Genetics of cognitive control: Implications for Nimh's research domain criteria initiative
Abstract
Cognitive control refers to a set of mental processes that modulate other cognitive and emotional systems in service of goal-directed adaptive behavior. There is growing support for the notion that cognitive control abnormalities are a central component of many of the neuropsychological deficits observed in individuals with mental illnesses, particularly those with psychotic disorders. NIMH's research domain criteria (RDoC) initiative, which is designed to develop biologically informed constructs to better understand psychopathology, designated cognitive control a construct within the cognitive systems domain. Identification of genes that influence cognitive control or its supportive brain systems will improve our understating of the RDoC construct and provide candidate genes for psychotic disorders. We examine evidence for cognitive control deficits in psychosis, determine if these measures could be useful endophenotypes, and explore work linking genetic variation to cognitive control performance. While there is a wealth of evidence to support the notion the cognitive control is a valid endophenotype for psychosis, its genetic underpinning remains ill characterized. However, existing work provides a promising foundation on which future endeavors might build. Confirming existing individual gene associations will go some way to expanding our understanding of the genetics of cognitive control, and by extension, psychotic disorders. Yet, to truly understand the molecular underpinnings of such complex traits, it may be necessary to evaluate genes in tandem, focusing not on single genes but rather on empirically derived gene sets or on functionally defined networks of genes. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Cognitive control refers to a set of mental processes that modulate other cognitive and emotional systems in service of goal-directed adaptive behavior. There is growing support for the notion that cognitive control abnormalities are a central component of many of the neuropsychological deficits observed in individuals with mental illnesses, particularly psychotic disorders, but not exclusively so, since similar abnormalities are also observed in individuals with attention deficit hyperactivity (ADHD) and other disorders. Furthermore, there is growing evidence that cognitive control impairments directly influence functional outcome. Converging behavioral and imaging work has implicated prefrontal cortex and catecholaminergic neuromodulatory systems in cognitive control. These same neural networks are thought to be a locus of dysfunction in psychosis. Psychotic illnesses (primarily schizophrenia, schizo-affective, and psychotic bipolar disorders) are clinically heterogeneous with overlapping genetic risk architectures, common clinical phenomenology and intersecting treatment regimens. These illnesses are among the leading causes of disability worldwide as current clinical definitions, which are poorly aligned with pathophysiology, are insufficient for treatment and prognosis. NIMH's research domain criteria (RDoC) initiative is designed to develop biologically informed constructs of psychosis and other mental illness to better understand pathology and ultimately to improve patient outcomes. Cognitive control was designated a construct of particular importance for the RDoC initiative due to the often profound impairments in this domain among individuals with psychosis. As similar, though less severe, deficits are observed in their unaffected relatives, at least a portion of psychotic cognitive control impairment is associated with genetic predisposition for these diseases. Thus, behavioral and imaging-based cognitive control endophenotypes are ideally suited to aid the functional characterization of putative psychosis risk genes, allowing us to move beyond a genotype–phenotype association to delineating system-level mechanisms of psychosis.
The goals of this review is to examine current evidence for cognitive control deficits in psychosis, to determine if these measures could be useful endophenotypes for the psychotic illnesses, and briefly explore work linking genetic variation to performance on cognitive control tasks. Before focusing on cognitive control, we briefly describe the rationale and implications of the RDoC effort.
THE RESEARCH DOMAIN CRITERIA INITIATIVE
As the etiopathology of major psychiatric disorders remains obscure, and these complex syndromes are extremely distal from their presumed causative risk genes and underlying molecular biological mechanisms, the National Institute of Mental Health sponsored the RDoC initiative designed to bypass the above problems by providing an alternative, non-disease-based structured conceptual framework [Insel et al., 2010; Cuthbert and Insel, 2013]. The RDoC framework consists of a schema for tying together various levels of biological and behavioral measurement, comprising data regarding genes, molecules, cells, neural circuits, physiology, behavior, self-reports, and experimental paradigms around core component processes, including attention, perception, language, and cognitive control. This overall conceptual system, that allows investigators to build from simpler to more complex phenomena, is based on research data focused of these different analytic levels/analysis units across the various functional dimensions/constructs, which in turn may be impaired or involved in a variety of classically defined psychiatric syndromes [Pearlson, 2015].
