used routinely in Pompe disease, but are nonspecific and have limited prognostic value. Direct measurement of the muscle glycogen for the purposes of diagnosis and monitoring requires invasive and sometimes risky biopsies, and has limited sensitivity in late-onset forms of the disease because of heterogeneity in the distribution of glycogen deposition [Winkel et al., 2005; Müller-Felber et al., 2007].
The discovery of the glucose tetrasaccharide, Glcα1-6Glcα1-4Glcα-4Glc (Glc4), excreted in the urine of a patient with Pompe disease [Hallgren et al., 1974] provided an alternative, noninvasive means of assessing glycogen storage in this disorder. Glc4 is a limit dextrin produced by the amylolytic digestion of glycogen and other branched glucose polymers, such as amylopectin, that contain α1-6 glycosidic linkages
The discovery of the glucose tetrasaccharide, Glcα1-6Glcα1-4Glcα-4Glc (Glc4), excreted in the urine of a patient with Pompe disease provided an alternative, noninvasive means of assessing glycogen storage in this disorder. Glc4 is a limit dextrin produced by the amylolytic digestion of glycogen and other branched glucose polymers, such as amylopectin, that contain α1-6 glycosidic linkages.
[Walker and Whelan, 1960; Ugorski et al., 1983]. Studies of the excretion of this tetrasaccharide have demonstrated that it is elevated in conditions associated with increased glycogen storage, including several of the glycogen storage disorders: GSD II [Lennartson et al., 1976; Lennartson et al., 1978; Kuriyama et al., 1985; Oberholzer and Sewell, 1990], GSD III [Lennartson et al., 1976, 1978; Oberholzer and Sewell, 1990], GSD VI [Oberholzer and Sewell, 1990], and GSD IX [Morava et al., 2005]; certain leukemias and sarcomas [Kumlien et al., 1988]; and pregnancy [Hallgren et al., 1977]. It was also reported to be elevated in conditions of muscle breakdown including Duchenne muscular dystrophy [Lennartson et al., 1976; Lundblad et al., 1979] and patients who had suffered muscle trauma [Kumlien et al., 1988], and in patients with acute pancreatitis [Kumlien et al., 1988]. As a result of these observations and other studies on the metabolic origin of Glc4, it was hypothesized that Glc4 is produced by intravascular degradation of glycogen released from damaged tissues by salivary and pancreatic α-amylases and neutral α-1,4-glucosidase activity [Ugorski et al., 1983]. In these early studies, molecular confirmation of the disorders generally was not available, some of the methods employed for Glc4 determination lacked specificity and sensitivity, and age-appropriate control ranges were not established.
The advent of enzyme replacement therapy (ERT) with recombinant human acid alpha-glucosidase (rhGAA) for both infantile and late-onset forms of Pompe disease [Kishnani et al., 2007; van der Ploeg et al., 2010] generated a need for improved diagnostic testing and the noninvasive assessment of glycogen accumulation in treated patients. To this end, we developed a stable isotope dilution-liquid chromatographic-tandem mass spectrometric method for the measurement of urinary Glc4 [Young et al., 2003, 2009]. This biomarker has been monitored in patients with infantile and late-onset Pompe disease on ERT, and has also been used as a diagnostic biomarker for patients evaluated for Pompe disease, in conjunction with acid alpha-glucosidase (GAA) activity measurements.
The information obtained from molecular biomarkers of disease contributes to the understanding of the disease process, but is usually insufficient to fully define the extent of disease pathology. The usefulness of imaging techniques such as echocardiography [Ansong et al., 2006; Kishnani et al., 2007; El-Gharbawy et al., 2011], computed tomography (CT) [Cinnamon et al., 1991; Arai et al., 1993; de Jager et al., 1998; Ishigaki et al., 2010; Ishigaki et al., 2011a, b], magnetic resonance imaging (MRI) [Pichiecchio et al., 2004, 2009; Dlamini et al., 2008; Ravaglia et al., 2008, 2010; Barker et al., 2010; Carlier et al., 2011], and ultrasound [Zaidman et al., 2011] as noninvasive tools to evaluate pathological changes in Pompe disease has been described.
