Metabolic disorders and mental retardation
Abstract
The metabolic and anatomical substrate of most forms of mental retardation is not known. Because the basis of normal brain function is not sufficiently understood, the basis of abnormal function is understood poorly. Even in disorders where the fundamental biochemical defect is known, such as phenylketonuria (PKU) and other enzyme defects, the exact basis for brain dysfunction is uncertain. The outcome for treated PKU, galactosemia, homocystinuria, and lysosomal disorders is not yet optimal. The various forms of nonketotic hyperglycinemia often respond poorly to current therapy. Less familiar disorders, with or without seizures, such as deficient synthesis of serine or creatine and impaired glucose transport into the brain, and disorders with variable malformations, such as Smith-Lemli-Opitz (SLO) syndrome and the congenital disorders of glycosylation (CDGs), may initially be thought to be a nonspecific form of developmental delay.
Less familiar disorders, with or without seizures and disorders with variable malformations may initially be thought to be a nonspecific form of developmental delay.
Simple tests of urine, blood, and cerebrospinal fluid may lead to a diagnosis, accurate genetic counseling, and better treatment. Metabolic brain imaging (magnetic resonance spectroscopy (MRS)) has also helped to reveal biochemical abnormalities within the brain. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Some forms of mental retardation have been known to have a biochemical basis for many years, but the biochemical mechanisms of brain damage, dysfunction, and destruction are still poorly understood, and the basis of most forms of retardation is not known. The purpose of this article is to illuminate selected areas of metabolic significance, including recent advances in our understanding of familiar disorders, some important disorders recently discovered, and diagnostic tests that can help reveal the causes of brain dysfunction. The emphasis is on new knowledge about well-known conditions, and newer disorders that are not yet well known.
Single-gene disorders that cause mental retardation may be transmitted in an autosomal dominant manner (or arise by new mutation), in an autosomal recessive manner, X-linked recessive or dominant, and by mitochondrial inheritance. Nearly all disorders of intermediary metabolism are autosomal recessive, although there are some familiar X-linked recessive ones. A few are autosomal or X-linked dominant.
The mechanisms for brain damage or dysfunction are not known for most metabolic disorders, and nearly all disorders that cause mental retardation have no obvious biochemical derangement. Known mechanisms of brain damage or dysfunction in genetic disorders include defects in energy production leading to problems ranging from gross abnormalities of brain formation to subtle abnormalities of function (e.g., disorders of oxidative phosphorylation/electron transport chain, glutaric aciduria type II); disorders of energy availability (creatine deficiency, impaired glucose transport); altered expression of genes leading to aberrant or arrested developmental programming (fragile X syndrome, Rett syndrome); abnormal levels of neurotransmitters leading to aberrant neurotransmission (nonketotic hyperglycinemia, serine disorders, biopterin disorders); and excess or unavailability of substrate, leading to disturbance of myelin formation or function (phenylketonuria, lysosomal storage disorders).
Relatively few metabolic conditions cause mental retardation in isolation: other neurological symptoms such as ataxia, chorea, dystonia, or seizures are commonly found. Movement disorder and seizures may follow developmental delay in infancy. We therefore discuss some of the less familiar disorders in which mental retardation is not the only symptom, but is prominent, and which may not be suspected without doing specific tests. We also discuss some of the biochemical disorders in which malformations occur. Somatic characteristics and dysmorphic features may be subtle or unappreciated, especially in young children, and become more obvious only with time. We will also discuss selected aspects of metabolic testing pertinent to mental retardation.
ASPECTS OF MENTAL RETARDATION IN SOME WELL-KNOWN DISORDERS
Phenylketonuria
Phenylketonuria (PKU) is one of the first metabolic diseases to be characterized. Untreated PKU leads to mental retardation and seizures. Newborn screening, begun on a large scale in the 1960s, allows the early institution of a diet that restricts phenylalanine (PHE) intake and supplements tyrosine. This therapy improves clinical outcome dramatically. However, there is imperfect correlation between PHE blood levels and outcome.