The RDoC framework consists of five distinct domains reflecting our current understanding of emotion, cognition, motivation, and social behavior (http://www.nimh.nih.gov/research-priorities/rdoc/development-and-definitions-of-the-rdoc-domains-and-constructs.shtml). These domains include negative valence systems, positive valence systems, cognitive systems, systems for social processes, and arousal/regulatory systems. Each domain is comprised of a set of functional constructs characterized in terms of genes, molecules, cells, circuits, physiology, behavior, and self-reports. The cognitive systems domain includes six constructs: attention, perception, declarative memory, language, cognitive control, and working memory (http://www.nimh.nih.gov/research-priorities/rdoc/cognitive-systems-workshop-proceedings.shtml). Although these constructs are heuristically useful, they are not entirely distinct and clearly overlap. For example, working memory plays a role in sustained attention and declarative memory may require some aspect of attention. Arguably, the most overlapping constructs are working memory and cognitive control [Miyake and Shah, 1999; Engle and Kane, 2004]. Specifically, working memory and cognitive control overlap in the subconstructs of updating and maintenance of goal representations (see below). However, while cognitive control is restricted to the maintenance of goal related information, the information maintained in working memory has no such restriction. Nonetheless, there is theoretical debate concerning the degree of separation between of these constructs [Kane and Engle, 2000; Botvinick et al., 2001].
Cognitive Control
Cognitive control is required for one to select, maintain, and update goal representations, monitor his or her performance, and adapt to changes in the environment. It is often defined as a set of mental processes that modulate other cognitive and emotional systems in the service of goal-directed behavior, particularly when prepotent modes of responding are not adequate to meet the demands of the current, often novel, context [Lesh et al., 2011]. Cognitive control involves multiple subcomponent processes often termed executive functions, including vigilance or sustained attention [Pennington and Ozonoff, 1996; Smith and Jonides, 1999]; initiation of complex goal-directed behaviors [Lezak, 1995]; inhibition of prepotent but incorrect responses [Smith and Jonides, 1999; Luna et al., 2010]; flexibility to shift easily between goal states [Ravizza and Carter, 2008]; planning necessary steps to achieve a goal [Smith and Jonides, 1999]; and working memory, the ability to hold information in mind and manipulate it to guide response selection [Goldman-Rakic, 1996]. Miller and Cohen [2001] propose that the prefrontal cortex supports cognitive control by actively maintaining “rules” online in order to evaluate incoming information and internal states to guide response selection toward a current goal. According to this view [Miller and Cohen, 2001], cognitive control mechanisms support the range of executive functions, including working memory, selective attention, stimulus response mapping, and performance monitoring [Shallice, 1988; Moseley et al., 1990; Carter et al., 1998; O'Reilly et al., 1999], and are not restricted to a particular cognitive domain [Smith and Jonides, 1999; Banich et al., 2009].
An inherent aspect of the cognitive control concept is its multifaceted nature. Not surprisingly, there is no quintessential measure of cognitive control performance. Rather, there are a number of different putative cognitive control tasks that each index aspects of cognitive control to varying degrees. When designating task paradigms indexing cognitive control, the RDoC workgroup focused on three subconstructs of cognitive control. The first involved goal selection, updating, representation and maintenance as a singular subconstruct. The second included response selection and inhibition or suppression. Finally, performance monitoring was designated as an independent subconstruct (http://www.nimh.nih.gov/research-priorities/rdoc/cognitive-systems-workshop-proceedings.shtml). This formulation of the cognitive (sub) domains involved in cognitive control is similar, but not identical, to the formulations developed by the RDoC working group on working memory and from the CNTRICS group [Barch and Smith, 2008; Barch et al., 2009a,2009b]. In Table I, we attempt to map commonly used executive function/cognitive control tasks to individual subconstructs. Examining this table, it becomes evident that no task uniquely indexes a single cognitive control subconstruct.