In this manuscript, we present new data on the sensitivity and specificity of Glc4 as a diagnostic biomarker, review the role of Glc4 in monitoring the treatment response, and discuss the application of imaging techniques for evaluating disease severity in Pompe disease.
MATERIALS AND METHODS
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. De-ionized water (DI-H2O) was prepared in-house. d3-Creatinine (Cambridge Isotopes, Andover, MA); Glc4 standard (Glycorex AB, Lund, Sweden); HPLC grade solvents (VWR, West Chester, PA); [13C6]-labeled glucose tetrasaccharide internal standard was synthesized as previously described [Young et al., 2003]; Whatman grade 903 filter paper (VWR, Batavia, IL); Sep-Pak® Vac 100 mg C18 cartridges (Waters Corporation, Milford, MA).
Analysis of Glc4
Glc4 was determined as the total hexose tetrasaccharide (Hex4) fraction in urine by liquid chromatography-electrospray tandem mass spectrometry [Young et al., 2003, 2009] using an Acquity UPLC-Quattro Micro tandem mass spectrometer (Waters Corporation) at the Duke Biochemical Genetics Laboratory (Durham, NC, USA), or on an API 3200 triple quadrupole mass spectrometer (AB Sciex, Toronto, Canada) equipped with a TurboIonSpray source and an Agilent 1200 Series inlet system (Agilent Technologies, Massy, France) at the Laboratoire des Maladies Héréditaires du Métabolisme, Hospices Civils de Lyon. Glc4 concentrations in urine were normalized to the creatinine concentration. Previously, we have determined that Glc4 accounts for at least 95% of the total Hex4 fraction in urine from patients with Pompe disease (unpublished observations). For the purposes of this manuscript Hex4 measurements will be referred to as Glc4, in keeping with previous reports on this biomarker.
Acid Alpha-Glucosidase (GAA) Activity Determination
For the majority of cases, GAA activity was determined in whole blood spotted and dried on filter paper using a fluorometric assay with 4-methylumbelliferyl-alpha-D-glucoside (4-MUG) as the substrate and acarbose as an inhibitor of maltose glucoamylase (MGA) [Zhang et al., 2006; Kallwass et al., 2007]. GAA activity was measured in two cases in cultured skin fibroblast sonicates or muscle biopsy homogenates using maltose or 4-MUG as the substrate [Goldstein et al., 2009].
GAA Gene Sequencing
All 19 coding exons and flanking intronic sequences of the GAA gene were amplified by polymerase chain reaction (PCR) using isolated genomic DNA. PCR was performed using HotStart-IT Taq Master Mix (2X; USB Corporation, Cleveland, OH) in a 30-µl reaction containing 50–500 ng genomic DNA and 0.3 µM primer mix. All PCR primers were tailed with M13 universal primers at the 5′ end. Amplicons were treated with ExoSAP-IT (USB Corporation), and bidirectionally sequenced, using 0.6 µM M13 forward/-20 or M13 reverse/-27 primer with the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Sequence products were treated with the Big Dye XTerminator Purification Kit (Applied Biosystems) and electrophoresed on the ABI 3130xl Genetic Analyzer (Applied Biosystems). Chromatograms were base called, assembled, and aligned using SeqScape software v2.5 (Applied Biosystems). Targeted mutation analysis was performed for some patients by amplifying targeted exons (2, 14, 18 or exons involving known family mutations) using genomic DNA for PCR amplifications and direct sequencing in both directions. Sequences were compared with the reference DNA sequence (NM_000152).
