Linkage analysis of Tourette syndrome in a large utah pedigree†‡
Nicola J. Camp and William M. McMahon contributed equally to this work.
How to Cite this Article: Knight S, Coon H, Johnson M, Leppert MF, Camp NJ, McMahon WM. 2009. Linkage Analysis of Tourette Syndrome in a Large Utah Pedigree. Am J Med Genet Part B 153B:656–662.
Abstract
Tourette syndrome (TS) is a neuropsychiatric disorder characterized by multiple motor and phonic tics. The heritability of TS has been well established, yet there is a lack of consensus in genome-wide linkage studies. The purpose of this study was to conduct a genome-wide linkage analysis on a unique, large, high-risk TS Utah pedigree. We examined a qualitative trait (TS1) where cases had a definitive diagnosis of TS as observed by a clinical interviewer (n = 66) and a quantitative phenotype based on the total Yale global motor and phonic tic severity scores (n = 102). Both parametric and non-parametric multipoint linkage analyses based on MCMC methods were performed using a 10 cM spaced micro-satellite autosomal marker set. Two regions of interest were identified under affecteds-only recessive models; a LOD score of 3.3 on chromosome 1p for Yale tic severity and a LOD score of 3.1 on chromosome 3p for the TS1 phenotype. Twenty-seven individuals shared linked segregating haplotypes for the 1p region. They had significantly higher Yale tic phonic scores than non-sharers (P = 0.01). There were 46 haplotype sharers on chromosome 3p with significantly higher percentage of females among these individuals compared to the non-sharers (P = 0.03). The significant linkage peaks on chromosomes 1p and 3p are in new areas of the genome for TS, and replication of these findings is necessary. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Tourette syndrome (TS) is a neuropsychiatric disorder characterized by multiple motor and phonic tics that wax and wane over a lifetime [Leckman et al., 2001]. The prevalence of TS is estimated to be between 26 and 115 per 10,000 [Scahill, 2004]. Males are more likely to have TS with a ratio as high as 4–1 [Robertson, 2000; Leckman et al., 2001]. The age at onset for motor tics ranges from 2 to 14 with a mean age of 7 years; vocal tics usually occur 2–3 years later [Robertson, 2000; Leckman et al., 2001]. Over half of all TS patients experience remission of symptoms in early adulthood [Robertson, 2000; Leckman et al., 2001].
The heritability of TS has been well established [Baron et al., 1981; Devor, 1984; Price et al., 1985; Pauls and Leckman, 1986; Pauls et al., 1991; McMahon et al., 1992, 1996, 2003; Eapen et al., 1993; Hasstedt et al., 1995; Nestadt et al., 2000]. Early segregation analysis suggested the existence of a single major gene [Baron et al., 1981; Devor, 1984] and an autosomal dominant mode of inheritance was proposed [Pauls and Leckman, 1986; Eapen et al., 1993]. However, reports of bilineal transmission [Kurlan et al., 1994; Hasstedt et al., 1995; McMahon et al., 1996; Hanna et al., 1999] and a lack of consensus from the many linkage studies undertaken to identify major genes suggests that the mode of inheritance is likely more complex.
The lack of consensus in genome-wide linkage studies for TS is characteristic for psychiatric disorders and points to a more complex disease model than a single major gene. However, the fact that large multigenerational pedigrees have arguably been the more successful study design in TS linkage studies [Barr et al., 1999; Merette et al., 2000; Curtis et al., 2004; Verkerk et al., 2006; The Tourette Syndrome Association International Consortium of Genetics (TSAICG), 2007] indicates that rarer, more highly penetrant susceptibility variants may exist for TS. Early linkage studies of large pedigrees were restricted to analyses based on one or two markers at a time due to the lack of software capable of performing multipoint analyses [Barr et al., 1999; Merette et al., 2000; Curtis et al., 2004]. Ignoring results obtained from the affected-pedigree-member method that have been shown to be invalid [Field and Kaplan, 1998], these studies indicated suggestive regions on chromosomes 5p, 19p [Barr et al., 1999] and 5q, 10p, and 13q [Curtis et al., 2004], and a significant region on chromosome 11q [Merette et al., 2000]. However, linkage scores from these analyses were based on only one or two markers, so that it is possible that the results are inflated, calling for caution when interpreting these earlier findings. Two more recent studies including multigenerational pedigrees have performed multipoint analyses using SIMWALK2 [Verkerk et al., 2006; TSAICG, 2007]. A suggestive region on chromosome 3q was found in one study [Verkerk et al., 2006]. Additionally, a suggestive region on chromosome 5p and a significant region on 2p were found in a large collaborative study [TSAICG, 2007]. TSAICG also included 308 sib-pairs; however the significant score on chromosome 2p was primarily driven by the multigenerational pedigrees.
