Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data
Abstract
Individuals with psychiatric disorders have elevated rates of autoimmune comorbidity and altered immune signaling. It is unclear whether these altered immunological states have a shared genetic basis with those psychiatric disorders. The present study sought to use existing summary-level data from previous genome-wide association studies to determine if commonly varying single nucleotide polymorphisms are shared between psychiatric and immune-related phenotypes. We estimated heritability and examined pair-wise genetic correlations using the linkage disequilibrium score regression (LDSC) and heritability estimation from summary statistics methods. Using LDSC, we observed significant genetic correlations between immune-related disorders and several psychiatric disorders, including anorexia nervosa, attention deficit-hyperactivity disorder, bipolar disorder, major depression, obsessive compulsive disorder, schizophrenia, smoking behavior, and Tourette syndrome. Loci significantly mediating genetic correlations were identified for schizophrenia when analytically paired with Crohn's disease, primary biliary cirrhosis, systemic lupus erythematosus, and ulcerative colitis. We report significantly correlated loci and highlight those containing genome-wide associations and candidate genes for respective disorders. We also used the LDSC method to characterize genetic correlations among the immune-related phenotypes. We discuss our findings in the context of relevant genetic and epidemiological literature, as well as the limitations and caveats of the study.
Abbreviations
-
- ADHD
-
- attention deficit-hyperactivity disorder
-
- BD
-
- bipolar disorder
-
- BH
-
- Benjamini–Hochberg
-
- CD
-
- Crohn's disease
-
- CRP
-
- C-reactive protein
-
- GWAS
-
- genome-wide association study
-
- GW hits
-
- genome-wide significant associations
-
- HESS
-
- heritability estimation from summary statistics
-
- LD
-
- linkage disequilibrium
-
- LDSC
-
- linkage disequilibrium score regression
-
- MHC
-
- major histocompatibility
-
- OCD
-
- obsessive compulsive disorder
-
- PBC
-
- primary biliary cirrhosis
-
- PGC
-
- Psychiatric Genomics Consortium
-
- QC
-
- quality control
-
- REML
-
- restricted maximum likelihood
-
- SLE
-
- systemic lupus erythematosus
-
- SNP
-
- single nucleotide polymorphism
-
- SZ
-
- schizophrenia
-
- UC
-
- ulcerative colitis.
1 INTRODUCTION
The biological bases of major psychiatric disorders have been studied for decades, yet they remain largely unresolved. Evidence from both clinical and biomedical literature has demonstrated that individuals with these conditions show differences in circulating immunologic markers, functional capacities of isolated immune cells, and atypical prevalence of clinical immune-related phenotypes compared to individuals not affected by psychiatric or neurodevelopmental disorders (Dowlati, 2010; Eaton et al., 2006; Fineberg & Ellman, 2013; Gesundheit et al., 2013; Gibney & Drexhage, 2013; Hess et al., 2016; Jones & Thomsen, 2013; Masi et al., 2015; Modabbernia, Taslimi, Brietzke, & Ashrafi, 2013; Rege & Hodgkinson, 2013). It remains unclear what roles (if any) altered immunologic functions may play in the major psychiatric phenotypes, though plausible mechanisms linking altered immune functions with neurobiological changes during early brain development and in fully developed adults have been identified (Deverman & Patterson, 2009; Felger & Lotrich, 2013; Meyer, 2014; Miller, Haroon, Raison, & Felger, 2013; Oskvig et al., 2012; Sekar et al., 2016; Shatz, 2009; Smith, Li, Garbett, Mirnics, & Patterson, 2007). While some studies have already suggested potential genetic bases for the immune dysregulation observed in a subset of psychiatric patients (Jung, Kohane, & Wall, 2011; Stringer, Kahn, de Witte, Ophoff, & Derks, 2014; The Network & Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015; Wang, Yang, Gelernter, & Zhao, 2015), the extent to which co-occurrence or segregation of clinical phenotypes may be influenced by similarities in genome-wide genetic risk signals warrants further examination. Genome-wide association studies (GWASs) and meta-analyses can shed light on the regions of the genome that tend to associate with a clinical phenotype, quantitative trait, or biomarker; this is accomplished through tagging and association-testing of single nucleotide polymorphisms (SNPs) that vary within the population. Recently developed methods like linkage disequilibrium (LD) score regression (LDSC; Bulik-Sullivan, Finucane, et al., 2015a) and heritability estimation from summary statistics (HESS; Shi, Mancuso, Spendlove, & Pasaniuc, 2017) allow for direct comparison of GWAS summary statistics for two different phenotypes for quantitative assessment of genetic correlation.
In the present study, we leveraged existing data to explore the genetic associations of a set of medical phenotypes that are enriched with immune and inflammatory processes; these included allergic conditions, classic autoimmune diseases, other inflammatory diseases, and vulnerability to infectious disease. We sought to cross-correlate the genetic associations of these phenotypes with the associations obtained from studies of a set of psychiatric and behavioral phenotypes. We hypothesized that some phenotype-pairs with evidence for increased clinical comorbidity might also share similarities in their genome-wide association profile, which would be reflected in our analyses as significant positive correlations. Additionally, in light of literature suggesting shared genetic risk among some immune and inflammatory disorders, we assessed genetic correlations within this set of phenotypes using the LDSC method; these findings are reported within the Supporting Information. Genetic correlations within the set of psychiatric phenotypes have been reported previously (Anttila, 2016; Bulik-Sullivan, Finucane, et al., 2015a; Zheng et al., 2016) and are not examined in the present study.
2 MATERIALS AND METHODS
2.1 Literature search
We searched the published literature (Pubmed, SCOPUS), data repositories (dbGaP and immunobase.org), and the downloads page of the Psychiatric Genomics Consortium (PGC) website (https://www.med.unc.edu/pgc/downloads) to identify phenotypes with potentially usable GWAS and GWAS meta-analysis summary statistics. For studies identified in the published literature, we contacted corresponding authors to request summary statistics. In order to facilitate cross-study comparison, we utilized studies that reported samples of European ancestry, broadly defined to include Central, Southern and Eastern Europe, Scandinavia, and Western Russia. Our initial search yielded a large number of datasets reflecting a wide range of behavioral and immune-related phenotypes (Supporting Information Table S1); the set of phenotypes ultimately retained for final analyses was selected based on criteria described below. When multiple studies were identified for a given phenotype, we pursued the studies with the largest effective sample sizes and ultimately used the available study with the largest heritability z-score. In several instances, data from the largest existing studies could not be shared or reflected a mixed-ancestry meta-analysis; in these cases, we deferred to the next largest European-ancestry study. We chose to retain datasets with an effective sample size greater than 5,000 individuals and with estimated SNP heritability z-score ≥3, in keeping with previous recommendations (Bulik-Sullivan, Finucane, et al., 2015a). This filter resulted in the exclusion of many relevant immune-related phenotypes, including eosinophilic esophagitis (Sleiman et al., 2014), granulomatosis with polyangiitis (Xie et al., 2013), IgA nephropathy (Kiryluk et al., 2014), HIV-related neurocognitive phenotypes (Levine et al., 2012), morning cortisol levels (Bolton, 2014), myeloid leukemias (Tapper et al., 2015), psoriatic arthritis (Ellinghaus et al., 2012), sarcoidosis (Fischer et al., 2012), and systemic sclerosis (Radstake et al., 2010). This also resulted in exclusion of several psychiatric and behavior phenotypes, including adolescent alcohol abuse (Edwards et al., 2015), anxiety-spectrum disorders (Otowa et al., 2016), borderline personality disorder (Lubke et al., 2014), language impairment (Jernigan et al., 2016), personality domains (five factor model; de Moor et al., 2012), post-traumatic stress disorder (Duncan, 2017a), and reading disability (Eicher et al., 2013). We also ultimately excluded data from studies of ethanol, opiate, and cocaine dependence (Gelernter, Kranzler, Sherva, Almasy, et al., 2014a; Gelernter, Kranzler, Sherva, Koesterer, et al., 2014b; Gelernter, Sherva, et al., 2014c), as genetic correlations involving these phenotypes were frequently outside the boundaries tolerated by the LDSC software, making them difficult to interpret; this may have been related to the ordinal-ranked phenotypes used in the GWASs. Finally, while relationships between tobacco-smoking behavior and other psychiatric phenotypes have been examined previously (Bulik-Sullivan, Finucane, et al., 2015a; Zheng et al., 2016), we chose to retain smoking data in order to assess relationships with a more complete set of immune-related phenotypes. The full list of phenotypes identified in the search and retained for analyses is shown in Supporting Information Table S1, along with identification of the study cohorts and consortia that generated these data, full citations of the respective publications, and indications of sample size, information regarding genomic inflation, and estimated SNP heritability.