Classical PKU results from impaired activity of the liver enzyme phenylalanine hydroxylase (PAH), which converts PHE to tyrosine (Fig. 1). The result is a raised level of PHE in the blood and other tissues and reduction in the level of tyrosine. Tyrosine is required for the production of a number of biologically active substances, including dopamine via L-DOPA. Impaired metabolism of tetrahydrobiopterin (BH4), a cofactor for hydroxylations involved in the metabolism of PHE, tyrosine, and tryptophan, leads to malignant hyperphenylalanemia, which is more difficult to treat than PKU.
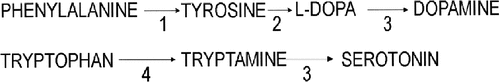
PHE metabolism, showing the relationship to tyrosine and dopamine. Enzymes: 1) PAH, 2) tyrosine hydroxylase, 3) aromatic L-amino-acid decarboxylase, 4) tryptophan hydroxylase. Tetrahydrobiopterin (BH4), a cofactor for reactions 1, 2, and 4, is metabolized to quinonoid dihydrobiopterin in these reactions and resynthesized by dihydropteridine reductase.
More than 400 mutations have been identified in the PAH gene. Deletions, insertions, point mutations, and splicing mutations have been described [http://data.mch.mcgill.ca/pahdb_new/; Eng et al., 2001]. The number of possible mutations and the fact that most individuals are compound heterozygotes account only in part for the large biochemical phenotypic variability seen in PKU patients and the variability in intelligence quotient (IQ) and brain magnetic resonance imaging (MRI) changes [Burgard et al., 1999]. In vivo PAH activity can only be predicted if both motations are identified [Okano et al., 1991]. Siblings who share identical mutations may have different clinical expression [Ramus et al., 1993, Ramus et al., 1999]. PHE tolerance does not seem to be dependent on enzyme activity in vivo [van Spronsen et al., 1998]. There are even individuals with markedly raised blood PHE levels who escape brain damage [Primrose, 1983].
Therapy for PKU involves adherence to a strict diet low in PHE, commencing as soon as the diagnosis is made [Smith et al., 1990]. Although treatment dramatically improves outcome, neuropsychological testing of treated patients can show a lower IQ than that of unaffected siblings and parents. Subtle deficits in higher cortical function have been observed in patients with apparently good metabolic control. The mechanisms of brain damage remain unclear. There may be direct neurotoxic effects of PHE [Huttenlocher, 2000] and effects related to insufficient tyrosine. A raised PHE level may impair transport into the brain of other large neutral amino acids that share the same transporter, including tyrosine and tryptophan, with resultant alteration in neurotransmitter levels. MRI studies in patients with PKU have demonstrated white matter abnormalities, even in patients who were thought to have adequate control, based on blood levels. The mechanisms for these changes seem to relate to dysmyelination [Thompson et al., 1990]. Studies using MR spectroscopy (MRS) have shown a correlation between the concentration of PHE in the brain and clinical outcome, suggesting that the transport of PHE across the blood-brain barrier (BBB) is a critical factor [Moller et al., 1998; Koch et al., 2000; Weglage et al., 2001].
Relaxation of the diet at school age was the initial practice, but this can lead to some loss of intelligence [Holtzman et al., 1986]. “Diet for life” does have major difficulties. Current recommendations in the United States emphasize that some form of dietary therapy should continue for life [National Institutes of Health Consensus Development Conference Statement, 2001]. Guidelines from the United Kingdom and Europe allow for relaxation of the diet after childhood, while acknowledging that raised levels of PHE may be associated with some subtle cognitive impairment [Recommendations on the dietary management of phenylketonuria, 1993; Burgard et al., 1999].
The developing brain is most vulnerable to raised levels of PHE, and good control within childhood has been shown to have the greatest influence on structural (MRI changes) and functional outcome (IQ).
The developing brain is most vulnerable to raised levels of PHE, and good control within childhood has been shown to have the greatest influence on structural and functional outcome.
Untreated maternal PKU leads to devastating damage to the fetus, including microcephaly, severe mental retardation, and congenital heart disease. The fetal blood level of PHE will be slightly higher than the mother's. The fetus of an affected mother will be an obligate carrier of PKU. If the father is a carrier, there is a 50% risk the fetus will also have PKU. Women with PKU must be on the diet before they conceive and continue it throughout the pregnancy, with frequent monitoring of the blood PHE level. With this practice, the outcome is excellent [Levy and Waisbren, 1983; Platt et al., 2000; Rouse et al., 2000].