| RDoC working memory | RDoC cognitive control | CNTRICS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | Active maintenance | Flexible updating | Interference control | Goal selection/updating | Goal maintenance | Response selection | Response inhibition | Performance monitoring | Dynamic adjustment | Rule generation/selection |
| AX-CPT | √ | √ | √ | √ | ||||||
| CANTAB ID/ED | √ | √ | √ | √ | √ | √ | √ | |||
| Switching stroop | √ | √ | √ | √ | ||||||
| Response reversal | √ | √ | √ | √ | √ | √ | ||||
| Stop signal task | √ | √ | ||||||||
| Go/no go | √ | √ | ||||||||
| Letter n-Back | √ | √ | √ | √ | ||||||
| Penn conditional exclusion | √ | √ | √ | √ | ||||||
| Dimensional change sort | √ | √ | √ | √ | ||||||
| Flanker (Simon) | √ | √ | ||||||||
| Antisaccade | √ | √ | √ | |||||||
| List sorting | √ | |||||||||
| Pattern processing speed | √ | √ | ||||||||
| Switching fluency | √ | √ | √ | |||||||
| Tower task | √ | √ | √ | √ | √ | |||||
| Digit-symbol substitution | √ | √ | ||||||||
| Letter-number sequencing | √ | √ | ||||||||
| Trail making test | √ | √ | √ | √ | √ | √ | √ | |||
Brain Systems Supporting Cognitive Control
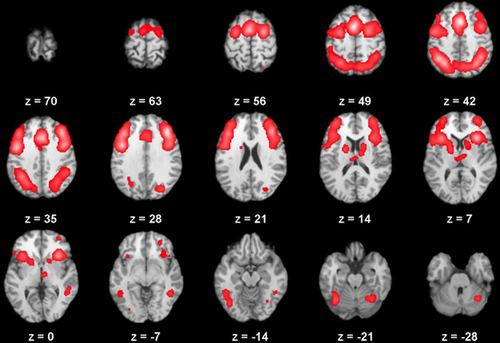
Although putatively distinct executive functions make up cognitive control, a common brain system appears to support this set of behaviors [Duncan and Owen, 2000], including the dorsolateral prefrontal cortex (DLPFC), medial frontal cortex (including the anterior cingulate cortex [ACC]), and parietal regions [Fuster, 1997; Cohen et al., 1999, 2000; Botvinick et al., 2001; Yarkoni et al., 2005; Bellebaum and Daum, 2007; D'Esposito, 2007]. This observation was confirmed in a recent meta analysis of 193 functional neuroimaging studies involving 2,832 healthy individuals performing various executive function measures noted a common pattern of activation in prefrontal, dorsal anterior cingulate, and parietal cortices across domains, supporting the idea that executive functions are supported by a superordinate cognitive control network [Niendam et al., 2012], see Figure 1. Within the cognitive control brain network, the DLPFC is believed to maintain rules for action and response selection [Watanabe, 1990, 1992; Asaad et al., 2000]. In contrast, the ACC is believed to detect response conflict and signal the DLPFC when control-related activity is needed [MacDonald et al., 2000; Egner and Hirsch, 2005; Kerns et al., 2005]. Finally, parietal regions are thought to provide the ability to shift attentional focus and information on learned stimulus–response pairings [Posner and Petersen, 1990; Miller and Cohen, 2001; Bunge et al., 2002, 2003]. Impaired cognitive control could result from insults to any of these regions or dysconnectivity between regions [Cocchi et al., 2013], leading to deficits across a range of putatively distinct executive functions. Recent work examining the interaction between brain regions in the cognitive control network suggest that this functional connectivity is closely related to task performance in healthy adults [Cocchi et al., 2013; Ham et al., 2013], tracks with development across adolescence and early adulthood [Vink et al., 2014], in children with autism [Lesh et al., 2013; Ambrosino et al., 2014], among individuals with substance use disorders [Cisler et al., 2013; Mitchell et al., 2013] or those with schizophrenia [Cole et al., 2011]. Indeed, recently Cole et al. [2013] provided compelling evidence that connectivity dynamics within the cognitive control network facilitates performance on a host of tasks, suggesting a central role for “flexible hubs” in cognitive control and adaptive behavior. Thus, commensurate with the behavioral definition of cognitive control, one ingle brain region supports all aspects of the concept, though it is possible that functional connectivity may be critical for adequate cognitive control processing.
