BDNF Val66Met polymorphism alters sympathovagal balance in healthy subjects†
How to cite this article: Yang AC, Chen T-J, Tsai S-J, Hong C-J, Kuo C-H, Yang C-H, Kao K-P. 2010. BDNF Val66Met Polymorphism Alters Sympathovagal Balance in Healthy Subjects. Am J Med Genet Part B 153B:1024–1030.
Abstract
A common polymorphism of the brain-derived neurotrophic factor (BDNF) gene (Val66Met) has been implicated in anxiety, which is associated with lower vagal activity. We hypothesize that the BDNF Val66Met polymorphism may have a modulatory effect on the cardiac sympathovagal balance. A total of 211 healthy Chinese-Han adults (58 male, 153 female, aged 33.3 ± 10.3 years) were recruited with three BDNF genotypes: Val/Val (47, 22.3%), Val/Met (108, 51.2%), and Met/Met (56, 26.5%). Autonomic function was assessed via an analysis of heart rate variability. Reductions in high-frequency power, an index for parasympathetic activity, and increases in the low-frequency/high-frequency ratio, an index for sympathovagal balance, were found in subjects bearing the Met/Met genotype as compared to the Val/Val group. These results suggest that an altered sympathovagal balance with relatively decreased parasympathetic activity is associated with the Met/Met genotype, suggesting a potential role for the studied BDNF polymorphism in modulating cardiac autonomic functions. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Brain-derived neurotrophic factor (BDNF), a secretory protein in the neurotrophin family, is essential for the survival, development, and maintenance of the neuronal systems [Maisonpierre et al., 1990; Tuszynski and Gage, 1994]. A single-nucleotide polymorphism in the human BDNF gene has been identified in which a valine at amino acid 66 is substituted with a methionine (Val66Met), leading to altered activity-dependent secretion of BDNF protein [Egan et al., 2003]. The BDNF Val66Met polymorphism has been found in studies of both humans and animals to affect anxiety traits and behaviors. An investigation of this polymorphism in human subjects found that the Met allele was associated with increased trait anxiety [Jiang et al., 2005]. In mouse models, transgenic mice with homozygous knock-in Met alleles exhibited increased anxiety-related behaviors that were not normalized by treatment with antidepressants [Chen et al., 2006].
Intriguingly, anxiety is a known risk factor for cardiovascular morbidity and is associated with autonomic dysfunction [Miu et al., 2009]. A body of research has emerged demonstrating that anxiety disorders, particularly panic disorder, generalized anxiety disorder and post-traumatic stress disorder, are associated with reduced vagal modulation or increased sympathetic activity [Friedman and Thayer, 1998; Carney et al., 2000; Dishman et al., 2000; Gorman and Sloan, 2000; Mellman et al., 2004; Bornas et al., 2005; Miu et al., 2009; Shinba et al., 2008; Mujica-Parodi et al., 2009].
Although the specific role of BDNF in the pathophysiology of anxiety remains to be identified, the associations between BDNF and anxiety and between anxiety and autonomic dysfunction suggest that BDNF may play a role in the autonomic system. In fact, several animal studies have suggested that BDNF may not only affect serotonergic neurons, but that it may also have neurotrophic or regulatory effects in neurons related to both the sympathetic and parasympathetic systems [Deng et al., 2000; Slonimsky et al., 2003; Zhou et al., 2004; Kasselman et al., 2006].
The potential modulatory effect of BDNF on neurons in the autonomic system leads to our hypothesis that the BDNF Val66Met polymorphism may affect autonomic function as characterized by cardiac sympathovagal balance. In the present study, we tested this hypothesis by employing an analysis of heart rate variability (HRV), a widely accepted tool for assessing autonomic function, to investigate the association of the BDNF polymorphism with the sympathovagal balance in a sample of healthy adults.
MATERIALS AND METHODS
Subjects
Two hundred thirty-five healthy Han Chinese volunteers were recruited from hospital colleagues at two medical centers: Taipei Veterans General Hospital and Kaohsiung E-DA Hospital, Taiwan. All subjects gave informed consent before commencement of the study. The protocol was approved by the Institutional Review Boards of the Taipei Veterans General Hospital (Taipei, Taiwan) as well as E-DA Hospital (Kaohsiung, Taiwan). Each subject was carefully reviewed for a history of medical disease and psychiatric illness as well as medication use. Subjects included in the study did not have a personal history of medical conditions (e.g., malignancy, heart failure, or diabetes mellitus), pregnancy, psychiatric illnesses or substance abuse/dependence. None of the subjects in this study were taking any medication. DNA samples for all subjects were obtained by drawing blood or by buccal swabs. Of these subjects, 214 were successfully contacted for ambulatory electrocardiogram (ECG) monitoring. Three additional subjects were excluded at this point due to the presence of frequent ectopic heartbeats. The final study sample consisted of 211 healthy subjects (58 males and 153 females, aged 33.3 ± 10.3 years).