Patient Cohort
Anonymized data were collected from the records of clinical laboratory testing for patients evaluated for Pompe disease between 2006 and 2011 at the Duke Biochemical Genetics Laboratory. Information collected included, at a minimum, the age of the patient in years at the time of the urine sample collection, urinary Glc4 concentration relative to creatinine, and GAA activity in dried blood spots, cultured skin fibroblasts and/or muscle biopsies. Other pertinent information such as clinical features, CK measurements, inter-current illness, and the results of target mutation or full gene sequencing was also included, when available. Additionally, de-identified data, including age in years and Glc4 concentrations prior to starting ERT, from 16 patients with late-onset Pompe disease were provided by the Laboratoire des Maladies Héréditaires du Métabolisme, Hospices Civils de Lyon. These data were used in combination with the data from the Duke Biochemical Genetics Lab for the evaluation of the relationship of levels of measured Glc4 with age.
Glc4 was compared with serum CK in patients for whom this information was available, with the objective of assessing the value of Glc4 as a biomarker of glycogen storage in the context of myofiber disruption, and understanding its relationship with a marker of muscle damage. Patients with normal GAA activity (n = 13), patients with deficient GAA activity (n = 11) and patients referred because of a suspicion of a glycogen storage disorder, but on whom information regarding GAA activity testing or other diagnostic evaluations were not available (n = 26), were included in the analysis.
Ethics Review
This study was approved by the Duke University Health System Institutional Review Board.
Statistical Analysis
Descriptive statistics and Pearson correlation coefficients were calculated using GraphPad Prism® 5.04 software (La Jolla, CA). Statistical significance was defined as P ≤ 0.05.
RESULTS
Sensitivity and Specificity of Urinary Glc4 in Patients Evaluated for Pompe Disease
The results of 208 evaluations for Pompe disease by the concurrent measurements of GAA activity in various tissues (dried blood spots, cultured skin fibroblasts, and/or muscle biopsy tissue), and urinary Glc4 were identified by a retrospective review of laboratory records. Sixty-eight patients were diagnosed with Pompe disease by the demonstration of a deficiency of GAA activity in dried blood spots (n = 67) or cultured skin fibroblasts (n = 1) (median age: 0.6 years; range: 11 days to 67 years). The diagnosis was confirmed for 55 patients by the documentation of two disease causing mutations through full gene sequencing. Prior to the availability of full gene sequencing, the diagnosis of Pompe disease was confirmed by demonstration of GAA deficiency in a second tissue type (n = 9), by a repeat analysis in a second sample of the same tissue (n = 1), and/or the demonstration of a mutation through targeted mutation analysis (n = 3). Glc4 concentrations obtained on this patient population were compared with age-matched control ranges that were recently reassessed (manuscript submitted) and are described in the footnote of Table I. In this cohort of 68 patients with deficient GAA activity and a diagnosis of Pompe disease, Glc4 was elevated in the majority of cases (n = 64), demonstrating 94% sensitivity of Glc4 in patients with Pompe disease across a wide age range (Table I). Four patients had Glc4 levels at or below the upper limit of the age-matched control range (Fig. 1b). These included three juvenile patients aged 1.7, 8.9, and 11 years, with no cardiac involvement and relatively mild presentations (hypotonia, abnormal sleep studies, or mild motor delay, respectively). The fourth patient was an 11-day-old infant with a Glc4 concentration close to the upper limit of the control range (17 mmol/mol CN; age-matched controls 95th centile: 20 mmol/mol CN).
| Age (years) | Urinary Glc4 (mmol/mol CN) | ||
|---|---|---|---|
| Patients with deficient GAA activity | Patients with normal GAA activity | Aged-matched upper control limita | |
| <0.5 | 38 (17–110), n = 31 | 7.9 (1.5–83), n = 50 | <20 |
| 0.5–1.0 | 53 (35–87), n = 10 | 4.7 (0.6–21), n = 15 | <15 |
| 1.0–3.0 | 55 (6.6–196), n = 4 | 3.7 (1.4–32), n = 17 | <7.8 |
| >3.0 | 12 (1.4–67), n = 23 | 1.4 (0.3–57), n = 58 | <3.1 |
- Glc4, glucose tetrasaccharide biomarker; CN, creatinine; GAA, acid alpha-glucosidase.
- Median and ranges are shown.