2.2 GWAS phenotypes retained for genetic correlation
For our psychiatric and behavior-related phenotypes, we ultimately retained GWAS summary data reflecting studies of Alzheimer's disease (Lambert et al., 2013), angry temperament (Mick et al., 2014), anorexia nervosa (Duncan et al., 2017b), attention deficit-hyperactivity disorder (ADHD; Demontis, 2017), autism (Anney, 2017), bipolar disorder (BD; Hou et al., 2016; Sklar et al., 2011), cigarette smoking (ever-smoked status; The Tobacco & Genetics Consortium, 2010), major depressive disorder (Ripke et al., 2013), trait neuroticism (Turley et al., 2018), obsessive-compulsive disorder (OCD; Arnold, 2017), Parkinson's disease (Pickrell et al., 2016), schizophrenia (SZ; Ripke, 2014), and Tourette Syndrome (personal communication from PGC Working Group). Collectively, these phenotypes were treated as a set. For phenotypes that are known or suspected to involve alterations to immune cells and/or inflammatory signaling, we ultimately retained GWAS data reflecting allergy (any, self-reported; Hinds et al., 2013; Pickrell et al., 2016), asthma (self-reported; Pickrell et al., 2016), atopic dermatitis (EArly Genetics and Lifecourse Epidemiology (EAGLE) Eczema Consortium, 2015), childhood ear infection (self-reported; Pickrell et al., 2016), celiac disease (Dubois, 2010), serum C-reactive protein (CRP; Dehghan, 2011), Crohn's disease (CD; Franke et al., 2010; Liu et al., 2015), hypothyroidism (self-reported; Pickrell et al., 2016), primary biliary cirrhosis (PBC; Cordell, 2015), psoriasis (Tsoi et al., 2015), rheumatoid arthritis (Okada et al., 2014), systemic lupus erythematosus (SLE; Bentham et al., 2015), susceptibility to pulmonary tuberculosis (Curtis et al., 2015), type 1 diabetes (Bradfield et al., 2011), and ulcerative colitis (UC; Anderson, 2011; Liu et al., 2015) These phenotypes were treated as a set in subsequent analyses.
2.3 Data pre-processing and analysis
Our primary analyses were performed using the LDSC software (https://github.com/bulik/ldsc; Bulik-Sullivan, Finucane, et al., 2015a). Briefly, this set of tools can be used with existing GWAS summary data in order to distinguish polygenicity from confounding caused by uncontrolled population stratification or cryptic relatedness among samples (Bulik-Sullivan, Loh, et al., 2015b), to estimate the heritability of a given phenotype (Bulik-Sullivan, Finucane, et al., 2015a), and to estimate the genetic correlation between two phenotypes based on two separate or related sets of summary statistics (Bulik-Sullivan, Finucane, et al., 2015a). In the latter application, the minimal requirements for each set of summary statistics include columns of data indicating SNP ID, the identities of reference and non-reference alleles, association p-value, effect size, test statistic (e.g., odds ratio, regression β, or Z-score), and sample size (per SNP or for all SNPs). For each pair of phenotypes, this tool compares the strength and direction of association signal at each locus while correcting for the correlation that would be expected based on genetic linkage alone, and it provides an estimate of the genetic correlation between phenotypes. This method relies on adjustment for the linkage between SNPs (i.e., covariance caused by genomic proximity); for our analyses, we used the set of LD scores provided by the software's creators, based on the 1000 Genomes Project's European sample (file = eur_w_ld_chr, URL = https://data.broadinstitute.org/alkesgroup/LDSCORE). Because minor allele frequencies (MAFs) and imputation quality scores were not available for all the obtained sets of GWAS results, we filtered the GWAS results to retain only SNPs that were included within the HapMap3 panel and had a MAF ≥ 5% within the 1000 Genomes Project Phase 3 European samples (Bulik-Sullivan, Finucane, et al., 2015a); this decision resulted in the exclusion of a sizable proportion of SNPs, but ensured equitable treatment of all datasets. The extended major histocompatibility complex (MHC) region contains high amounts of long-range LD, making it challenging to accurately map association signals in this region. For this reason, and following the work of others (Bulik-Sullivan, Finucane, et al., 2015a; Zheng et al., 2016), we excluded this region from our analyses (chromosome 6, base positions 25 × 106 to 35 × 106). Additional SNP quality control (QC) routines followed those implemented by the GWAS authors and the defaults employed with the LDSC munge_sumstats.py function; this function checks alleles to ensure that the supplied alleles match those in the HapMap3 reference panel. For each dataset, we estimated the phenotype's heritability. The results of this analysis, along with features of each GWAS dataset (sample size, number of QC-positive SNPs, genomic inflation factor, etc.), are shown for all phenotypes in Supporting Information Table S1. All phenotypes with sample size ≥5,000 and estimated SNP heritability z-score ≥3 were retained for correlation analysis (indicated in Supporting Information Table S1 in green highlight). Pair-wise genetic correlations were assessed between retained phenotypes based on the intersection of QC-positive SNPs, and heatmaps were constructed to depict these relationships. For correlation coefficients returned within the bounds of the LDSC software, p-values were corrected using the Benjamini–Hochberg (BH) method for the total number of unique tests depicted in each correlation matrix. Within the main text, we describe only correlations that survived family-wise multiple-test correction. Correlations are reported as the coefficient ± standard error. For phenotype-pairs showing statistically significant genetic correlations, we re-evaluated the genetic correlations and estimated heritability using the HESS method (https://github.com/huwenboshi/hess; Shi et al., 2017)
2.4 Characterization of genetically correlated loci and associated genes
For psychiatric-immune phenotype-pairs showing significant genetic correlations after BH correction for multiple testing, we used the HESS software to estimate partitioned heritability and genetic correlations based on smaller LD-based segments of the genome (average size = 1.5 Mb). We report the number and identity of genomic partitions (based on HG19 reference) displaying significant local genetic correlations and apply correction for the total number of partitions (≈1,694, after MHC removal). Because presently available methods are poorly suited for fine-mapping the loci mediating a genetic correlation, we prioritized reporting correlated loci that also contain genome-wide significant associations for the relevant phenotypes (i.e., associations with p < 5 × 10−8; subsequently called GW hits). We report GW hits contained within the present datasets, but also cross-reference these findings with those contained in immunobase.org, in order to identify loci associated with multiple immune-related disorders. We report the HGNC symbols for candidate genes proposed to mediate those associations. The full list of genes contained within each correlated loci is provided in Supporting Information Table S3. Additionally, we used HESS to examine patterns of local genetic correlation in relationship to GWAS hits to make inferences about putative causal directionality between the phenotype-pairs. For all HESS analyses, we used the 1000 Genomes Project Phase 3 European reference panel and the LD-independent genome partitions recommended by the software developers (Berisa & Pickrell, 2015). Following the developers’ practices, we assumed no sample overlap for comparisons of data generated by different consortia (Shi et al., 2017).