Finally, although general IQ in patients with PKU is slightly lower than that in their siblings, other functions, especially executive functions, may also be impaired in PKU and may be worsened by relaxation of the diet. Reinstating therapy in adults may reverse these problems. Even in older, previously untreated individuals there can be some improvement in behavior with treatment [Fitzgerald et al., 2000].
Galactosemia
In galactosemia there is defective conversion of galactose to glucose, via a pathway catalyzed by the enzymes galactokinase, galactose-1-phosphate uridyl-transferase, and uridine diphosphate galactose 4-epimerase. Transferase (UDPGT or GALT) deficiency is the main form. Clinical manifestations following the ingestion of galactose include failure to thrive, vomiting and diarrhea, liver dysfunction, cerebral edema, mental retardation, and vulnerability to Escherichia coli sepsis [Levy et al., 1977]. Allelic heterogeneity within the GALT gene contributes to the large phenotypic variability that is seen in classical galactosaemia. Q188R is the most common mutation in white Europeans, accounting for 60–70% of mutant chromosomes [Tyfield et al., 1999]. However, genotype-phenotype correlations are not precise [Kaufman et al., 1994; Novelli and Reichardt, 2000] and may be beyond explanation by simply a single-gene mutation [Tyfield, 2000].
Galactose is required for synthesis of glycoproteins, glycosphingolipids, and similar substances. It is not an essential nutrient, as it can be made from glucose. In untreated galactosemia the erythrocyte galactose level will be several-fold elevated. Red cell galactose-1-phosphate levels are also elevated, and galactitol is raised in the blood [Ning and Segal, 2000].
The major dietary source of galactose is milk, so using a synthetic formula will reduce the blood galactose level. Dietary therapy prevents cataracts, liver failure, and sepsis. However, patients demonstrate a delay in speech acquisition [Waisbren et al., 1983], subtle speech dyspraxia throughout life [Waggoner et al., 1990; Nelson et al., 1991], and diminished IQ [Schweitzer et al., 1993]. Ataxia, tremor, and movement disorders may also occur [Koch et al., 1992]. In females, ovarian failure is frequent [Kaufman et al., 1986]. An international review of classical galactosemia revealed that expectant treatment with restricted milk intake during pregnancy and no postnatal galactose exposure in families at risk does not prevent ovarian failure and cognitive deficits [Waggoner et al., 1990]. These findings are much more likely in classical galactosemia, in which enzyme activity is close to zero, than in less severe variants.
It is unclear whether neurological and ovarian damage in individuals with transferase deficiency and dietary restriction relate to prenatal events or occur via long-term intoxication either by endogenous [Gitzelmann et al., 1975] or exogenous [Acosta and Gross, 1995] sources. The endogenous production is greater than dietary sources once milk and its derivatives are eliminated. Accumulated galactose and its intracellular metabolites, especially galactitol, are clearly toxic. Elevated galactose-1-phosphate impairs glycosylation of various glycoproteins, similar to what occurs in the congenital disorders of glycosylation (CDGs). Ovarian and pubertal failure occurs in CDG type Ia.
Lysosomal Storage Diseases
The lysosomal storage diseases are a heterogeneous group of conditions in which there is progressive accumulation of undegraded catabolites. Deficient or defective transport or metabolism results in failed recycling and intralysosomal storage of material in various tissues, particularly the reticuloendothelial system and the nervous system. Progressive central nervous system (CNS) damage occurs in most of these conditions, which now number about 40.
Therapies for these devastating conditions have attempted to improve enzyme activity and reduce the quantity of material stored. Methods attempted include the use of bone marrow transplant, enzyme replacement, and substrate restriction [reviewed by Wraith, 2001]. The development of treatments for some of the lysosomal storage disorders means that early diagnosis will become essential.
The development of treatments for some of the lysosomal storage disorders means that early diagnosis will become essential.
A newborn screening test for lysosomal dysfunction is currently being studied [Meikle et al., 1999].
Bone marrow transplant (BMT) has been used with increasing frequency as a therapy for the lysosomal storage diseases. Clinical improvement may be due to replacement of tissue macrophages and blood cells, intercellular enzyme transfer, uptake of donor-derived enzyme in plasma, and the clearance of accumulated substrate.