Self-Report Mood/Personality Trait Measures
Mood state was assessed by self-report using Zung's depression rating scale [Zung, 1965]. Scores on Zung's depression scale range from 20 through 80, and a score above the cut-off threshold of 49 indicates depressed mood. Three factors derived from Zung's depression scale, namely cognitive, mood and somatic dimensions, were also evaluated for comparisons [Passik et al., 2000]. In addition, we administered the Tridimensional Personality Questionnaire [Cloninger, 1987], which measures three personality dimensions: novelty seeking, harm avoidance, and reward dependence. A validated Chinese version of the Tridimensional Personality Questionnaire was employed in this study [Chen et al., 2002].
Laboratory Methods
Genotyping of the BDNF gene Val66Met polymorphism was performed using the PCR–RFLP method. In brief, the DNA fragments of interest were amplified via PCR with the primers 5′-ACTCTGGAGAGCGTGAAT-3′ and 5′-ATACTGTCACACACGCTC-3′. The Val66Met polymorphism was differentiated with the NlaIII restriction enzyme. Partial digestion was minimized by an internal restriction site and a control sample of digestible homozygous Val/Val.
ECG Monitoring and Analysis of Heart Rate Variability
Holter recordings (MyECG E3-80 Portable Recorder, Microstar, Inc., Taipei, Taiwan) were used to obtain two hours of ECG signals. The E3-80 device continuously recorded three channels of ECG signals at a sampling rate of 250 Hz. The ECG signals were automatically processed and analyzed by open source HRV algorithms [Goldberger et al., 2000]. All ECG monitoring took place in the daytime, and participants were asked to avoid smoking and to stay in a resting state while being monitored.
Time domain measures of HRV include the mean heart rate and standard deviation of the normal interbeat intervals (SDNN), the root mean square successive difference between adjacent normal interbeat intervals (RMSSD), and the percentage of adjacent intervals that varied by greater than 50 msec (pNN50) [Mietus et al., 2002]. The SDNN assesses the overall variability of interbeat intervals. The RMSSD and pNN50 measure the short-term variation of interbeat intervals, which is mainly modulated by parasympathetic innervation [Goldberger et al., 2001].
Conventional spectral HRV measures [Task-Force, 1996] include high-frequency power (0.15–0.40 Hz), low-frequency power (0.04–0.15 Hz), and very-low-frequency power (0.003–0.04 Hz). Low-frequency power is suggested to be modulated by both sympathetic and parasympathetic activities, whereas high-frequency power is mainly modulated by parasympathetic activity [Katona and Jih, 1975; Pomeranz et al., 1985]. The low-frequency/high-frequency ratio was computed as a measure of the sympathovagal balance toward sympathetic activity [Malliani et al., 1994; Task-Force, 1996]. The physiological mechanism underlying very-low-frequency power is disputed, but has been suggested to be mediated partly by the renin–angiotensin–aldosterone system [Akselrod et al., 1981; Task-Force, 1996; Taylor et al., 1998], as well as by the parasympathetic modulation [Taylor et al., 1998; Kleiger et al., 2005].
Statistical Analysis
We calculated allele and genotype frequencies and performed Hardy–Weinberg equilibrium tests for each BDNF genotype. The spectral HRV indices were log transformed to produce normalized distributions. Chi-squared tests were used to compare categorical variables. Differences in continuous variables were compared for individual genotypes using one-way analysis of variance followed by the Bonferroni post hoc test for corrections of multiple between-group comparisons. In order to control the effects of non-genetic factors, a general linear model (GLM) was used with age, gender, and body mass index being entered as variables or covariates. Partial correlation analysis was applied, controlling for age, to determine the associations between HRV indices and self-reported mood scale or personality traits. A P value of less than 0.05 (two-tailed) was required for statistical significance.