3 RESULTS
3.1 Genome-wide correlations between psychiatric and immune-inflammatory phenotypes
All pair-wise LDSC genetic correlations between psychiatric and immune-related phenotypes are depicted in Figure 1. Notably, 21 correlations survived BH correction for multiple testing (denoted with **) and 6 survived a more stringent Bonferroni correction (denoted with ***). Full results for these analyses are provided in Supporting Information Table S2. Significant positive relationships were observed between ADHD and each of: CRP (rg = 0.23 ± 0.06, p = 2.0 × 10−4), childhood ear infections (rg = 0.20 ± 0.05, p = 2.0 × 10−4), psoriasis (rg = 0.23 ± 0.07, p = 1.0 × 10−3), rheumatoid arthritis (rg = 0.16 ± 0.05, p = 9.0 × 10−4), and tuberculosis susceptibility (rg = 0.36 ± 0.11, p = 1.6 × 10−3). Anorexia nervosa showed a negative genetic correlation with CRP (rg = −0.30 ± 0.08, p = 1.0 × 10−4). BD was positively correlated with each of: celiac disease (rg = 0.31 ± 0.09, p = 4.0 × 10−4), CD (rg = 0.21 ± 0.05, p = 3.7 × 10−5), psoriasis (rg = 0.25 ± 0.08, p = 3.8 × 10−3), and UC (rg = 0.23 ± 0.06, p = 2.0 × 10−4). Major depressive disorder was positively correlated with hypothyroidism (0.33 ± 0.09, p = 5.0 × 10−4). Similarly, neuroticism was positively correlated with hypothyroidism (rg = 0.25 ± 0.06, p = 7.2 × 10−5), in addition to childhood ear infection (rg = 0.13 ± 0.04, p = 8.0 × 10−4). OCD was negatively correlated with type 1 diabetes (rg = −0.32 ± 0.11, p = 5.4 × 10−3). Smoking behavior was positively correlated with CRP (rg = 0.31 ± 0.07, p = 3.6 × 10−5) and with rheumatoid arthritis (rg = 0.17 ± 0.05, p = 2.3 × 10−3). SZ showed positive genetic correlations with CD (rg = 0.12 ± 0.03, p = 2.0 × 10−4), PBC (rg = 0.14 ± 0.05, p = 2.0 × 10−3), SLE (rg = 0.15 ± 0.04, p = 2.0 × 10−4), and UC (rg = 0.14 ± 0.04, p = 2.0 × 10−4). Finally, we observed a positive genetic correlation between Tourette syndrome and allergy (rg = 0.24 ± 0.06, uncorrected p = 2.7 × 10−5). Additionally, several large-magnitude correlations attained a nominal threshold of statistical significance (e.g., autism-allergy and OCD-celiac); these correlations tended to have a higher standard error and were generated using relatively smaller GWAS sample sizes. As such, they may be more likely to reflect false positives and should be regarded with appropriate skepticism.
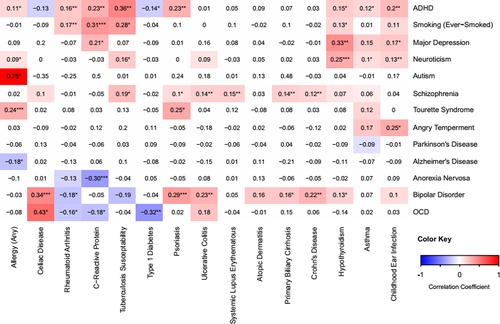
For phenotypes involved in correlations that survived multiple test correction, estimated SNP heritability is shown in Table 2. For these phenotypes, we reassessed SNP heritability and the magnitude of genome-wide genetic correlations using the HESS method (Tables 1 and 2). Correlation coefficients were not correlated between the two methods (Pearson r = 0.25, p = 0.25; Supporting Information Figure S1) and the absolute value of the difference was negatively related to sample size (r = −0.45, p = 0.035; Supporting Information Figure S2), which is consistent with the software developer's guidelines (Shi et al., 2017). LDSC-based correlations among the immune-related phenotypes are reported in the Supporting Information Text and Table S5.
| Psychiatric phenotype | Immune-related phenotype | LDSC correlation ± error, uncorrected p-value | HESS correlation ± error |
|---|---|---|---|
| ADHD | CRP | 0.23 ± 0.06, p = 2.0 × 10−4 | 0.21 ± 0.04 |
| ADHD | Childhood ear infection | 0.20 ± 0.05, p = 2.0 × 10−4 | 0.14 ± 0.03 |
| ADHD | Psoriasis | 0.23 ± 0.07, p = 1.0 × 10−3 | 1.99 ± 0.20 |
| ADHD | Rheumatoid arthritis | 0.16 ± 0.05, p = 9.0 × 10−4 | 0.29 ± 0.04 |
| ADHD | Tuberculosis susceptibility | 0.36 ± 0.11, p = 1.6 × 10−3 | 0.87 ± 0.25 |
| Anorexia nervosa | CRP | −0.30 ± 0.08, p = 1.0 × 10−4 | −0.53 ± 0.12 |
| BD | Celiac disease | 0.34 ± 0.08, p = 4.5 × 10−5 | 1.91 ± 0.12 |
| BD | CD | 0.22 ± 0.06, p = 5.0 × 10−4 | 1.31 ± 0.07 |
| BD | Psoriasis | 0.29 ± 0.07, p = 2.7 × 10−5 | 5.76 ± 0.58 |
| BD | UC | 0.23 + 0.07, p = 1.5 × 10−3 | 1.59 ± 0.08 |
| Cigarettes (ever-smoked) | CRP | 0.31 ± 0.07, p = 3.6 × 10−5 | 2.24 ± 0.73 |
| Cigarettes (ever-smoked) | Rheumatoid arthritis | 0.17 ± 0.05, p = 2.3 × 10−3 | 0.51 ± 0.22 |
| Major depression | Hypothyroidism | 0.33 ± 0.09, p = 5.0 × 10−4 | 0.45 ± 0.09 |
| Neuroticism | Childhood ear infection | 0.13 ± 0.04, p = 8.0 × 10−4 | 0.06 ± 0.01 |
| Neuroticism | Hypothyroidism | 0.25 ± 0.06, p = 7.2 × 10−5 | 0.03 ± 0.01 |
| OCD | Type 1 diabetes | −0.32 + 0.11, p = 5.4 × 10−3 | 0.98 ± 0.18 |
| SZ | CD | 0.12 ± 0.03, p = 2.0 × 10−4 | 0.31 ± 0.03 |
| SZ | PBC | 0.14 ± 0.05, p = 2.0 × 10−3 | 0.88 ± 0.05 |
| SZ | SLE | 0.15 ± 0.05, p = 1.2 × 10−3 | 0.12 ± 0.02 |
| SZ | UC | 0.14 ± 0.04, p = 2.0 × 10−4 | 0.56 ± 0.03 |
| Tourette's syndrome | Allergy (any) | 0.24 ± 0.06, p = 2.7 × 10−5 | 0.29 ± 0.07 |
- Note. This table displays psychiatric-immune phenotype-pairs showing genome-wide genetic correlation with the linkage disequilibrium score regression (LDSC) method after correction for the total number of genetic correlations depicted in Figure 1 using the Benjamini–Hochberg (BH) method. We also report the genome-wide correlation estimates produced by the heritability estimation from summary statistics (HESS) method. ADHD = attention deficit-hyperactivity disorder; BD = bipolar disorder; CD = Crohn's disease; CRP = C-reactive protein; OCD = obsessive compulsive disorder; PBC = primary biliary cirrhosis; SLE = systemic lupus erythematosus; SZ = schizophrenia; UC = ulcerative colitis.