- a The upper limit of the aged-matched control ranges were calculated as the 95th centile of control group values. Age group <0.5 years: median = 6.6 mmol/mol CN, n = 111; age group 0.5–1.0 years: median = 3.4 mmol/mol CN, n = 54; age group 1–3 years: median = 2.1, n = 44; age group >3 years: median = 0.8, n = 302.
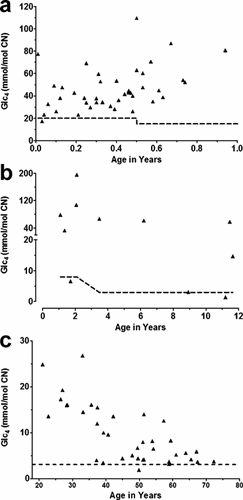
Correlation of urinary Glc4 with age in untreated patients with a confirmed deficiency of GAA. Dashed lines represent the upper limit of age-matched control ranges: 0–6 months age (95th centile: 20 mmol/mol CN, n = 109), 6–12 months (95th centile: 15 mmol/mol CN, n = 56), 1–3 years (95th centile: 7.8 mmol/mol CN, n = 37), >3 years (95th centile, 3.1 mmol/mol CN, n = 309). a: Age group <1 year: Glc4 was significantly positively correlated with age (Pearson r = 0.413, P < 0.05; n = 42). b: Age group 1–17 years: Glc4 was not significantly correlated with age (r = −0.477, P = 0.138; n = 11). Note: the oldest patient in this group was 12 years old and hence the X-axis scale was not extended beyond 12 years to improve clarity of the graph. c: Age group ≥18 years: Glc4 was significantly negatively correlated with age (r = −0.768, P < 0.05; n = 21).
One hundred forty patients evaluated for Pompe disease had a GAA activity that measured in the normal control range (median age: 1.4 years; range: 1 day to 68 years). One hundred eighteen of these patients also had Glc4 concentrations within the control range (Table I) demonstrating Glc4 has a specificity of 84% for Pompe disease. Of the 22 patients with normal GAA activity and elevated Glc4 (median age: 2.5 years; range 2 days to 57 years), 6 had mild elevations within 20% of the upper control limit. Repeat testing was performed for only one of these 22 patients, with the repeat value being within control limits.
In addition, the data showed the positive predictive value of urinary Glc4 as a biomarker in this cohort of patients with a high clinical suspicion of Pompe disease was 74% and the negative predictive value was 97%.
Correlation of Urinary Glc4 With Age in Untreated Patients With Pompe Disease
The relationship of urinary Glc4 concentrations with age in untreated patients with a deficiency of GAA is shown in Figure 1a–c. There was an overall significant decrease in Glc4 values with age (Pearson r = −0.628, P < 0.05, n = 90) in untreated patients with Pompe disease, with adult patients typically excreting lower concentrations of Glc4 than infants or older children. A closer examination of Glc4 concentrations in different age groups revealed a significant positive correlation with age for infants with Pompe disease <1 year of age (Pearson r = 0.413, P < 0.05, n = 42) (Fig. 1a). In contrast, no significant correlation was observed for juvenile patients aged 1–17 years (Pearson r = −0.477, P = 0.14, n = 11) (Fig. 1b). Glc4 values were negatively correlated with age for untreated adult patients aged ≥18 years (Pearson r = −0.727, P < 0.05, n = 37), (Fig. 1c).
Correlation of Urinary Glc4 With CK
Glc4 values were compared with CK measurements for 50 patients (median age 18 years, range: 9 days to 68 years) for whom date-matched values were available. The results of GAA activity testing were available for 24 of these patients. In the remaining patients GAA activity testing was not referred to our laboratory and these data were not available. The clinical indication for referral was either myopathy (n = 8) or was not stated. Glc4 was not correlated with CK values when the entire cohort was included in the analysis (Pearson r = 0.072, P = 0.62, n = 50). However, when the patients with a deficiency of GAA and a diagnosis of Pompe disease were analyzed as a separate group (median age 37 years, range: 1–67 years), a significant positive correlation of Glc4 with CK values was observed (Pearson r = 0.646, P = 0.03, n = 11). In contrast, those patients with normal GAA activity (median age 28 years, range: 9 days to 59 years) showed no correlation (Pearson r = 0.025, P = 0.94, n = 13). Several patients with elevated CK had normal Glc4 concentrations (10 of 33; 30%). Likewise, a similar proportion (5 of 12; 29%) of patients with normal CK values had elevated Glc4.