The purpose of this study was to conduct a genome-wide linkage analysis on a unique, large, high-risk TS Utah pedigree containing 260 individuals with 238 having genotype data and 108 reporting TS or chronic tics. A strictly defined dichotomous TS phenotype and a quantitative tic severity score were considered and both parametric and non-parametric multipoint linkage analyses were performed.
MATERIALS AND METHODS
Ascertainment and Diagnosis
Due to the unique structure of the large, high-risk TS pedigree studied and issues concerning identifiability, we cannot illustrate this family with a pedigree drawing. Descriptions of the pedigree have previously been presented elsewhere [McMahon et al., 1992, 2003]. Briefly, this pedigree was originally ascertained through a 10-year-old male proband with TS. The grandfather of the proband is the founder of the current pedigree, and although deceased, was reported to have chronic tics. The pedigree founder married 10 women and had 43 offspring. Across the total four generations descendant from this initial founder, there are over 500 descendents. Review of genealogical records indicate no evidence for consanguinity. Here we have studied descendents from five wives. These five branches are saturated with TS and contain 260 individuals (including the original proband and 36 marry-ins) and a total of 108 with reported TS or tics. Assessment and diagnosis of TS and associated conditions was done by a consistent team of expert clinicians through an interview of the individual and/or their parents. Interviews span the past 25 years, with pedigree members from earlier generations assessed in the 1990s and those in the later generations assessed in the 2000s. TS diagnosis classification was based on criteria of the Diagnostic and Statistical Manual of Mental Disorders, IV and the developed criteria by the Tourette Syndrome Classification Study Group 1993. The Yale Global Tic Severity Scale (YGTSS) and the Shapiro Checklist were captured to measure tic severity [Shapiro et al., 1978; Leckman et al., 1989]. Obsessive–compulsive disorder (OCD) diagnosis and severity were reported.
Phenotype
Our primary analyses consisted of one qualitative and one quantitative phenotype. The qualitative trait (TS1) considered was a strict TS definition that consisted of cases with a definitive diagnosis of TS where tics were observed by the interviewer (n = 66). Our quantitative phenotype considered tic severity based on the total YGTSS global motor and phonic tic severity scores as reported for their worst ever TS severity (n = 102). Secondary analyses consisted of two further qualitative phenotypes of decreasing stringency from our primary trait: TS2 included all cases in TS1 in addition to cases who had a definitive diagnosis of TS by self-report of tics, but where tics were not observed by the clinical interviewer (n = 79); and TS3 additionally considered those with multiple chronic tics without TS (n = 108). This final definition matches that used by the recent TSAICG study 2007.
Genotyping and Marker Characteristics
Genotyping was done at the Centre National de Genotypage in Evry, France following standard DNA amplification protocols. There were a total of 368 autosomal STR genetic markers genotyped that passed performance thresholds and these were at an average spacing of 10 cM. A total of 238 of the 260 individuals in the pedigree were successfully genotyped. Error checking of the genotypes was done using PedCheck [O'Connell and Weeks, 1998] and any genotypes causing misinheritances were zeroed (see Supplementary Table I). Furthermore, there were 41 individuals for whom genotyping was available but for whom a complete TS assessment or diagnosis was not available, leaving 197 individuals with both genotype and phenotype data. All genotype data were used to determine inheritance states for the linkage analysis, but LOD scores were based on sharing of individuals with phenotype of interest.
The second-generation Rutgers' linkage map was used [Matise et al., 2007]. An EM algorithm which takes into account the pedigree structure was used to estimate the allele frequencies [Thomas and Camp, 2006].
Data Analysis
A Markov Chain Monte Carlo (MCMC) software, MCLINK, was used to calculate the multipoint LOD scores for large pedigrees [Thomas et al., 2000].
Both parametric and non-parametric multipoint linkage analyses were performed. For the parametric analyses of the qualitative phenotypes, an “affecteds only” analysis was done using general models. For the recessive model, the disease allele frequency was set at 0.01 with a penetrance function of (0.01, 0.01, 0.90), and for the dominant model the allele frequency was set at 0.005 with a penetrance function of (0.01, 0.90, 0.90). For the quantitative analysis, the penetrance function, which depends on each individual's trait value, was determined by estimating the mean and variance of two normal distributions from the data using an EM algorithm and deriving a ratio of odds between the distributions.