| Phenotype | Data source | Estimated genome-wide SNP heritability ± error (LDSC/HESS) | GWAS N | QC-Positive SNPS (MHC Excluded) |
|---|---|---|---|---|
| ADHD | Demontis (2017) | 0.24 ± 0.02/0.26 ± 0.02 | 53,293 | 1,004,958 |
| Allergy (any, self-report) | The 23andMe Research Team | 0.08 ± 0.01/0.15 ± 0.01 | 181,000 | 1,060,611 |
| Anorexia nervosa | Duncan et al. (2017b) | 0.26 ± 0.04/0.09 ± 0.04 | 14,477 | 1,054,719 |
| BP | Hou et al. (2016) | 0.20 ± 0.02/0.14 + 0.02 | 40,225 | 1,052,397 |
| Childhood ear infection | The 23andMe Research Team | 0.07 ± 0.01/0.10 ± 0.01 | 122,000 | 1,060,612 |
| Celiac disease | Dubois (2010) | 0.30 ± 0.05/0.13 ± 0.04 | 15,283 | 271,764 |
| Cigarettes (ever-smoked) | Tobacco and Genetics Consortium | 0.07 ± 0.01/0.01 ± 0.02 | 74,035 | 963,355 |
| CD | Liu et al. (2015) | 0.47 ± 0.06/0.33 ± 0.03 | 21,389 | 1,062, 075 |
| CRP | Dehghan (2011) | 0.13 + 0.02/0.11 ± 0.02 | 66,185 | 965,855 |
| Hypothyroidism (self-report) | The 23andMe Research Team | 0.05 + 0.01/0.08 ± 0.01 | 135,000 | 1,060,612 |
| Major depression | PGC Depression Working Group | 0.14 ± 0.03/0.07 ± 0.04 | 18,759 | 967,534 |
| Neuroticism | Social Science Genetics Consortium | 0.09 ± 0.01/0.44 ± 0.01 | 168,105 | 1,053,712 |
| OCD | PGC OCD/TS Working Group | 0.29 ± 0.05/0.09 ± 0.04 | 10,215* | 1,054,746 |
| PBC | Cordell (2015) | 0.37 + 0.06/0.17 ± 0.04 | 13,239 | 940,715 |
| Psoriasis | Tsoi et al. (2015) | 0.82 ± 0.13/0.09 ± 0.04 | 5,116* | 1,037,355 |
| Rheumatoid arthritis | Okada et al. (2014) | 0.14 + 0.02/0.10 ± 0.01 | 58,284 | 1,051,805 |
| SZ | PGC Schizophrenia Working Group | 0.47 ± 0.02/0.62 ± 0.01 | 77,096 | 1,061,529 |
| SLE | Bentham et al. (2015) | 0.27 ± 0.05/0.27 ± 0.03 | 23,210 | 1,056,783 |
| Tourette syndrome | PGC OCD/TS Working Group | 0.35 ± 0.04/0.08 ± 0.05 | 13,341* | 1,041, 689 |
| Tuberculosis susceptibility | Curtis et al. (2015) | 0.18 ± 0.05/0.02 ± 0.05 | 11,936 | 819,917 |
| Type 1 diabetes | Bradfield et al. (2011) | 0.18 ± 0.03/0.15 ± 0.03 | 26,890 | 854,164 |
| UC | Liu et al. (2015) | 0.25 ± 0.03/0.23 ± 0.03 | 27,432 | 1,062,094 |
- Note. This table displays phenotype names, data sources, and estimated SNP heritability using the linkage disequilibrium score regression (LDSC) and heritability estimation from summary statistics (HESS) methods, as well as the GWAS sample size and number of SNPs surviving quality control. Full publication references, consortia names, links to web resources, and additional details on the original studies are provided in Supporting Information Table S1. GWAS N denoted with * indicates the median N for all SNPs. ADHD = attention deficit-hyperactivity disorder; BD = bipolar disorder; CD = Crohn's disease; CRP = C-reactive protein; OCD = obsessive compulsive disorder; PBC = primary biliary cirrhosis; PGC = Psychiatric Genomics Consortium; QC = quality control; SNP = single nucleotide polymorphism; SLE = systemic lupus erythematosus; SZ = schizophrenia; UC = ulcerative colitis.
3.2 Characterization of loci contributing to psychiatric-immune genetic correlations
For psychiatric-immune phenotype-pairs that demonstrated a significant genome-wide correlation with the LDSC method (i.e., those in Table 1), we used the HESS software to examine the genetic correlation within the ~1,694 partitioned genomic loci. The number of correlated loci before and after BH multiple test correction are depicted in Table 3; detailed results for these analyses, including local heritability, correlation strength, and the lists of gene symbols within each loci are provided in Supporting Information Table S3. Only SZ displayed robust local genetic correlations with immune-related phenotypes, including thirty-two loci with CD, 37 loci with PBC, 20 loci with SLE, and 8 with UC (Table 3, depicted in Figure 2). Upon closer examination of the loci implicated between SZ and CD, we noticed that five of these loci contained GW hits, including one locus on chromosome 4q24 (4:100678360–103221356; highlighted green in Figure 2) that contained GW hits for both SZ and CD within the present data, and with four other autoimmune diseases (immunobase.org); these signals are near autoimmunity candidate genes NFKB1 and MANBA, as well as proposed SZ candidate gene SLC39A8, among others contained within the locus (see Supporting Information Table S3). The locus on 10p12.3 (10:18725659–18816236, highlighted green) contains a GW hit for SZ attributed to calcium channel gene CACNB2. Another locus mediating a significantly correlated locus on 12q12 (12:39227169–40816185, highlighted green) contains a GW hit for CD attributed to LRRK2. When examining the loci implicated between SZ and PBC, we observed three harboring GW hits for the former and three harboring signals for the latter, including loci within 3p24.3 (3:16282442–17891118, highlighted orange) containing PLCL2 and within 11q23.3 (11:117747110–119215476, highlighted orange), containing candidate genes CXCR5, DDX6, and TREH. Among the loci implicated between SZ and SLE, we observed two harboring GW hits for the former and three harboring hits for the latter. One such locus within 1q21 (1:148361253–151538881, highlighted yellow) contains a SZ association signal localizing near candidate gene APH1A. Another locus within 1q23 (1:159913048–162346721, highlighted yellow) contains a GW hit for SLE, as well as several other autoimmune diseases, associated with candidate gene FCGR2A. Similarly, a locus within 22q11.21 (22:19912358–22357325, highlighted yellow) containing multi-disease association signal is associated with MAPK1 and UBE2L3. Among the loci implicated between SZ and UC, one within 11q13.1 (11:63804569–65898631) harbored GW hits for multiple autoimmune disorders.