DISCUSSION
Glc4 as a Diagnostic Biomarker
In this retrospective review of clinical and laboratory data from pediatric and adult patients evaluated for Pompe disease, we have determined that urinary Glc4 had high sensitivity (95%) and specificity (84%) as a diagnostic biomarker within this at-risk group. This is in agreement with our previous observation that Glc4 was a highly predictive biomarker of Pompe disease in infantile patients enrolled in the pivotal clinical trials of ERT with rhGAA
In this retrospective review of clinical and laboratory data from pediatric and adult patients evaluated for Pompe disease, we have determined that urinary Glc4 had high sensitivity (95%) and specificity (84%) as a diagnostic biomarker within this at-risk group. This is in agreement with our previous observation that Glc4 was a highly predictive biomarker of Pompe disease in infantile patients enrolled in the pivotal clinical trials of ERT with rhGAA.
[An et al., 2005; Young et al., 2009]. In the current study, while the majority of patients diagnosed with Pompe disease had elevated Glc4 concentrations, a small number of juvenile patients with a milder phenotype relative to other patients of a similar age, and an 11-day-old infant excreted a normal concentration of Glc4. We have previously shown that urinary Glc4 concentrations were correlated with measures of glycogen accumulation in quadriceps muscle biopsies [Young et al., 2009]. It is therefore not unexpected that some patients would have normal Glc4 excretion when evaluated at a point early in the disease process, when the degree of glycogen accumulation and muscle fiber damage may be limited. Disease severity is highly variable in Pompe disease and influenced by several factors including the degree of residual enzyme activity [Hirschhorn and Reuser, 2000], the age of onset, and the duration of disease [Hagemans et al., 2005; Winkel et al., 2005; Kishnani et al., 2006]. Therefore it is important to interpret urinary Glc4 concentrations in the context of the clinical presentation.
Glc4 Excretion Correlates With Age and Phenotype
As observed in this and a previous study [An et al., 2000], infants with Pompe disease typically excrete higher Glc4 concentrations than adults with the disease. A positive correlation of Glc4 with age was observed for patients less than 1 year of age, and is similar to trends in serum enzyme biomarkers of muscle and liver damage (AST, ALT, and LDH) in untreated infantile patients [van den Hout et al., 2003]. This finding is consistent with the rapidly progressive nature of the infantile form of the disease
As observed in this and a previous study, infants with Pompe disease typically excrete higher Glc4 concentrations than adults with the disease. A positive correlation of Glc4 with age was observed for patients less than 1 year of age, and is similar to trends in serum enzyme biomarkers of muscle and liver damage (AST, ALT, and LDH) in untreated infantile patients. This finding is consistent with the rapidly progressive nature of the infantile form of the disease.
[van den Hout et al., 2003; Kishnani et al., 2006]. Patients with infantile Pompe disease evaluated at a very early age (i.e., ≤1 month of age) may not have Glc4 elevations, as observed in this study for one patient aged 11 days. In a cohort of patients with infantile Pompe disease identified through newborn screening, Glc4 values were either close to, or above, the upper normal control limits at the age of 1 month or younger (manuscript submitted).
The declining trend of Glc4 with age in the adult group suggests that the degree of glycogen accumulation is lower in adults diagnosed at a later age. One limitation of this study is a lack of information on disease duration or clinical manifestations. It cannot be determined, therefore, whether the lower values represent milder disease, or an advanced disease state of muscle fiber atrophy and replacement with fibrofatty tissue devoid of glycogen. Another limitation is the lack of information on inter-current illness, such as infection, and how this may affect the Glc4 value. We have observed temporary increases in Glc4 for some patients with infantile Pompe disease on ERT during inter-current illness (unpublished observations).