MCLINK calculates a multipoint LOD score, called the TLOD that we report for all parametric analyses. For a TLOD score, the inheritance vectors in the pedigree (which define identical by decent status) are determined based on all markers, but the LOD is estimated at each marker position and maximized over the recombination fraction at each point. The retention of the recombination fraction maximization in the statistic maintains robustness to model misspecification not usually characteristic of other multipoint statistics [Abkevich et al., 2001]. The non-parametric analysis was conducted using the methods of Camp et al. 2001 with the P-values calculated based on simulated null distribution values. The P-values from the NPL analysis were translated to a LOD score scale for ease of comparison between results.
Due to the multiple models, standard LOD threshold values need to be adjusted. This was done using the method proposed by Camp and Farnham 2001 After accounting for the multiple models and two primary phenotypes, LOD scores of 1, 2, and 3, corresponding to 10:1, 100:1, and 1,000:1 odds in favor for the alternative hypothesis, were adjusted to thresholds of 1.6, 2.6, and 3.6, respectively. False discovery rates (FDR) results for linkage peaks are also reported for thresholds of LOD scores ≥3, ≥2, and ≥1.
For each linkage peak with LOD ≥2.6 from the primary analysis, individuals carrying the linked segregating haplotype (haplotype sharers) were determined and characteristics of sharers and non-sharers were compared using Fisher's exact tests and Wilcoxon rank sum tests.
RESULTS
Primary Linkage Analyses
Genome-wide multipoint linkage results for phenotypes TS1 and the YGTSS tic severity quantitative trait are shown in Figure 1. Two regions exceeding 2.6 were identified, indicating odds in favor of the alternative hypothesis greater than 100:1 after adjustment for multiple testing. Table I contains results for all peaks with a LOD score above 1.6 and Table II contains the FDR results.
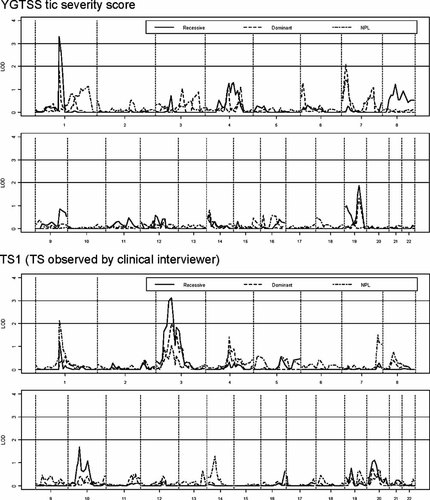
Primary linkage analysis results for TS1 and quantitative trait based on the YGTSS tic severity score.
| Chromosome band | cM position | Marker | Phenotype | Model | LODa |
|---|---|---|---|---|---|
| 1p31-p22 | 112.71 | D1S207 | YGTSS severity | Rec | 3.3 |
| 112.71 | D1S207 | TS1 | NPL | 2.1 | |
| 112.71 | D1S207 | YGTSS severity | Dom | 1.9 | |
| 3p23-p13 | 71.83 | D3S1289 | TS1 | Rec | 3.1 |
| 71.83 | D3S1289 | TS1 | Dom | 2.0 | |
| 7p21 | 21.10 | D7S513 | YGTSS severity | Dom | 2.1 |
| 10p12 | 90.34 | D10S197 | TS1 | Rec | 1.7 |
| 19q13 | 75.28 | D19S902 | YGTSS severity | Rec | 1.9 |
- a LOD ≥1.6 indicates a 10:1 ratio in favor of the alternative hypothesis; LOD ≥2.6 indicates a 100:1 ratio in favor of the alternative hypothesis, corrected for multiple models and phenotypes.
- a Expected indicates the number of markers expected to exceed the LOD score under the null distribution.
- b Corresponding FDR q-value indicating the proportion of the findings exceeding the LOD score that would be expected under the null (i.e., expected/observed).
On chromosome 1p a LOD score of 3.3 (recessive model) was found for the YGTSS tic severity phenotype, with the peak occurring at marker D1S207 (112.71 cM). A lower score was found for precisely the same marker with the dominant model (YGTSS tic severity, LOD = 1.9). The same region was also identified using the NPL analysis with the TS1 phenotype (LOD = 2.1).
A linkage signal was also detected under a recessive model on chromosome 3p for the TS1 phenotype, with the peak LOD score of 3.1 occurring at D3S1289 (71.83 cM). The same marker also resulted in linkage evidence under the dominant model (TS1, LOD = 2.0).