| Phenotype pair | No. of correlated loci (BH p < .05/p < .05) with GWS hits and associated genes contained within correlated loci (BH p < 0.05) |
|---|---|
| ADHD-CRP | 0/7 |
| ADHD-CEA | 0/3 |
| ADHD-psoriasis | 0/5 |
| ADHD-RA | 0/5 |
| ADHD-tuberculosis susceptibility | 0/0 |
| Anorexia nervosa-CRP | 0/0 |
| BD-celiac disease | 0/30 |
| BD -CD | 0/12 |
| BD -psoriasis | 0/3 |
| BD -UC | 0/5 |
| Cigarettes (ever-smoked)-CRP | 0/0 |
| Cigarettes (ever-smoked)-RA | 0/0 |
| Major depression-HPT | 0/0 |
| Neuroticism-CEA | 0/14 |
| Neuroticism-HPT | 0/15 |
| OCD-type 1 diabetes | 0/1 |
| SZ-CD | 32/251 SZ 4:102921704–103198082** (ACTR3BD4, BDH2, CENPE, SLC39A8, SLC9B1, SLC9B2) CD 4:103188709–103198082** (CENPE) CD 8:126529074–126568355 (FAM84B) CD 10:64301873–64588424 (No Genes) CD 12:40337163–40815560 (CNTN1, LRRK2, MUC19, RNU6-713P); CD 21:16790941-16841303 (No Genes) |
| SZ-PBC | 37/256 SZ 1:30427639–30437268 (No Genes) SZ 10:18725659–18816236 (AIFM1P1, CACNB2) PBC 3:16955259–16955259** (PLCL2) PBC 11:118579747–118743772** (ARCN1, CXCR5, DDX6, MIR6716, PHLDB1, RNU6-1157P, RNU6-376P, TREH, TREHP) PBC 22:39670851-39747780 (CACNA1I, ENTHD1) |
| SZ-SLE | 20/200 SZ 1:149999764–150507233 (ANP32E, APH1A, C1orf54, CA14, CIART, MIR6878, MRPS21, OTUD7B, PLEKHO1, PRPF3, RN7SL480P, RNU2-17P, RPRD2, TARS2, VPS45) SZ 2:58377014–58383820 (FANCL, VRK2) SLE 1:161444369–161501904 ** (FCGR2A) SLE 7:128562446–128771234 (CALU, CICP14, FAM71F1, FAM71F2, IMP3P2, RN7SL81P, RNA5SP242, RNA5SP243, RNU6-177P) SLE 8:11332026–11394233 (FAM167A-AS1, RN7SL293P, RNU6-1084P, SLC35G5, TDH) SLE 22:21910280-21999229** (MAPK1, PPM1F, PRAMENP, TOP3B, UBE2L3) |
| SZ-UC | 8/205 UC 11:63804569–65898631** (CCDC88B, RPS6KA4, TRPT1, FLRT1) |
| Tourette's syndrome-allergy | 0/0 |
- Note. This table summarizes findings of local genetic correlation analysis, including the number of significantly correlated loci before and after Benjamini–Hochberg (BH) correction for multiple testing (shown in bold). Loci that showed robust correlations were interrogated for co-localization with significant genome-wide associations (GWS hits, with p < 5 × 10−8). The chromosomal coordinates containing GWS signal are provided, along with associated genes. Proposed candidate genes are highlighted with bold text. ADHD = attention deficit-hyperactivity disorder; BD = bipolar disorder; BH = Benjamini–Hochberg; CD = Crohn's disease; CEA = childhood ear infection; HPT = hypothyroidism; NC-H = comparison; OCD = obsessive compulsive disorder; PBC = primary biliary cirrhosis; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; SZ = schizophrenia; UC = ulcerative colitis.
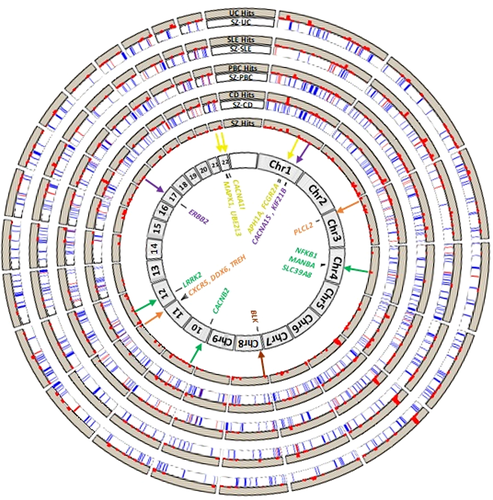
We also sought to examine whether the specific loci might be implicated across multiple psychiatric-immune disorder pairs (Figure 2). An analysis limited to only those surviving BH correction for multiple testing yielded only two loci shared by multiple disease pairs. The first locus (within 3p24.3; 3:21643707–22204244) was identified in correlations of SZ with PBC and with CD; it contained no GWS hits and two genes of unclear consequence ZNF385D and ZNF385D-AS2. The second locus within 8p32.1 (8:11278998–13491775, highlighted brown) was identified in correlations of SZ with PBC and with SLE; this locus contained numerous genes and is adjacent to a GWS hit for SLE associated with candidate gene BLK. When we broadened the scope to examine all loci implicated in nominally significant correlations (uncorrected p < 0.05), we find several that are common to multiple psychiatric-immune disorder pairs (Table 4). The most widely implicated locus was shared among the five pairs of psychotic and inflammatory bowel disorders (within 17q12; 17:36809344–38877404, highlighted purple) and contains a GW hit for BD ascribed to candidate gene ERBB2. There were another eight loci that were implicated in four disorder pairs. Among these, one located within 1q32.1 (1:200137649–201589975, highlighted purple) contains GW hits for multiple autoimmune disorders (including celiac disorder, CD, multiple sclerosis, and UC) and is near candidate genes CACNA1S and KIF21B. The full list of loci implicated across multiple disorder pairs is available in Supporting Information Table S3. The results of the HESS analysis of putative causal directionality (Table 5) indicated that local genetic correlations were stronger in the loci containing GW hits for SZ (rg ≈ 0.41 ± 0.12) as compared with those containing hits for the paired autoimmune diseases (rg ≈ 0.17 ± 0.13).
| Locus | No. of pairs | Phenotype pairs | GWS associations and nearby genes |
|---|---|---|---|
| 17:36809344–38877404 | 5 | BD-CD, BD-UC, SZ-CD, SZ-PBC, SZ-UC | BP 17:37839493–37893484 (ERBB2)/CD 17:37912377–38064876/SLE 17:38007190–38007319/PBC 17:37912377–38080865/UC 17: 37903731–38089717 (RNU6-489P, TBC1D3C, TBC1D3D, TBC1D3K, TBC1D3L) |
| 1:200137649–201589975 | 4 | BD-CD, BD-UC, SZ-PBC, SZ-UC | CD 1:200599616–201069559**/UC 1: 200864267–201024059** (C1orf106, CACNA1S, GPR25, KIF21B) |
| 2:69139564–70755198 | 4 | BD-CD, SZ-CD, SZ-SLE, SZ-UC | None |
| 3:38356116–40221298 | 4 | Neuroticism-HPT, SZ-PBC, SZ-SLE, SZ-UC | None |
| 6:17386405–19207487 | 4 | SZ-CD, SZ-PBC, SZ-SLE, SZ-UC | None |
| 8:11278998–13491775 | 4 | Neuroticism-HPT, SZ-CD, SZ-PBC, SZ-SLE | Neuroticism 8:11281273–11895516/SLE 8:11426026–11546260** (BLK, C8orf49, CTSB, FAM167A, FAM167A-AS1, FDFT1, GATA4, LINC00208, MTMR9, NEIL2, RN7SL293P, RNU6-1084P, SLC35G5, SUB1P1, TDH) |
| 8:9640787–10463197 | 4 | Neuroticism-HPT, SZ-PBC, SZ-SLE, SZ-UC | Neuroticism 8:9793601–10459000 (LINC00599, MIR124-1, MSRA) |
| 11:27020461–28481593 | 4 | SZ-CD, SZ-PBC, SZ-SLE, SZ-UC | None |
| 22:19912358–22357325 | 4 | SZ-CD, SZ-PBC, SZ-SLE, SZ-UC | CD 22:21916166–21985094**/SLE 22:21910280–21999229** (CCDC116, MAPK1, RIMBP3, UBE2L3, YDJC) |
- Note. This table depicts the loci that showed significant (uncorrected p < .05) correlations across multiple pairs of phenotypes. Bold font denotes phenotype-pairs for which the locus survived BH multiple test correction. The ** symbol denotes loci at which multiple autoimmune disorders show an association reaching genome-wide significance (per immunobase.org). Bold font is also used to indicate proposed candidate genes. BD = bipolar disorder; CD = Crohn's disease; GWS = genome-wide significance defined as p < 5 × 10−8; HPT = hypothyroidism; PBC = primary biliary cirrhosis; SLE = systemic lupus erythematosus; UC = ulcerative colitis.