Specificity of Glc4 and Comparison With CK, a Secondary Biomarker of Muscle Damage
The causes of the Glc4 elevations observed in a minority of patients with normal GAA activity are unknown, but could be associated with dietary factors such as ingestion of glucose polymers (glycogen or amylopectin) or treatment with IV glucose, both of which have been shown to cause temporary increases in Glc4 excretion [Kumlien et al., 1989]. Alternatively these elevations may result from pathological processes that damage glycogen-rich tissues. It should be noted that repeat Glc4 testing was not performed for the majority of these patients and it is not possible to determine whether these elevations were temporary.
The lack of correlation between CK and Glc4 in the overall patient population evaluated (patients with a confirmation of Pompe disease and those without) indicates that Glc4 is not elevated in all instances when there is evidence for muscle damage. Investigations on the correlation of Glc4 with CK in our previous studies of patients with infantile Pompe disease have shown inconsistent results. In 18 patients with infantile Pompe disease Glc4 was not significantly correlated with CK at baseline prior to the start of ERT, or after 52 weeks on ERT [Young et al., 2009]. However a significant correlation was observed at 12 weeks on ERT. Other investigations have shown that some, but not all, long-term survivors with infantile Pompe disease on ERT may show a significant positive correlation of Glc4 with CK over time (unpublished observations). The reason for these inconsistencies is unclear and further studies are needed to determine the association of Glc4 in disorders or conditions in which the integrity of myofibers is compromised, but in which glycogen may not be abnormally accumulated.
Comparison of Glc4 With CK and Other Serum Biomarkers for Monitoring Patients With Infantile Pompe Disease on ERT
We have previously reported trends in Glc4 excretion correlated with the motor response to ERT in infantile Pompe disease [An et al., 2005; Young et al., 2009]. All patients treated showed a reduction of Glc4 to varying degrees within 4 weeks of initiating therapy. Patients who had a reduction of Glc4 into, or close to, the control range early in the course of treatment, and sustained low levels of Glc4 excretion during 2 years of treatment, had the best clinical outcomes; these patients maintained motor gains over 2–3 years of treatment. In contrast Glc4 levels, although somewhat reduced at 4 weeks compared with baseline, remained moderately to markedly elevated for patients who made minimal or no motor gains on treatment. A group of intermediate responders made motor gains in the first year of treatment that corresponded with a reduction of Glc4 that was comparable to that observed in the group with the best response. During the second to third year of treatment, however, the intermediate responders made no further gains, or declined, and exhibited a corresponding increase in Glc4 excretion. In contrast, serum enzyme biomarkers of tissue damage (CK, AST, or ALT) did not normalize during the course of treatment for any of the patients and none showed as clear a separation between the response groups as did Glc4.
Patients with late-onset Pompe disease on therapy may also show downwards trends in Glc4 on treatment, although changes are typically less pronounced compared with infantile Pompe disease (unpublished observations). In a double-blind placebo controlled study of ERT in late-onset Pompe disease, a statistically significant reduction in Glc4 relative to baseline values was observed at 78 weeks for patients on ERT, compared with the placebo group (manuscript in preparation). This corresponded with a significant, modest improvement in clinical end-points (6-min walk test and forced vital capacity) in the treated group.
Hence we conclude that Glc4, as a surrogate biomarker of glycogen accumulation, is useful for monitoring the response to ERT in Pompe disease.
Imaging Techniques for Assessing Disease Severity in Pompe Disease
While primary and secondary biomarkers in Pompe disease provide an overall measure of the extent of glycogen accumulation and tissue damage, details on the distribution of the accumulated glycogen and structural tissue damage must be ascertained using other approaches. Many of the structural complications of Pompe disease can be diagnosed noninvasively by a variety of traditional imaging modalities. For example, echocardiography can assess decreases in left ventricular diastolic function and changes in ventricular mass due to glycogen deposition [Forsha et al., 2011]. Echocardiography, CT, and magnetic resonance angiography (MRA) have been used to detect and characterize thoracic aortic aneurysms and one of their life-threatening consequences, aortic dissection [El-Gharbawy et al., 2011]. Zaidman et al. [2011] employed focused extremity muscle ultrasound to measure muscle strength and function in patients with Pompe disease.