RESULTS
Demographic Data
Demographic and clinical data for subjects with the three BDNF genotypes are presented in Table I. The Val66Met genotype distribution (Val/Val: n = 47, 22.3%; Val/Met: n = 108, 51.2%; Met/Met: n = 56, 26.5%) was in Hardy–Weinberg equilibrium. The three BDNF groups did not differ in age, gender ratio, smoking status, body mass index, self-report depression scale, or personality traits. Of note, most volunteers were recruited from hospital colleagues, and the rate of smoking was low (n = 2, 0.9%). It is also notable that seven (3.3%) of the enrolled subjects were classified as having mild depression (Zung's depression scale between 50 and 59). However, they had no clinical depression as evaluated by a psychiatrist during the enrollment phase of the study.
| Val/Val (n = 47) | Val/Met (n = 108) | Met/Met (n = 56) | F or χ2 | P | |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, years (SD) | 32.6 ± 9.9 | 32.6 ± 10.0 | 35.1 ± 10.9 | 1.25 | 0.290 |
| Gender, M/F | 13/34 | 30/78 | 15/41 | 0.02 | 0.990 |
| Current smoker, n | 0 | 2 | 0 | 1.93 | 0.381 |
| Body mass index, kg/m2 | 21.8 ± 3.61 | 22.1 ± 3.8 | 22.0 ± 3.8 | 0.07 | 0.937 |
| Depression scale | |||||
| Zung's Self-Rating Depression Scale | 33.4 ± 11.6 | 31.0 ± 10.4 | 35.1 ± 11.2 | 1.83 | 0.165 |
| Cognitive factor | 17.8 ± 6.7 | 16.3 ± 4.9 | 17.5 ± 6.2 | 0.91 | 0.407 |
| Mood factor | 5.7 ± 3.1 | 5.0 ± 3.1 | 5.8 ± 3.2 | 0.88 | 0.417 |
| Somatic factor | 6.4 ± 2.7 | 6.5 ± 2.8 | 7.3 ± 2.7 | 1.55 | 0.216 |
| Personality dimension | |||||
| Novelty seeking | 15.0 ± 3.9 | 15.8 ± 4.3 | 15.7 ± 3.4 | 0.43 | 0.653 |
| Harm avoidance | 14.5 ± 6.9 | 15.7 ± 6.7 | 15.9 ± 7.7 | 0.35 | 0.703 |
| Reward dependence | 18.4 ± 3.3 | 19.2 ± 3.5 | 19.1 ± 3.7 | 0.48 | 0.620 |
- BDNF, brain-derived neurotrophic factor.
- Data represent mean ± 1 standard deviation unless otherwise noted.
- F ratios from one-way analyses of variance (df = 2,210); χ2 from contingency tables.
Correlations Between Self-Reported Mood/Personality Scale and Heart Rate Variability
A weak but significantly negative correlation existed between harm avoidance, an index of anxiety traits, and HRV indices in the entire study sample (n = 211), including SDNN (r = −0.23, P = 0.008), very-low-frequency power (r = −0.19, P = 0.029), and low-frequency power (r = −0.22, P = 0.012). There were no correlations between HRV indices and reward dependence, an index of social attachment, and novelty seeking, an index of exploration and impulsivity, or the self-reported Zung's depression scale and its sub-factors.
Association of BDNF Genotypes With Heart Rate Variability
The HRV indices for the three BDNF genotypes are presented in Table II. To exclude potential confounding factors of HRV indices, we first tested the association between HRV indices and non-genetic confounders, including age, gender, and body mass index. Pearson's correlation analysis showed that age was significantly correlated with pNN50 (r = −0.21, P = 0.003), very-low-frequency power (r = −0.29, P < 0.001), low-frequency power (r = −0.47, P < 0.001), and high-frequency power (r = −0.34, P < 0.001). The main effect of gender was significant only for the low-frequency/high-frequency ratio (F = 10.71, df = 1, 210, P = 0.008), but there was no significant BDNF-by-gender interaction in any HRV variable. Body mass index had no correlation with any HRV variable. Therefore, only age was entered as a covariate in the GLM with HRV indices as dependent variables.