Biochemical measurements do not correlate directly with clinical outcome. The interpretation of results is complicated by the heterogeneity of groups studied, varying enzyme levels, and the stage of disease at the time of transplant [Hoogerbrugge et al., 1998]. Following BMT and engraftment in mocopolysaccosidosis type 1 (μPS 1-Hurler), organomegaly, cardiac, and respiratory symptoms improve. There may be stabilization in neurological and bone symptoms, but these systems may not improve [Whitley et al., 1993; Hoogerbrugge et al., 1995]. Because of this, transplantation is recommended prior to neurological symptoms and less than 2 years of age.
The results in other storage diseases are varied. A patient with juvenile globoid cell leukodystrophy has demonstrated improved neuropsychological function. The neurological and neuropsychological features of X-linked adrenoleukodystrophy may stabilize [Shapiro et al., 1995]. The results in aspartylglucosaminuria have been promising but are not yet certain [Autti et al., 1997a,b]. Neurological progression is unaltered in Tay-Sachs, Sandhoff [Hoogerbrugge et al., 1995], Farber [Yeager et al., 2000], Hunter diseases, and metachromic leukodystrophy.
Enzyme replacement therapies do not have the problems of matching and rejection that accompany transplantation. Enzyme therapy has been successfully used in the treatment of nonneuronopathic Gaucher disease for many years and may have a role in the neuropathic form. Phase III trials have shown promise in the treatment of Fabry [Eng et al., 2001] and Pompe [Van den Hout et al., 2001] diseases, which have minimal or no CNS involvement, and have recently begun for Hurler syndrome, in which CNS involvement is a major issue [Kakkis, 2001]. Gene therapy, to introduce a gene for a missing enzyme product into blood, liver, or other cells, has shown initial promise in animal models [Marechal et al., 1993; Caillaud and Poenaru, 2000], but no human lysosomal disorder has been treated by this technique to date. Limitation of synthesis of complex sphingolipids may also have a role in slowing the progression of Gaucher disease and others [Platt et al., 2001].
Nonketotic Hyperglycinemia (Glycine Encephalopathy)
The typical severe form of nonketotic hyperglycinemia (NKH) presents with seizures, coma, and apnea in the neonatal period. The plasma glycine level is usually moderately elevated, but the cerebral spinal fluid (CSF) glycine is significantly elevated, leading to an increase in the CSF/plasma glycine ratio (normally less than 0.06). Death may occur in the neonatal period. Long-term survival may occur, usually with minimal mental development, but surprisingly little gross brain destruction.
There is uncertainty regarding the extent of prenatal damage. The placental circulation cannot lower the plasma glycine level sufficiently to lower the CSF/brain glycine level to the normal range. The corpus callosum may be absent. However, newborn infants with NKH are typically unremarkable for the first few days.
The glycine cleavage system, consisting of four peptides, produces ammonia, CO2, and hydroxymethyltetrahydrofolate. This is the main mechanism of glycine disposal in the brain. Glycine can also be converted by serine hydroxymethyltransferase to the amino acid L-serine (Fig. 2). The production of D-serine (see Serine Biosynthesis Defects, below) may be impaired in NKH [Iwama et al., 1997; Mothet et al., 2000].
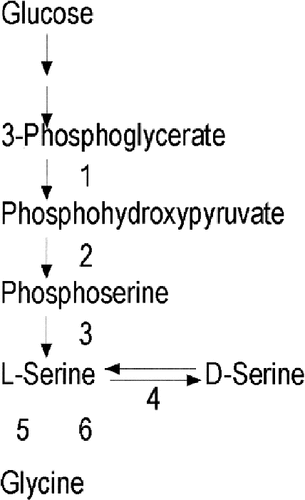
Serine pathway. Serine is synthesized from 3-phosphoglycerate, in the Embden-Meyerhof glycolytic pathway. Enzymes: 1) 3-phosphoglycerate dehydrogenase, 2) phosphopyruvate aminotransferase, 3) phosphoserine phosphatase, 4) serine racemase, 5) serine hydroxymethyltransferase and glycine cleavage system, and 6) serine hydroxymethyltransferase.
Lowering the plasma glycine level by the use of sodium benzoate can lead to improved seizure control. The CSF glycine level will fall, but not to the normal level. Benzoate dosage and toxicity must be carefully monitored, because of the narrow therapeutic window.