| Phenotype 1, phenotype 2 | Local Genetic correlation ± error at loci reaching GWS only for phenotype 1 | Local genetic correlation ± error at loci reaching GWS only for phenotype 2 | Suggested direction |
|---|---|---|---|
| SZ-CD | 0.37 ± 0.09 | 0.11 ± 0.08 | SZ → CD |
| SZ-PBCs | 0.58 ± 0.18 | 0.26 ± 0.17 | SZ → PBC |
| SZ-SLE | 0.26 ± 0.13 | 0.16 ± 0.16 | SZ → SLE |
| SZ-UC | 0.43 ± 0.09 | 0.16 ± 0.10 | SZ → UC |
- Note. Depicts the results of HESS analysis of putative causal directionality. Within this analysis, local genetic correlations are examined within loci containing GWS associations for each phenotype. The phenotype for which GWS loci produce the larger local correlations suggests that genetic liability for this phenotype may contribute to genetic risk for the other, especially when the correlation error bounds of the second phenotype overlap with zero. When both phenotypes show correlations overlapping with zero, no directionality is supported. CD = Crohn's disease; GWS = genome-wide significance defined as p < 5 × 10−8; PBC = primary biliary cirrhosis; SLE = systemic lupus erythematosus; UC = ulcerative colitis.
4 DISCUSSION
In contrast to previous studies examining large sets of medical, anthropomorphic, metabolic, and behavioral phenotypes (Anttila, 2016; Bulik-Sullivan, Finucane, et al., 2015a; Ohn, 2017; Shi et al., 2017; Zheng et al., 2016), the present study performed a focused comparison of psychiatric and immune-related phenotypes using two methods to estimate genetic correlation from summary statistics. We used updated versions of psychiatric GWASs (Anney, 2017; Arnold, 2017; Demontis, 2017; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011), and compiled a more comprehensive set of immune-related phenotypes, while simultaneously reducing the burden imposed by multiple testing. Additionally, this analysis reflects the first application of the LDSC and HESS method for some of these phenotype-pairs. We identified several genome-wide correlations that were robust to multiple testing. Furthermore, we used the HESS method to validate genome-wide correlations and to conduct a quantitative analysis that localizes correlations to regions of the genome. We prioritized the reporting of findings based on co-localization with GW hits. As such, this study provides a quantitative map of genetic relationships between psychiatric and immune-related disorders and serves, along with previous work (Wang et al., 2015), as a starting point for identifying and characterizing potentially pleiotropic loci.
Prominent among the LDSC genome-wide significant findings was a cluster of modest positive correlations involving BD (rgs ranging 0.25 to 0.33) and SZ (rgs ranging 0.12 to 0.15) in conjunction with immune-related disorders involving the gastrointestinal tract (i.e., CD, PBC, UC). These findings are consistent with available epidemiological evidence indicating that the presence of one set of disorders portends increased risk for a diagnosis from the other class of disorders, though the causality and temporality of these relationships is not clearly established (Benros, 2013; Cucino & Sonnenberg, 2001; Dickerson et al., 2011; Eaton, Pedersen, Nielsen, & Mortensen, 2010; Marrie et al., 2017a, Marrie et al., 2017b; Sidhom et al., 2012). Positive genetic inter-correlations among these phenotypes are also consistent with recent work demonstrating that the positive correlation between BD and SZ are significantly mediated by both CNS and immunologic tissues (Lu et al., 2017). Our local genetic correlation analyses were inadequately powered to detect loci relevant to most of the psychiatric-immune disorder pairs, including BD. However, comparisons with SZ yielded 97 loci that were robust to multiple test correction, 18 of which also were shown to harbor GW hits in previous studies. In several instances, these GW hits localize near genes with functions that are pleiotropic and relevant to both brain and immune system phenotypes. For example, we identified a SZ-CD correlated locus at 4q24 (4:100678360–103221356) that contained GW hits for both SZ (putatively attributed to SLC39A8) and several autoimmune diseases (putatively attributed to NFKB1 and MANBA); others have proposed that associations at this locus may exert pleiotropic effects on a wide range of phenotypes (additionally including body mass index, serum levels of manganese, N-terminal pro-B-type natriuretic peptide, and HDL-cholesterol) through a functional variant found in European populations affecting the SLC39A8 cation transporter (Costas, 2017; Li et al., 2016). A locus within 11q23.3 (11:117747110–119215476) was significantly correlated between SZ and PBC and harbors a region of GW hits for multiple autoimmune disorders attributed to PLCL2, a catalytically inactive phospholipase-like protein thought to influence intracellular signaling, calcium homeostasis, and GABA-ergic receptor trafficking in immune and neuronal cell types, among others (Murakami, Matsuda, Harada, & Hirata, 2017; Takenaka et al., 2003; Toyoda et al., 2015). A de novo missense mutation affecting this gene was identified in an exome sequencing study of SZ affected individuals, though no replication appears to have been reported (Xu et al., 2011). Similarly, a correlated locus within 22q13.1 (22:39307894–40545797, highlighted yellow in Figure 2) contains GW hits for PBC, which overlaps with voltage-gated calcium channel gene CACNA1I; this gene has been implicated by both GWAS and rare-variant studies of SZ (Andrade et al., 2016; Ripke, 2014). Another correlated locus within 11q23 (11:118579747–118743772) contained GW hits for multiple autoimmune disorders and is suspected to exert pleiotropic effects through several genes, whose functions include repression of aberrant interferon signaling (DDX6; Lumb et al., 2017), chemokine signaling between T-helper and B-cells (CXCR5; Papp, Szabó, Szekanecz, & Zeher, 2014; Vaeth et al., 2014), and enzymatic break down of microbial disaccharides (TREH; Muller et al., 2013). Notably, functional genomic studies have identified DDX6 as a gene that is perturbed during neuronal differentiation of samples derived from individuals with schizophrenia (Maschietto et al., 2015), and as a peripheral blood marker of cerebrospinal fluid serotonin metabolite levels (Luykx et al., 2016), supporting its relevance to psychiatric phenotypes.
We also examined loci that showed a nominal genetic correlation across multiple disorder pairs, and found these loci also harbored GW hits for respective phenotypes. The locus at 17q12 shared among multiple disorders contains a GW hit for BD (17:36809344–38877404) ascribed to candidate gene ERBB2 (Hou et al., 2016). This gene and its relatives encode receptor tyrosine kinases that interact with a family of growth factors called neuregulins to regulate the assembly of neural circuitry, myelination, neurotransmission and synaptic plasticity. A large body of evidence implicates both ligands and receptors from these families as susceptibility genes for SZ and BD (Mei & Nave, 2014). Notably, ERBB2 overlaps with GW hits for multiple autoimmune disorders, though these have been attributed to different genes in the region. Another locus at 1q32.1 (1:200137649–201589975) contains GW hits for multiple autoimmune disorders (including celiac disease, CD, multiple sclerosis, and UC) and is near candidate genes C1orf106, CACNA1S, GPR25, and KIF21B. Genetic disruptions of voltage-gated calcium channels, including CACNA1S, are well-established susceptibility factors in psychiatric and neurological disorders (Heyes et al., 2015; Schmunk & Gargus, 2013). KIF21B encodes a neuronal motor protein implicated in GABAA receptor trafficking (Labonte, Thies, & Kneussel, 2014), in addition to having a suspected role in regulating inflammatory signaling in several lymphocyte subtypes (Goris, Boonen, D'hooghe, & Dubois, 2010).