Centrality of Cognitive Control Dysfunction in Psychosis
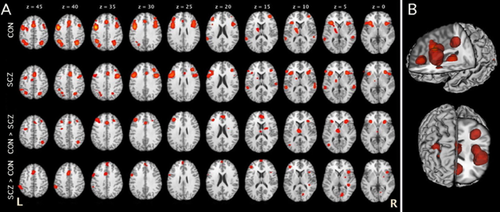
Schizophrenia and psychotic bipolar disorder are associated with neuropsychological deficits across a wide range of cognitive domains, such as working memory, language function, executive function, episodic memory, processing speed, attention, inhibition, and sensory processing [Saykin et al., 1991, 1994; Robinson et al., 2006; Glahn et al., 2007; Arts et al., 2008; Mesholam-Gately et al., 2009; Knowles et al., 2010]. Although it is possible that this diverse array of cognitive impairments reflects multiple dissociable neurophysiological deficits or that individuals with schizophrenia have a generalized deficit across all cognitive domains, a more parsimonious theory suggests that disruption of a single superordinate system that regulates, but is distinct from, other cognitive abilities could result in the pattern of impairments observed in psychosis [Glahn et al., 2006; Minzenberg et al., 2009; Lesh et al., 2011; Barch and Ceaser, 2012]. Indeed, cognitive control impairments appear to be a locus of dysfunction in psychotic illnesses. Specifically, there is growing support for the notion that an inability to actively represent and dynamically adjust goal-related information in order to guide behavior due to dysfunction within the DLPFC and/or its connectivity with other brain regions is core to the pathophysiology of psychotic illnesses [Barch et al., 2001; Lesh et al., 2011; Barch and Ceaser, 2012; Lesh et al., 2013]. Supporting this theory is a substantial task-based fMRI literature associating cognitive control/executive dysfunction with aberrant DLPFC activity in medicated [Holmes et al., 2005] and unmedicated [Barch et al., 2001; MacDonald et al., 2005] psychotic patients relative to healthy controls [Barbalat et al., 2009; Minzenberg et al., 2009]. Indeed, our recent meta-analysis of 41 functional neuroimaging studies in schizophrenia (a prototypical psychotic illness) found reduced activity in DLPFC, rostral/dorsal ACC, thalamus, and inferior/posterior parietal areas among patients across a wide variety of executive tasks [Minzenberg et al., 2009], see Figure 2. Furthermore, there is growing evidence for reduced connectivity between the DLPFC and other cognitive control related brain regions during task performance [Yoon et al., 2008; Sambataro et al., 2013] and rest [Cole et al., 2011; Fornito et al., 2011; Repovs et al., 2011; Meda et al., 2012; Anticevic et al., 2013]. Similarly, gamma synchrony is disrupted when first-episode [Minzenberg et al., 2010] or previously treated [Cho et al., 2006] psychotic patients perform cognitive control tasks. Finally, the magnitude of cognitive control deficits are reduced when psychotic patients are administered agents that modulate the dopamine [Barch and Carter, 2005; McClure et al., 2010] or GABA [Lewis et al., 2008] neurotransmitter systems. These findings suggest that a better understanding of neurobiological roots of cognitive control dysfunction in psychotic illnesses could lead to improved treatment of these disorders, as cognitive impairments dramatically influence patient's quality of life and psychosocial functioning [Green, 1996; Bowie et al., 2010; Nuechterlein et al., 2011].