| Val/Val (n = 47) | Val/Met (n = 108) | Met/Met (n = 56) | F | P | Post hoc | |
|---|---|---|---|---|---|---|
| Time domain | ||||||
| Mean heart rate, beats/min | 80.9 ± 15.5 | 84.8 ± 12.4 | 83.4 ± 10.3 | 1.63 | 0.198 | — |
| Standard deviation of normal interbeat intervals, msec | 81.8 ± 22.9 | 74.7 ± 22.4 | 71.3 ± 21.6 | 2.90 | 0.057 | GG > AA |
| Root mean square successive difference between adjacent normal interbeat intervals, msec | 35.0 ± 13.0 | 30.8 ± 14.7 | 25.1 ± 9.3 | 7.53 | 0.001 | GG ∼ GA > AA |
| Percentage of adjacent normal interbeat intervals that varied by greater than 50 msec, % | 13.6 ± 11.5 | 11.2 ± 12.1 | 5.8 ± 5.9 | 7.50 | 0.001 | GG ∼ GA > AA |
| Frequency domain | ||||||
| Very-low-frequency power, ln(ms2/Hz) | 8.72 ± 0.52 | 8.50 ± 0.53 | 8.43 ± 0.59 | 4.18 | 0.017 | GG ∼ GA > AA |
| Low-frequency power, ln(msec2/Hz) | 7.57 ± 0.62 | 7.35 ± 0.65 | 7.25 ± 0.67 | 3.19 | 0.043 | GG > AA |
| High-frequency power, ln(msec2/Hz) | 6.80 ± 0.74 | 6.48 ± 0.91 | 6.11 ± 0.77 | 8.54 | <0.001 | GG ∼ GA > AA |
| Low-frequency/high-frequency ratio, ln(msec2/Hz) | 2.75 ± 1.36 | 3.15 ± 1.68 | 3.80 ± 1.47 | 6.07 | 0.003 | GG ∼ GA < AA |
- BDNF, brain-derived neurotrophic factor; GG, Val/Val; GA, Val/Met; AA, Met/Met.
- Data represent the unadjusted mean ± 1 standard deviation of heart rate variability variables. Power spectral estimates were log transformed due to skewed distributions. F ratios from one-way analyses of variance (df = 2,210) followed by Bonferroni post hoc comparisons.
Comparisons of HRV indices for each genotype are shown in Table II. Significant between-genotype differences (df = 2, 210) were seen in RMSSD (F = 7.53, P = 0.001), pNN50 (F = 7.50, P = 0.001), very-low-frequency power (F = 4.18, P = 0.017), low-frequency power (F = 3.19, P = 0.043), high-frequency power (F = 8.54, P < 0.001), and the low-frequency/high-frequency ratio (F = 6.07, P = 0.003). The three BDNF groups did not differ in mean heart rate and SDNN.
Scatter plots of spectral measures for each group are shown in Figure 1. An increasing trend was identified in which the Met/Met group had the lowest very-low-frequency power, low-frequency power, and high-frequency power, followed sequentially by the Val/Met group and Val/Val group. Conversely, a descending trend in the low-frequency/high-frequency ratio was observed with decreasing number of Met alleles.
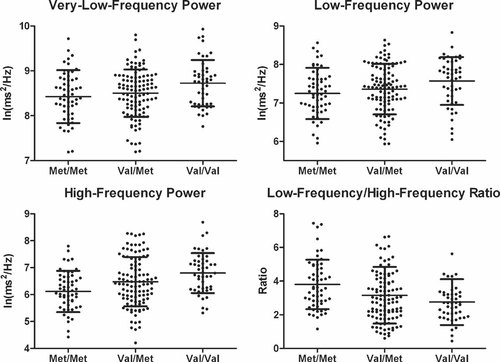
Association of brain-derived neurotrophic factor polymorphism with spectral components of heart rate variability.
Post hoc analyses following by GLM analysis were then performed to assess the differences in HRV indices between the three BDNF genotypes. Compared to the Val/Val group, subjects with the Met/Met genotype had significant reductions in RMSSD (P = 0.001), pNN50 (P = 0.001), very-low-frequency power (P = 0.016), low-frequency power (P = 0.037), and high-frequency power (P < 0.001). Conversely, the low-frequency/high-frequency ratio was significantly increased in the Met/Met group compared to the Val/Val group (P = 0.001). Similarly, compared to the Val/Met group, the Met/Met group showed reductions in RMSSD (P = 0.025), pNN50 (P = 0.008) and high-frequency power (P = 0.028), and increases in low-frequency/high-frequency ratio (P = 0.033). The Val/Val and Val/Met groups differed with borderline significance only in high-frequency power (P = 0.091), but did not differ in the other time and spectral HRV components.