Perhaps of greater impact to the long-term management of patients with Pompe disease, is a growing body of work in the use of imaging biomarkers to detect and stage fibrofatty muscular degeneration in the Pompe disease population. In particular, the use of MRI may bypass a number of the limitations of traditional serum/urine analysis, biopsy, and functional testing. Specifically, a whole-body MRI approach provides a global assessment of muscle degeneration while localizing specific muscle groups involved, and may allow for quantification of the degree and distribution of muscle involvement. Carlier et al. [2011] recently described an MRI-based technique for screening involved muscle groups and qualitative evaluation of their degree of involvement. MR-spectroscopy techniques have also been used to elucidate muscle energetics and metabolite changes, including in vivo measurement of muscle glycogen by 13C-NMR spectroscopy, though such examinations are focused to particular muscle groups and can be time-intensive [Wary et al., 2010; Laloui et al., 2011].
Due to the relative robustness of MRI signal derived from lipid molecules, fatty replacement of the musculature is one of the most promising MRI biomarkers for screening paradigms in Pompe disease. In addition, it may be possible to evaluate specific muscle groups whose disease status carries important implications for quality of life and mortality, such as the diaphragm and tongue, which are not accessible for biopsy. Although there are several case series exploring the appearance of fibrofatty muscle degeneration in Pompe disease and other neuromuscular diseases [Chan and Liu, 2002; Pichiecchio et al., 2004; Dlamini et al., 2008; Carlier et al., 2011] there is a paucity of quantitative or longitudinal data to evaluate the utility of MRI in assessing disease progression, response to treatment, or correlate imaging findings with functional and survival outcomes. The migration of techniques developed in fatty liver imaging to the musculature may overcome some of the current limitations of imaging and provide fast, whole-body, quantitative evaluation of muscle degeneration in the near future [Boll et al., 2010; Bashir et al., 2011].
In summary, urinary Glc4 is a noninvasive biomarker of glycogen accumulation that is useful for the diagnosis and monitoring of patients with Pompe disease. It has high sensitivity for Pompe disease, but can be elevated in other conditions, and thus for diagnostic purposes it must be measured in conjunction with GAA activity testing. Trends in Glc4 are informative for patients with infantile Pompe disease on ERT: a reduction into or close to the control range during the first few weeks of treatment is a good prognostic indicator. Patients who maintain low levels usually have the best responses in terms of overall ventilator-free survival and motor response. Adults with late-onset Pompe disease typically excrete lower concentrations of Glc4 compared with patients with infantile Pompe disease. In late-onset Pompe disease Glc4 excretion is significantly reduced in response to ERT, although changes may be small. Glc4 and other biomarkers of the overall disease burden do not provide specific information on the distribution of affected tissues. Magnetic resonance spectroscopy and imaging techniques offer alternative, noninvasive approaches to molecular biomarkers for assessing in detail the disease severity in Pompe disease.
Acknowledgements
The authors would like to acknowledge patients and their families, and physicians referring samples to the laboratory. We also acknowledge Amie Vaisnins-Carroll, B.S., Kelley Boyd B.S., Gwen Dickerson B.S., Denise Peterson, Jian Dai B.S., Shelby Currier, B.S., and Eileen Winchester, B.S. for technical assistance and data management, and Genzyme Corporation for previous funding for the development of the Glc4 assay and monitoring patients on ERT.
Sarah P. Young, David S. Millington, Priya S. Kishnani, Deeksha S. Bali and Monique Piraud, have received research/grant support from Genzyme Corporation. Priya S. Kishnani has received honoraria from Genzyme and is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Corporation. Pascal Laforet has received honoraria and scientific support from Genzyme, and is a member of the Pompe Disease Advisory Board.