Secondary Linkage Analyses
For the less stringent qualitative phenotypes (TS2 and TS3) no additional peaks were identified (Supplementary Fig. 1), although, on several chromosomes these phenotypes did identify linkage evidence in the same regions as TS1 or YGTSS tic severity analyses. However, in all cases, the findings for TS2 and TS3 were less significant than for TS1 and/or YGTSS tic severity.
Haplotype Sharing: Chromosome 1p Region
For the linkage peak on 1p, we identified the individuals with high YGTSS tic severity score that shared the linked segregating chromosomal segment contributing to the 3.3 recessive linkage score, and identified these as “haplotype sharers.” We used a YGTSS tic severity score cut-off of at least 7 for examining this peak. There were a total of 37 individuals who had scores of at least 7 of these there were 27 sharers and 9 non-sharers under a recessive model and one individual whose sharing (under a recessive model) was undetermined due to missing parental phenotype data. We compared clinical characteristics of sharers and non-sharers. There were no significant differences between the sharers and non-sharers with respect to gender, age at ascertainment, OCD diagnosis, or YGTSS motor tic severity score. We found that the chromosome 1 haplotype sharers, however, did have a significantly higher YGTSS tic phonic score compared to non-sharers (11.2 vs. 8.4; P = 0.01). Sharers and non-sharers had the same rate of echopraxia (22.2%), and not statistically significantly different rates of echolalia (51.9% vs. 33.3%, P = 0.45), and palilalia (37.0% vs. 11.1%; P = 0.22). Most (n = 20, 74.1%) of the chromosome 1 sharers were also haplotype sharers on chromosome 3p.
Haplotype Sharing: Chromosome 3p Region
Of the 66 genotyped individuals with definite TS observed (TS1), seven were not considered for recessive sharing because of the married-in spouses as both of their haplotypes could not be shared with other cases (n = 5) or undeterminable due to missing parental phenotype information (n = 2). Thus, of the remaining 59 individuals, 46 shared linked segregating haplotypes on chromosome 3p, under a recessive model, and 13 were non-sharers. Characteristics were compared. There were no significant difference between the chromosome 3 haplotype sharers and non-sharers with respect to age at ascertainment, YGTSS global tic severity score, and OCD diagnosis. The haplotype sharers had a significantly higher percentage of females (52.2%) compared to the non-sharers (15.4%) (P = 0.03). While not statistically significant due to small sample size, it is interesting to note that sharers compared to non-sharers were more likely to report echopraxia (28.3% vs. 0.0%; P = 0.52), echolalia (47.8% vs. 23.1%; P = 0.20) and palilalia (32.6% vs. 7.7%; P = 0.09). Coprolalia and copropraxia were reported by two sharers and none of the non-sharers.
DISCUSSION
We found two linkage peaks of interest that have not previously been reported in the TS genetics literature. These two peaks correspond to three markers with multipoint LOD scores ≥3 which together have an FDR q-value of 0.08. This indicates that less than one of these markers is expected to be a false discovery, after accounting for multiple testing. The first peak (LOD = 3.3), for quantitative tic severity, was on chromosome 1p31-p22 under a recessive model. The sharers of this peak had higher YGTSS tic phonic scores. The second peak was on chromosome 3p23-p13 (LOD = 3.1) under a recessive model using a stringent phenotype of definite TS as observed by interviewer. The haplotype sharers at this peak were more likely to be female, with more echo-phenomena.
Both linkage peaks were found under a recessive model. Statistically, it is quite clear why such a model might generate superior linkage evidence. A large number of TS individuals share a haplotype for the chromosome 3 and chromosome 1 loci segregating from the pedigree founder whose family reported as having chronic tics. A large number of these also share a second haplotype at these loci from their marry-in parent (many of whom are also affected). This assortative mating and the presence of a segregating founder haplotype contributed to the significance of a recessive model. It is important to note that while it is likely that the underlying model that identifies the linkage peak is closer to the true underlying inheritance model, it is not a true representation of that unknown model. Our suggestion that the model for chromosomes 1 and 3 may be closer to a recessive mode is consistent with Hasstedt et al. 1995 and McMahon et al. 1996 prior segregation analyses of this pedigree which indicated a penetrance level of 28% in heterozygotes compared to a 99% penetrance level for homozygotes. Furthermore, the recessive mode of inheritance applies only to these linkage peaks and given locus heterogeneity it is possible that TS may follow dominant modes of inheritance for other loci as proposed in prior segregation analyses as performed in other populations [Pauls and Leckman, 1986; Eapen et al., 1993].