While it is tempting to speculate about these observations, we must acknowledge limitations and caveats of the present approach. Current methods for assessing genetic correlations are not well suited for fine-mapping shared liability across disorders; other methods are better suited for this task, including extensions of GWAS that model multiple phenotypes simultaneously (Cotsapas, 2011; Porter & O'Reilly, 2017; Turley et al., 2018; Wang et al., 2015). With respect to local genetic correlations, we have prioritized reporting of loci that co-localize with GW hits. However, this implies that the presence of the GW hit is contributing to the observed correlation, which we have not demonstrated presently. As such, our discussion of potentially pleiotropic loci and candidate genes should be considered anecdotal at this time. One indirect approach to assessing the role of GW hits in a local genetic correlation might be to re-estimate the local correlation after the removal of the smaller region of GW signal from the original datasets. When we conducted this analysis for the SZ-CD pair, we found that the number of significant loci (BH p < 0.05) was reduced from 32 to 8, suggesting that GW hits likely play an important role in many of the local genetic correlations. Future studies will be able to combine larger GWAS sample sizes with new methods aimed at stratifying genetic correlations by biological annotations (e.g., tissue type or signaling pathways) in order to more precisely define the parts of the genome that mediate a genetic correlation (Lu et al., 2017).
Several methods have now been used to examine quantitative SNP-based genetic relationships between psychiatric and immune-related phenotypes, including restricted maximum likelihood (REML) co-heritability, polygenic risk scores, genetic analysis incorporating pleiotropy and annotations, and other permutation-based methods (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Lee et al., 2013; Pouget et al., 2016; Wang et al., 2015). Different approaches rest on unique assumptions, test different sets of hypotheses, and appear prone to generating sometimes conflicting results. Using several approaches that were not dependent on the directionality of a given SNP's effect, Wang and colleagues concluded that many (24 of 35) pairs of psychiatric and immune-related phenotypes shared a statistically significant proportion of risk-associated loci; among these findings was a significant genetic overlap between BD (as well as SZ) and UC (Wang et al., 2015). However, many of the other relationships identified in that study were not significant in the present study. Another recent study demonstrated that polygenic risk scores reflecting additive risk for several autoimmune diseases can explain a small proportion of variance in SZ case-control status, yet the genome-wide significant SNPs from the autoimmune GWASs were not over-represented among SZ's genome-wide significant hits when permutation-based analysis was performed (Pouget et al., 2016). The apparent disagreement between different approaches for assessing shared genetic liability thus underscores the value of examining the consensus across studies and methods (Pouget et al., 2016).
The LDSC approach featured here attempts to quantitate similarities and differences in association signals across the entire genome. Some of our phenotype-pairs have been examined previously using genome-wide assessment methods, yielding apparently contradictory findings (Anttila, 2016; Bulik-Sullivan, Loh, et al., 2015b; Zheng et al., 2016). For example, a previous study implementing a REML-based approach did not find significant SNP-based co-heritabilities between CD and the major psychiatric phenotypes (Lee et al., 2013). Additionally, the first study implementing the LDSC method found no significant correlation (rg = 0.08 ± 0.08, uncorrected p = 0.33) between BD and UC (Bulik-Sullivan, Finucane, et al., 2015a); this study used a smaller dataset for BD (Sklar et al., 2011; N = 16,731) and a different version of the UC dataset (reported as Jostins et al., 2012; N = 27,432). A similar non-correlation is also reported in LD-Hub (http://ldsc.broadinstitute.org/), using what appears to be the same datasets, although referencing a related article (Liu et al., 2015; N = 27,432). The analyses portrayed in our main text utilized a larger BD dataset (Hou et al., 2016; N = 40,225), the same dataset for UC (Liu et al., 2015; N = 27,432), and uniform criteria for SNP retention based on inclusion in the HapMap3 panel and MAF ≥ 5% within the 1000 Genomes Project Phase 3 European samples. In order to resolve apparent discrepancies, we obtained additional versions of the available data for BD, SZ, CD, and UC and pre-filtered under both inclusive (imputation INFO score ≥ 0.9 or all SNPs, when INFO score unavailable) or exclusive criteria (MAF > 5% within the 1000 Genomes Project Phase 3 European samples). We found that correlations between SZ and each of CD, PBC, and UC tended to be more positive and more significant (i.e., reaching a BH-corrected threshold) when using the SZ data filtered at MAF > 5% (Supporting Information Figure S3). A similar pattern held true for inclusive vs. exclusive pre-filtering for the BD dataset generated by Sklar et al., but this was not the case for the larger Hou et al., dataset. A side-by-side comparison of the effects of different pre-filtering decisions for the BD, SZ, CD, and UC datasets in relation to the other phenotypes is provided in Supporting Information Figure S4. These observations indicate that decisions pertaining to SNP inclusion can have a considerable effect on the result of the LDSC analysis; this idea is further supported by the observation that stratified genetic correlation analyses based on MAF thresholds can produce different levels of statistical significance and opposite patterns of correlation directionality (Lu et al., 2017). Thus, our study suggests that genetic correlations between psychiatric and immune-related disorders may be more significant when analyses are restricted to common variation. Reassuringly, the developers of the HESS method use the same datasets examined presently, and also report positive genetic correlations between SZ and the inflammatory bowel disorders (Shi et al., 2017). The results of the HESS analysis of putative causal directionality indicate that the local genetic correlations are higher in loci occupied by SZ GW hits, as compared to the loci harboring hits for the paired autoimmune disorders (Shi et al., 2017). This pattern is consistent with the hypothesis that genetic liability toward SZ tends to impart a greater genetic risk for the corresponding paired disorder, rather than the opposite directional hypothesis. A related interpretation may be there is an unobserved intermediate phenotype (e.g., a shared biological pathways/mechanism) that is pleiotropic for both measured phenotypes, but more strongly influences the SZ phenotype. This pattern of findings could also be caused by the presence of a confounding factor (e.g., smoking, socioeconomic status) that portends risk for both phenotypes (Shi et al., 2017). Thus, we caution against over-interpretation of these findings. Extensions of Mendelian randomization methods to incorporate two GWAS samples using multi-allelic risk stratifying instruments will be better suited to address these hypotheses (Hartwig, Davies, Hemani, & Davey Smith, 2016), especially as future GWASs provide well-powered genetic estimates of potentially relevant intermediate phenotypes (e.g., brain structure morphometry, circulating immune cell phenotypes, and serum cytokine levels (Ahola-Olli, 2017; Astle et al., 2016; Hibar et al., 2015). Other limitations of the HESS method, including assumptions related to sample overlap and ancestry stratification, are discussed extensively by the method's developers (Shi et al., 2017).