Glycine is a neurotransmitter and a modulator of the N-methyl-D-aspartate (NMDA)-type glutamate receptor, a calcium channel. This receptor can be modulated by dextromethorphan. Treatment of NKH using this drug has been successful in a modest number of infants, with diminished seizure frequency and improved neurological performance and developmental outcome [Hamosh, 1998].
Atypical forms are less common, with only developmental delay and some seizures. There may be minimal elevation of the concentration of glycine in the plasma, and hence urine and the CSF/plasma ratio may be normal [Jackson and Applegarth, 1999], so the defect can only be confirmed by molecular or enzymatic methods. Response to sodium benzoate may be gratifying [Neuberger et al., 2000].
Hypothyroidism
The importance of thyroid hormone in normal brain function is well known. Recognition of congenital hypothyroidism by newborn screening has resulted in earlier treatment; the development of a practical way to correct environmental iodine deficiency by treating irrigation water has been a major advance for iodine-deficient regions [Cao et al., 1994].
Urea Cycle Disorders and Hyperammonemias
The severe forms of these disorders can cause significant or fatal brain dysfunction. The mild forms, which may cause mental retardation in isolation, may not be suspected without biochemical evaluation.
The mild forms, which may cause mental retardation in isolation, may not be suspected without biochemical evaluation.
Findings include hyperammonemia, raised levels of orotic acid on urine organic acid analysis, and raised glutamine with plasma amino acid analysis [Poplawski et al., 2002]. Central hyperammonemia may not be reflected in the periphery.
Disorders of Oxidative Phosphorylation
The disorders of mitochondrial energy production involving the electron transport chain (ETC) often cause progressive brain damage, with variable loss of function and loss of tissue. Leigh sydrome of intemittent ataxia, oculomotor palsies, and loss of reflexes may be due to defects of the ETC or pyruvate dehydrogenase. Nearly always the disorders that involve the brain lead to neurologic findings, often accompanied by myopathy, cardiomyopathy, or renal tubular dysfunction (Fanconi syndrome). Intermittent or episodic symptoms are frequent. Mild disorders may not cause peripheral symptoms, and increased CSF lactate can be the only biochemical manifestation. Consequently, consideration should be given to obtaining CSF, especially if the history or family history suggests the possibility of a mitochondrial disorder.
Homocysteinuria
Homocystinuria due to deficient cystathionine beta-synthase (CBS) deficiency is one of many causes of increased plasma homocysteine. Despite its name, plasma is the preferred diagnostic sample. Manifestations may include tall stature, a Marfanoid habitus, and lens dislocation. As in all conditions with hyperhomocysteinemia, thrombotic strokes may occur, or small vascular occlusions. Because the physical manifestations may be subtle, it is essential to test for homocystinuria as part of the workup of mental retardation.
Because the physical manifestations may be subtle, it is essential to test for homocystinuria as part of the workup of mental retardation.
A large properties proportions of patients with CBS deficiency respond well to pyridoxine. Disorders of folate metabolism may cause mental retardation or psychiatric symptoms, and can also raise the blood homocysteine level [Fowler, 2001].
Cholesterol Biosynthesis Disorders
Defects in the cholesterol biosynthetic pathway cause a variety of structural defects and biochemical derangement, most importantly Smith-Lemli-Opitz (SLO) syndrome, due to deficient activity of 7-dehydrocholesterol (7-DHC) reductase. It may cause only mental retardation and 2,3 syndactyly of the toes, although a characteristic face (ptosis, upturned nose, broad nasal tip), genital hypoplasia (in males), and other external and internal anomalies are often found [Cunniff et al., 1997].
It may cause only mental retardation and 2,3 syndactyly of the toes, although a characteristic face, genital hypoplasia, and other external and internal anomalies are often found.
Plasma cholesterol may be low in infancy but normal later. The serum level of 7-DHC (usually analyzed by gas chromatography) is elevated. The malformations and mental retardation seen in the disorders of cholesterol synthesis demonstrate the importance of cholesterol and related steroids for brain and organ formation. Altered function of hedgehog signaling proteins is one mechanism involved [Porter et al., 1996].