Cognitive Control As a Psychosis Endophenotype
Although most psychiatric genetic studies exclusively focus on diagnostic categories, this endpoint is relatively distant from the causal neuronal disruptions that lead to the symptoms of mental illnesses [Insel et al., 2010; Glahn et al., 2014]. Endophenotypes provide an index of genetic liability for illness that can be used to characterize the pathways through which genetic variation gives rise to clinical phenomena [Gottesman and Gould, 2003]. Criteria for endophenotypes include the following: (1) heritability; (2) association with the illness (in part due to presumed shared underlying genetic influences); (3) independence from clinical state; (4) impairment that co-segregates with the illness within families; (5) representing reproducible biological measurements; and (6) occurrence in non-affected family members at rates higher than in the population at large [Gershon and Goldin, 1986; Leboyer et al., 1998; Lenox et al., 2002; Gottesman and Gould, 2003]. We have previously suggested that the criteria for an endophenotype can be more precisely reduced to evidence for heritability and evidence for pleiotropy with the illness [Blangero et al., 2003; Glahn et al., 2012; Glahn et al., 2014]. This requirement of pleiotropy implies that endophenotypes are directly comparable to allied phenotypes discussed in other areas of complex disease genetics [Almasy and Blangero, 2001].
Behavioral cognitive control measures are strong candidate endophenotypes for psychotic disorders as they are highly heritable [Ando et al., 2001; Wright et al., 2001; Greenwood and Parasuraman, 2003; Glahn et al., 2010b] and impaired in individuals with psychotic disorders and their non-diseased relatives [Snitz et al., 2006; Glahn et al., 2010b]. Similarly, there is evidence for reduced activation of and connectivity within the cognitive control network in unaffected first-degree relatives of individuals with psychosis [Fusar-Poli et al., 2007; MacDonald et al., 2009; Meda et al., 2012; Khadka et al., 2013; Sambataro et al., 2013], suggesting that disrupted functional neuroimaging indices of cognitive control may be psychosis endophenotypes.
Genetics of Cognitive Control: Initial Findings
Identifying genes the influence cognitive control should provide strong empirically validated candidate genes for psychotic illnesses. While many cognitive control measures have been included in candidate gene studies [Payton, 2009], attempts to replicate these findings typically fail [Houlihan et al., 2009]. For example, a meta analysis of the impact of the catechol-O-methyltransferase (COMT) gene demonstrated that the Val108/158 Met genotype appears to influence n-back performance, with the Val allele associated with better performance [Barnett et al., 2008]. However, there was a high degree of heterogeneity between studies and questions were raised about the methodological soundness of the meta analysis [Wacker, 2011]. A more recent meta analysis examining the impact of the COMT polymorphism on functional activation evoked during working memory/cognitive control (primarily n-back) tasks found no evidence that the Val/Met variant influences brain activation [Nickl-Jockschat et al., 2015]. Together, these meta-analyses provide a cautionary tail for investigators focused on candidate gene or single polymorphism association studies, even for genes that appear to hold a great deal of promise [Egan et al., 2001]. Thus, given concerns about non-replication and a general pessimism about the candidate-gene approach in general [Flint and Mott, 2001; Tabor et al., 2002; Munafo et al., 2005], we do not review these findings here.
A number of investigators have included cognitive control (though this has been referred to as executive function or working memory) tests in genome-wide association studies of common variants (see [Knowles et al., 2014b] for a recent review). For example, Cirulli et al. [2010] examined executive function tests in 1,086 healthy individuals, but did not find a genome-wide significant variant. Similarly, no genome-wide significant variants were identified when examining performance on Cambridge Neuropsychological Test Automated Battery (CANTAB) in 750 healthy individuals [Need et al., 2009]. Papassotiropoulos et al. [2011] localized a common variant, rs10930201, which appears to impact short-term memory performance across four unique samples (samples sizes = 333, 254, 922, and 523, respectively), thought was not genome-wide significant in any single sample. The variant is located within the gene encoding voltage-gated sodium channel type 1a (SCN1A), which encodes the α subunit of the type I voltage-gated sodium channel and was further associated with brain activation within cognitive control regions.
Using genome-wide linkage analysis in a sample of 1,269 Mexican American individuals from extended pedigrees, Knowles et al. [2014a] identified two genome-wide significant loci on chromosome 8 (8q21.11-13 and 8q24.22) for working memory factor score. Post hoc association analysis in the region beneath the linkage peak at 8q21.13 revealed two common variants that were associated with working memory nearby the HEY1 gene [Knowles et al., 2014a]. HEY1 is a transcription target of Notch and makes up part of the hairy and enhancer of split (HES) and Hairy/Enhancer-of-split-related with YRPW-like motif (HEY, also named HERP) gene families [Fischer and Gessler, 2007; Bray and Bernard, 2010].