DISCUSSION
The key finding emerging from this study is that subjects bearing the BDNF Met/Met genotype had reductions in RMSSD, pNN50, and high-frequency power and increases in the low-frequency/high-frequency ratio, indicating an altered sympathovagal balance with reduced parasympathetic modulation and possibly increased sympathetic activity. To our knowledge, this is the first study to investigate the role of BDNF genetic variants in human autonomic functions. Our findings support the hypothesis that sympathovagal balance is altered by the BDNF polymorphism. There are several implications of our findings. First, dysregulation of the autonomic system, particularly low parasympathetic (vagal) activity, is associated with the onset and poor prognosis of cardiovascular diseases [Camm et al., 2004; Fei et al., 1996; Tsuji et al., 1996]. Recent evidence suggests that nerve growth factor and BDNF are involved in the development of cardiovascular disease and related disorders [Chaldakov et al., 2004; Liu et al., 2006]. Our finding of reduced vagal activity in the Met/Met genotype may implicate a risk factor for onset of cardiovascular events in the long run. Second, the Val/Met heterozygote group showed an intermediate distribution of high-frequency power and the low-frequency/high-frequency ratio after adjusting confounders (Fig. 1), suggesting the presence of codominant inheritance or a gene–dose relationship. Third, our findings complement conventional approaches of using self-report questionnaires, which often cannot effectively separate one phenotype from another. There is evolving evidence that heart rate is genetically determined [Singh et al., 1999]. Although the exact mode of genetic transmission is unclear, an analysis of HRV nevertheless provides quantitative phenotypic markers of autonomic nervous system function [Singh et al., 1999] to investigate the pathophysiology of complex traits and diseases.
The Role of BDNF in the Autonomic Nervous System
Acetylcholine is an essential neurotransmitter in the parasympathetic system. It has been reported that choline acetyltransferase, the enzyme synthesizing acetylcholine, can be activated by BDNF [Burgess and Aubert, 2006], indicating a potential modulatory role of BDNF in the parasympathetic system. Moreover, several findings have emerged to support the modulatory effect of BDNF in the sympathetic system, including the following: (1) BDNF has been found to modulate the cholinergic properties of sympathetic neurons [Slonimsky et al., 2003], (2) Variation of BDNF synthesis in a mouse model is correlated with synaptic innervations to sympathetic neurons [Causing et al., 1997], and (3) BDNF is suggested to have a potential role in pathophysiology in human autoimmune diseases associated with sympathetic overactivity [Kasselman et al., 2006]. Our findings, focusing on the study of humans, complement the above research by providing evidence of the impact of BDNF polymorphism on autonomic functions.
BDNF, Heart Rate Variability, and Personality Traits
Consistent with other reports [Tsai et al., 2004; Frustaci et al., 2008], the link between BDNF and trait anxiety is inconclusive in the present study as trait anxiety, measured by harm avoidance, did not differ among BDNF groups. Furthermore, our results showed no correlations between trait anxiety and autonomic-related HRV measures (e.g., RMSSD, pNN50, high-frequency power, or low-frequency/high-frequency ratio). Therefore, we are not able to assess the relationship between neuroticism and altered sympathovagal balance in this study sample. However, since low vagal tone is associated with anxiety, and BDNF has already been implicated in both depression and anxiety disorders [Martinowich et al., 2007], we cannot exclude the possibility that the Met/Met genotype with low vagal activity will have a higher incidence of mood/anxiety disorders in the long run.
LIMITATION
There are limitations to the present study. As the study design was cross-sectional, we cannot directly evaluate the long-term impact of the BDNF polymorphism on autonomic function and the incidence of anxiety. Thus, the observational nature of our study does not allow us to draw conclusions on the causality of the link between anxiety and sympathovagal imbalance. In terms of HRV analysis, a debate exists regarding how effective the low-frequency/high-frequency ratio is for separating sympathetic from parasympathetic influences on heart rate [Berntson et al., 1997]. Indeed, our results indicate more significant reductions in high-frequency power than low-frequency power when comparing the Met/Met group to the Val/Val group. Therefore, we cannot exclude a contribution of differences in sympathetic activity to our findings.
CONCLUSIONS
In conclusion, despite the lack of associations between BDNF polymorphism and trait anxiety, subjects bearing the Met/Met genotype exhibited reduced parasympathetic modulation and possibly increased sympathetic activity. A longitudinal investigation of the impact of this BDNF-associated autonomic imbalance on incidence of anxiety disorders and cardiovascular diseases should be conducted in the future.
Acknowledgements
This work was supported by grants V95ER3-003, V96ER3-002, V97ER3-003, and V97C1-132 from Taipei Veterans General Hospital, Taiwan, and National Science Council of Taiwan (NSC 95-2314-B-075-111). The authors wish to thank Zi-Hui Lin, Hui-Chung Chien and Meng-Wei Wang for their excellent technical assistance.