Cholesterol supplementation, sometimes given with the bile acid ursodeoxycholic acid, can improve motor and intellectual function in SLO syndrome. Some children have learned to walk, and some have regained speech. Head banging and other agitation may cease, and some patients have commented on how much better they feel [Tint et al., 1997; Kelley, 1998, 2000; Kelley and Hennekam, 2000].
SELECTED NEWER DISORDERS
Serine Biosynthesis Defects
At least three disorders of serine synthesis have been discovered. Deficiencies of 3-phosphoglycerate dehydrogenase (3PGPH) and phosphoserine phosphatase (PSPH) deficiency have been defined. The latter was first reported in a child with Williams syndrome and intractable seizures, who had the typical deletion in the elastin locus at 7q11.23 [Jaeken et al., 1997]. However, the gene for PSPH has been tentatively located at 7p15.2. These findings have not been reconciled.
The serine deficiency disorders are characterized by congenital microcephaly, seizures, and mental retardation.
The serine deficiency disorders are characterized by congenital microcephaly, seizures, and mental retardation.
Megaloblastic anemia may be present. In contrast to most other metabolic diseases, the serine synthesis disorders are recognized by finding a low level of this amino acid. Serine occurs in the diet and is synthesized from 3-phosphoglycerate, derived from glucose (Fig. 2). Because blood serine levels vary dramatically in relation to meals, and the deficiency in blood is mild, determining the fasting CSF serine level is the best way to recognize these disorders. Glycine and 5-methyltetrahydrofolate may also be low in blood and CSF. Brain imaging shows hypomyelination and white matter attenuation. MRS shows increased choline. Treatment with L-serine and glycine results in major improvement with diminution of seizures and improvement in the EEG, myelination, and development [Jaeken et al., 1996, 1997; de Koning et al., 1998, de Koning et al., 1999, 2000].
Nearly all biologically important amino acids are the L-isomer, and the D-isomer is inactive. However, D-serine, synthesized from L-serine by a racemase, is important in brain metabolism, as an agonist of the NMDA glutamate receptor [Schell et al., 1997; De Miranda et al., 2000]. The usual amino acid analysis does not distinguish between D and L forms. The production of D-serine may be impaired in NKH [Iwama et al., 1997; Mothet et al., 2000].
Purines and Pyrimidines
Most of the disorders of nucleotide metabolism (more than 20) do not present with isolated mental retardation, although it is a common feature. Defects of immune function or blood cell formation, movement disorders, and gout are among the usual problems. Analysis of urine for purine and pyrimidines can detect several mental retardation disorders that would not be suspected by other means. Abnormalities of purine and pyrimidine production are best detected in urine (first morning specimen), but a 24-hr sample may also be helpful. Bacterial activity and dietary purine intake can lead to misleading results [Hoffmann et al., 2002].
Adenylosuccinate lyase deficiency typically presents with severe epilepsy, psychomotor retardation, acquired microcephaly, and autistic features. Seizures are not always present. Urine and blood have detectable amounts of succinyladenosine and succinylaminoimidazole carboxymide riboside (SAICAR). The diagnostic metabolites of purine and pyrimidine disorders can be detected by a variety of methods, including capillary electrophoresis [Adam et al., 1999], proton nuclear MRS [Wevers et al., 1999], and tandem mass spectrometry [Ito et al., 2000].
Molybdenum cofactor deficiency, resulting in combined sulfite oxidase deficiency and xanthine oxidase deficiency, and isolated sulfite oxidase deficiency usually cause severe seizures, but milder forms are known. Sulfite oxidase deficiency can be detected by finding sulfite in fresh urine, or S-sulfocysteine on amino acid analysis. Xanthine oxidase deficiency will cause low uric acid production. This is also a feature of superactivity of cytosolic 5-prime nucleotidase, an X-linked disorder that leads to pyrimidine nucleotide depletion and developmental delay, perhaps with autistic features.
The three disorders of pyrimidine catabolism present with combinations of developmental delay and seizures. There is great variation in the severity of both of these features. Dihydropyrimidine dehydrogenase (DPD) deficiency has elevations of only thymine and uracil [Braakhekke et al., 1987] (Fig. 3). A variant of DPD with deficient activity is well known as a cause of neurotoxicity following 5-fluorouracil [Diasio, 2001; Raida et al., 2001]. Isolated elevation of uracil occurs in ornithine transcarbamylase (OTC) deficiency, due to excess production of orotic acid.