Our study also identified many phenotype-pairs that demonstrated significant genome-wide correlations using the LDSC method, but for which HESS-based genome-wide and local genetic correlations could not be identified. This is unsurprising, given that the sample sizes for these phenotypes were generally below the recommended sample size for HESS analyses (N ≥ 50,000; Shi et al., 2017) Nonetheless, some of these relationships are supported by evidence from clinical and epidemiological studies, and thus may warrant follow-up using larger sample sizes and alternative methods for assessing genetic relationships. For example, we observed a modest positive correlation between self-reported hypothyroidism and major depression (rg = 0.33 ± 0.09, p = 5.0 × 10−4), as well as trait neuroticism (rg = 0.25 ± 0.06, p = 7.2 × 10−5). This could be consistent with two different sets of clinical observations. The first is that symptoms of depression are common in individuals with hypothyroidism, and that subclinical hypothyroidism could play a role in a subset of persons diagnosed with major depression; thus cross-contamination of GWAS samples could lead to a biased positive correlation. However, the second observation is that there is an increased incidence of major depression and depressive symptomatology in persons with autoimmune thyroiditis receiving hormone replacement therapy (Dayan & Panicker, 2013; Giynas Ayhan, Uguz, Askin, & Gonen, 2014). It is worth noting that GWAS data for allergy, asthma, hypothyroidism, childhood ear infection, and Parkinson's disease were obtained through 23andMe, Inc. These data are based on self-report, and thus could be more susceptible to bias stemming from misdiagnosis or misreporting, though previous work supports their validity (Tung et al., 2011). None the less, the samples sizes are an order of magnitude larger than many other datasets, resulting in smaller standardized errors and better power for the detection of weak genetic correlations. It is yet unclear whether small magnitude genetic correlations like these might be clinically meaningful. The LDSC correlations observed presently were relatively weak magnitude (rgs ≈ 0.12 to 0.30) and of modest statistical significance (1 × 10−5 ≤ uncorrected p ≤ 5 × 10−3), when compared to the strongest genetic correlations observed within each group of datasets (e.g., SZ-BD rg = 0.87 with p = 7.4 × 10−94; CD-UC rg = 0.71 with p = 3.5 × 10−36).
Several other significant genetic correlations are supported in the clinical and epidemiological literature. For example, we found a positive correlation between ADHD and rheumatoid arthritis (rg = 0.16 ± 0.05, p = 9.0 × 10−4); this finds support in large registry-based studies indicating an increase in ADHD diagnosis in individuals with autoimmune disease (Nielsen, Benros, & Dalsgaard, 2017), children with mother's affected by autoimmune disease (Nielsen et al., 2017), and children of mothers with rheumatoid arthritis (Instanes et al., 2017). Registry-based studies also provide support for increased incidence of ear infections (rg = 0.20 ± 0.05, p = 2.0 × 10−4) and psoriasis (rg = 0.23 ± 0.07, p = 1.0 × 10−3) among individuals with ADHD (Adesman, 1990; Hegvik, Instanes, Haavik, Klungsøyr, & Engeland, 2017; Nielsen et al., 2017; Silva, Colvin, Hagemann, Stanley, & Bower, 2014). On the other hand, ADHD was positively correlated with CRP (rg = 0.23 ± 0.06, p = 2.0 × 10−4), though a relatively large epidemiological study finds no association in affected individuals (Vogel et al., 2017). The negative correlation between anorexia nervosa and CRP (rg = −0.30 ± 0.08, p = 1.0 × 10−4) is borne out in a recent meta-analysis of relevant studies (Solmi et al., 2015). Another negative correlation between OCD and type 1 diabetes (rg = −0.32 ± 0.11, p = 5.4 × 10−3) finds no support within a limited body of literature (Sivertsen, Petrie, Wilhelmsen-Langeland, & Hysing, 2014). However, the positive correlation between Tourette syndrome and allergy (rg = 0.24 ± 0.06, p = 2.7 × 10−5) is consistent with evidence of increased comorbidity between these phenotypes (Chang, 2011; Yuce et al., 2014). There is a paucity of clinical studies directly assessing the relationship between SZ and PBC (rg = 0.14 ± 0.05, p = 2.0 × 10−3). On the other hand, the correlation between SZ and SLE (rg = 0.15 ± 0.04, p = 2.0 × 10−4) appears to be supported by both epidemiological evidence of increased comorbidity (Tiosano et al., 2016) and the well-documented (although rare) phenomenon of CNS lupus presenting with SZ-like symptoms (Pego-Reigosa & Isenberg, 2008), which may contribute to misdiagnosis. Finally, positive correlations involving cigarette smoking behavior and CRP (rg = 0.31 ± 0.07, p = 3.6 × 10−5), as well as rheumatoid arthritis (rg = 0.17 ± 0.05, p = 2.3 × 10−3), are perhaps unsurprising given considerable evidence of elevated CRP in persons who smoke (Ohsawa et al., 2005), and increased incidence of smoking behavior among individuals diagnosed with rheumatoid arthritis (Di Giuseppe, Discacciati, Orsini, & Wolk, 2014). These findings may indicate a need for more adequate statistical treatment of smoking behavior in GWAS studies.
The present study identified a number of intriguing and previously unreported genetic correlations, some of which appear to localize near established risk factors for complex disease. On the whole, these findings are consistent with the idea that similar signatures of common genetic variation may increase risk for both psychiatric and immune-related disorders. However, it is important to keep in mind that these findings do not necessarily imply causality or even shared genetic etiology. SNP-based genetic correlations could arise from a wide variety of underlying factors, including the possibility that the relationship between phenotypes is mediated by behavioral or cultural factors, or influenced by a heritable but unexamined underlying trait that confers risk to both phenotypes (Anttila, 2016; Bulik-Sullivan, Finucane, et al., 2015a). Other factors that could contribute to genetic correlations include effects mediated by parental genotypes and their influence on parental behaviors that impact the offspring (Coop & Pickrell, 2016). Additionally, GWAS studies of psychiatric phenotypes typically do not screen affected cases on the presence of other medical conditions (and vice versa), thus over-representation of a given phenotype in the sample of another phenotype could bias the data toward the detection of a genetic correlation. Finally, estimates of genetic similarities could be influenced by misdiagnosed cases (Wray, Lee, & Kendler, 2012). Other general limitations of this method (in comparison with other approaches) have been discussed previously elsewhere (Anttila, 2016; Bulik-Sullivan, Finucane, et al., 2015a). In light of the exploratory nature of the present study, another critique pertains to the lack of clearly identified positive and negative control comparisons. Additionally, the clinical significance of weak or modest genetic correlations is yet unclear. Future work could shed light on this topic by comparing the strength of reported genetic correlations with estimates of effect size from epidemiological associations, in order to create an atlas of concordance and shed light on the sensitivity and specificity of these genetic methods. One final critique of this approach is that it falls short of identifying plausible genetic and biological mechanisms that mediate potentially pleiotropic loci. Future work incorporating expression quantitative trait loci, differentially expressed or methylated genes, or enriched ontological and functional terms may provide a clearer context for assessing biological similarities between phenotypes. Despite these limitations, the present study indicates that shared aspects of common genetic variation may underlie long-recognized epidemiological links between psychiatric and immune-related disorders and serves as a start point for the identification and characterization of potentially pleiotropic loci.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of all the individuals (patients, families, research participants, clinicians and diagnosticians, research associates, and data analysts) and consortia whose efforts made possible the GWAS studies and meta-analyses featured in the present study. For most of the phenotypes examined in the present study, clinical and genetic data were collected across numerous sites, each with their own unique patients, staff, and funding sources. While we attempted to provide more thorough recognition of the required acknowledgments for each individual phenotype in our supplementary note, we realize that it is not possible to recognize every individual and funding mechanism that made these studies possible, and we apologize for this. We gratefully acknowledge 23andMe, Inc., its staff, and its customers who consented to participate in research. We also gratefully acknowledge the developers of the LDSC and HESS software. We gratefully acknowledge Susan Service for her assistance preparing and analyzing the data supporting the original association studies of neurocognitive impairment and dementia in HIV-affected adults.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to this study.