Using a gene-set enrichment of analysis, Heck et al. [2014] recently identified a link between neuronal excitability and performance on an n-back task. A voltage-gated cation channel activity gene set was significantly associated with working memory performance in a discovery sample and in one of the replication samples. The authors extended this finding to show that alleles from the gene-set correlated with working-memory associated brain activation in brain regions previously shown to be important for working-memory performance.
Taken together, these results suggest that the localization of genetic loci and the eventual identification of genes influencing cognitive control is possible, though this work is still in its infancy.
CONCLUSIONS
Cognitive control, a paradigmatic RDoC construct, delineates linked mental processes modulating other cognitive and emotional systems to subserve goal-directed adaptive behaviors. Although this construct is not captured by any single task, we argue that various measures of cognitive control constitute strong candidate endophenotypes for psychotic disorders because of their demonstrated heritability, impairment across psychosis disorder patients, and their unaffected close relatives, independence of clinical state and reproducibility of measurement. Currently, RDoC-related literature apportions cognitive control into three component processes: (1) goal selection/updating representation and maintenance; (2) response selection/innovation/suppression; and (3) performance monitoring. Each of these component processes is then associated with specific (albeit partially overlapping) neural circuitry, behaviors, self-reports, and test paradigms. To date, few genome-wide significant findings have been reported for cognitive control measures and none have replicated. Furthermore, none of the existent findings can be easily examined in terms of component processes. Although these classifications are based on a large body of literature, the tripartite division is in part conjectural and may change as further evidence emerges. As argued above, the candidate-gene approach as a whole has been problematic, despite the listing of various genes associated with the three cognitive control components on the RDoC web-page (http://www.nimh.nih.gov/research-priorities/rdoc/cognitive-systems-workshop-proceedings.shtml).
We have argued elsewhere that just as complex cognitive domains do not depend on a single underlying brain region, but rather on interactions among more complex neural circuits, it is likely that cognitive control is genetically complex with multiple genes of small effect influencing behavior [Meda et al., 2012; Glahn et al., 2014]. It is possible that large numbers of such genes act collectively in networks [Meda et al., 2012], though currently, no analytic methods for appropriately testing empirically derived gene networks is universally agreed upon. We previously conjectured that such collective interactions are unlikely to be revealed through univariate analyses such as genome-wide association but are more likely to yield to multivariate approaches such as parallel independent component analysis [Meda et al., 2014]. However, such multivariate analytic approaches require replication.
Making progress delineating the genetic influences on cognitive control will likely require several parallel avenues of research. First, to the extent that large-scale common variant association studies will be informative, sample sizes of individuals with directly comparable behavioral measures need to increase to the tens of thousands [Visscher et al., 2012]. Yet, collecting that much data prospectively is costly and difficult and it is unlikely that enough different groups have collected samples with directly comparable cognitive control measures to easily combine data for a meta analysis. Indeed, a large cognitive genomics consortium recently published a GWAS study with over 5,000 samples examining the genetic architecture general cognition (“g” factor typically considered an index of intelligence), but was unable to localize a single genome-wide significant locus [Lencz et al., 2014]. A second paper from the same group with over 20,000 subjects was similarly disappointing [Trampush et al., 2015]. These studies focus on an empirically derived general cognitive factor rather than a more tightly defined cognitive domain, in part, because each of the participating studies combined in the meta analysis used different neuropsychological tests and combining these measures can be difficult [Donohoe et al., 2013; Knowles et al., 2014a]. Family-based studies could require smaller samples for identifying cognitive control genes [Knowles et al., 2014b], as they are often able to study common and rare variation. Finally, gene network or multivariate approaches could provide important insights in to the genetics of cognitive control, thought the caveats described above may limit success.
Overall, current evidence suggests that cognitive control impairments exist in patients with affective and non-affective psychotic illnesses and their relatives. Moreover, impairments constitute useful endophenotypes whose underlying biological and behavioral characteristics are helpfully delineated within the RDoC schema whose genetic and molecular biological underpinnings provide an exciting and important target for future research.