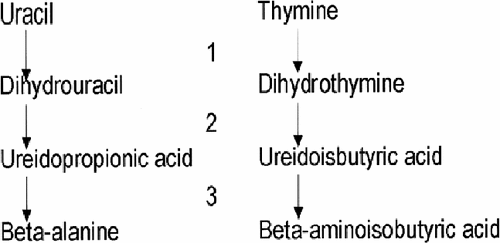
Pyrimidine pathway. Enzymes: 1) DPHDH, 2) dihydropyrimidinase, and 3) ureidopropionase.
Besides neurological problems, anal atresia, facial, and digital anomalies were present in one infant with dihydropyrimidinase deficiency, while another had diarrhea due to microvillus atrophy [Henderson et al., 1993; Assmann et al., 1997]. Ureidopropionase (beta-alanine synthase) deficiency has recently been described in a young girl with severe developmental delay, microcephaly, dystonic movements, and hypotonia [Moolenaar et al., 2001].
Creatine Deficiency
Creatine, which stores energy as creatine phosphate, is essential for normal brain function. Creatine deficiency will result in an absence of the creatine phosphate peak on brain MRS. Clinical manifestations include mental retardation with speech delay and autistic/hyperactive features, hypotonia, and seizure disorder.
Creatine is synthesized from arginine by the enzymes L-arginine:glycine amidinotransferase (AGAT) and guanidinoacetate methyltransferase (GAMT) (Fig. 4). Creatine is actively transported into the brain and excreted in the urine as creatinine. If synthesis of creatine is impaired, the urinary creatinine excretion will usually be low enough that urinary metabolites that are quantitated in terms of creatinine excretion, such as amino and organic acids, will spuriously appear to be present in excess (e.g., generalized amino/organic aciduria).
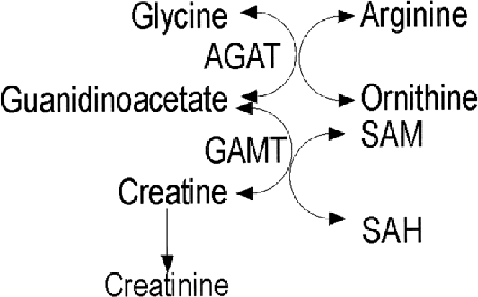
Creatine synthesis. Creatine is synthesized from arginine and glycine via guanidinoacetate. The synthesis of creatine phosphate is not shown. Creatine enters into the brain via a transporter and is also synthesized there. Creatinine is the end product of creatine metabolism. SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Severe cases of GAMT deficiency may show movement problems (hyperkinetic ballismus and dystonia). Brain MRI may show generalized cortical atrophy, and sometimes hyperintensity of the basal ganglia. Creatinine is low in the CSF [Stockler-Ipsiroglu, 1997], while guanidinoacetate is increased in CSF and urine, and may be detected by brain MRS.
Creatine monohydrate supplementation will restore brain creatine levels over several months. Clinical response may be obvious, but the neurotoxicity of guanidinoacetate in GAMT may persist. Arginine restriction and ornithine supplementation may be helpful [Stockler et al., 1994, 1996a,b; Verhoeven et al., 2000].
For the X-linked creatine transporter deficiency, high-dose creatine may be helpful, but there is a risk of crystallization in the kidneys. Supplementing the creatine precursor arginine (for transport into brain by a different transporter), has been proposed as an alternate therapy [Salomons et al., 2001].
Succinic Semialedehyde Dehydrogenase and GABA Transaminase Deficiencies
Succinic semialdehyde dehydrogenase deficiency is revealed by finding 4-hydroxybutyric aciduria on organic acid analysis. This is a disorder of gamma-aminobutyric acid (GABA) metabolism. GABA (4-aminobutyric acid), a neurotransmitter/neuromodulator that can be toxic, is increased in the CSF. Symptoms range from mild/moderate mental retardation to a severe seizure disorder. Ataxia, oculomotor apraxia, and autistic behavior may be present [Chambliss et al., 1998]. A mouse model, which upon weaning has lethal seizures, can be treated successfully with taurine [Hogema et al., 2001]. Taurine therapy has not yet been reported in humans. Two families with deficient activity of GABA transaminase (GABAT) have been reported. The first pair of children had severe psychomotor retardation and accelerated somatic growth. CSF GABA was increased, as were homocarnosine and beta-alanine. Leukodystrophy was found at autopsy. Another patient had seizures and developmental delay, but normal somatic growth [Medina-Kauwe et al., 1999].
Glucose Transporter Deficiency
Glucose is actively transported into the brain. Failure of this system leads to inadequate energy availability for the brain, mental retardation, and seizures, most commonly a form of absence epilepsy. Adequate fuel provision from ketones via a ketogenic diet can greatly improve seizure control and development. The diagnosis is made by finding that the CSF glucose level is less than 40% of a concurrent blood level, in the absence of infection [Boles et al., 1999].
CDGs
These disorders were formerly called carbohydrate-deficient glycoprotein syndromes. Many have dysmorphic features and malformations, including cerebellar atrophy, as well as major developmental delay and other neurological symptoms. Serum cholesterol may be low, especially in infancy, and thyroid-binding globulin deficiency may be ascertained on newborn thyroid screening. Isoelectic focusing of transferrin is the standard screening test [reviewed in Jaeken and Matthijs, 2001].
Deficiency of Leukotriene C4
The leukotrienes are a group of powerful inflammatory modulators.
Leukotriene A4 (LTA4) is derived from membrane-bound arachidonic acid by the actions of phospholipase A2 and 5-lipo-oxygenase. LTC4 is derived from LTA4 by the addition of a cysteinyl residue from glutathione, by the enzyme leukotriene C4 synthase (LTC4S). LTC4 is further metabolized to LTD4 and LTE4, which are biologically active, and are the main forms found in urine (Fig. 5).
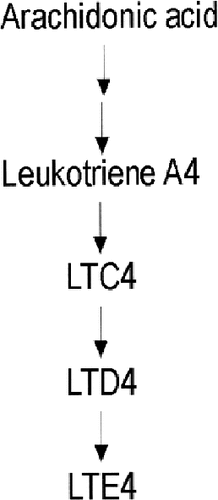
LTC4 metabolism. LTA4 is derived from membrane-bound arachidonic acid by the actions of phospholipase A2 and 5-lipo-oxygenase. LTC4 is derived from LTA4 by the addition of a cysteinyl residue from glutathione, by the enzyme LTC4 synthese. LTC4 is further metabolized to LTD4 and LTE4, which are also biologically active, and are the main forms found in urine.
Deficiency of LTD4 and LTE4 in urine, and LTC4 in CSF, has been found in at least two infants with severe hypotonia and psychomotor retardation, failure to thrive, and microcephaly. The absence of deep tendon reflexes was noted in one. Ventilator support was required for one of the infants. The outcome was fatal in 6 months in both cases [Mayatepek et al., 1993, 1999, 2000; Mayatepek and Hoffmann, 1994, Mayatepek and Hoffmann, 1995].
CONCLUSION
We have discussed some metabolic disorders that are associated with mental retardation. Metabolic testing must be considered, even when the yield is likely to be low, as the implications for the patient and the parents can be very large [Van Buggenhout et al., 2001; Poplawski et al., 2002].
Metabolic testing must be considered, even when the yield is likely to be low, as the implications for the patient and the parents can be very large.
The first step is a careful history, family history, and physical examination, which may suggest a specific disorder or category. Tests to be considered could include urine testing for amino acids, organic acids, pyrimidines and purines, guanidinoacetate, creatinine, glycosaminoglycans (mucopolysaccharides), sulfite, and uric acid. Blood testing might include amino acids, electrolytes (to calculate the anion gap), lactate/pyruvate, very long chain fatty acids, cholesterol and 7-DHC, lysosomal enzymes appropriate to the clinical picture, total homocysteine, and transferrin isoelectric focusing. Consideration should be given to lumbar puncture for glucose, organic acids, amino acids, lactate/pyruvate, creatinine, and neurotransmitters, depending on the clinical problem. Leukotrienes in the urine should be analyzed if severe unexplained seizures are occurring. Imaging may show structural abnormalities, sometimes associated with metabolic derangements, and MRS may show regions of the brain that have excess lactate or a deficiency of creatine phosphate.