The 1-LOD support interval for the chromosome 1p peak is approximately from 108.79 to 129.53 cM. However, examining the region identified by the haplotype sharers identifies a smaller region from 112.71 and 129.53 cM. This covers the region from 1p31.1 to 1p21.2 and contains 96 genes. This region has not been examined in previous TS studies. Five signal transducer genes (F3, GNG5, GPR88, PKN2, TGFBR3) reside within this region, but none of these genes have been studied in other neuropsychiatric disorders. The marker, D1S207, under the peak on this chromosome has shown linkage to the autoimmune disease of psoriasis [Veal et al., 2001]. This may be of interest as some recent studies have proposed an autoimmune model for TS, termed pediatric autoimmune neuropsychiatric disorder or PANDAS [Hoekstra et al., 2002]. A recent blind cohort study did find significant exacerbation of tics in relationship to infections [Kurlan et al., 2008] and this is worthy of note as the linkage peak was found using a phenotype related to severity of tics. The sharers under the linkage peak on chromosome 1 had higher phonic tic severity scores. Furthermore, a large percentage of sharers had echolalia (52%) and palilalia (37%). In fact, the percentage with echolalia is larger than previously reported percentage (46%) of clinical patients [Lees et al., 1984]. This evidence may indicate that the linkage peak is associated with verbal aspects of TS.
Our linkage peak on 3p is in the same region as the chromosomal translocation reported in 1990 by Brett et al. although their own subsequent linkage study of this region failed to find significant linkage [Brett et al., 1990, 1996]. Our study represents the first linkage evidence for TS in this region. The 1-LOD support interval at 3p delineated the linkage evidence between 50.35 and 88 cM. Examination of the haplotype sharing in this region further narrowed the linkage to be between 50.35 and 71.83 cM. This is a large region from 3p24.1 to 3p14.3 and containing over 286 known genes. None of these genes have been previously examined in TS. However, a recent genome-wide association analysis of ADHD, a co-morbidity of TS, did find significant association at a marker in this region (rs9845475, P = 3.95E − 6) [Lasky-Su et al., 2008]. There are total of 39 signal transducer genes in this region. Several of these signal transducer genes have been studied in schizophrenia including CCR5 [Rasmussen et al., 2006], GRM3 [Mossner et al., 2008], and TGFBR2 [Numata et al., 2008]. Another of these signal transducer genes, CTNNB1 has been linked to memory [Maguschak and Ressler, 2008]. The signal transducer gene ras homolog gene family, member A (RHOA) is in this region has been shown to be associated with smoking initiation [Chen et al., 2007]. This might be a candidate gene as smoking has been shown to alleviate tic symptoms in a mouse model [Hayslett and Tizabi, 2005] and in a small trial in the use of nicotine chewing gum in TS patients [Orth et al., 2005].
While not statistically significant, sharers of the haplotype on chromosome 3p were more likely to have the complex motor tic of echopraxia (30%). This rate of echopraxia is almost 10% higher than previously reported rates in clinical patients [Lees et al., 1984]. These haplotype sharers may represent distinct subset of TS and is reflective of the use of a stringent phenotypic definition of TS by observation of the clinical interviewer. This phenotype definition has not been used in linkage studies of TS. It was chosen because of the larger number of individuals in the pedigree with tics and the concern over phenocopies or over reporting of tics due to the overall acceptance of tics in the family.
Due to the large overlap of chromosome 1 haplotype sharers with chromosome 3 haplotype sharers, we investigated protein or gene interactions for all the genes found in these two regions using the web tool BioGrid, but found no reported interactions involving genes from these two regions [Breitkreutz et al., 2008].
We have found several linkage peaks in new areas of the genome. The large number of haplotype sharers on chromosome 3 and chromosome 1 strengthens the evidence for linkage under these peaks. However, due to cost constraints we were unable to add additional markers for fine mapping under the linkage peaks and thus we were not able to further narrow the regions identified. These findings were from a single unique pedigree, so it is not surprising that the linkage evidence does not overlap with peaks in other studies. However, subsequent fine mapping to determine good candidate genes within such a pedigree may reveal pathways or mechanisms that will be of interest more generally for TS.
Acknowledgements
We wish to express our deep gratitude to the family members who donated their time, blood and hope to improving our understanding of TS. We would like to thank Dr. Francis Filloux, Dr. Ben van de Wetering, Dr. David Pauls, Dr. Alice Carter, and Jubel Morgan for their help in the clinical assessment of individuals from this pedigree. We like to acknowledge Dr. Christine Bétard and Dr. Diana Zelenika from the Centre National de Génotypage, Evry, France for their contribution of genotyping. The Tourette Syndrome Association provided pilot study funds and addition support for portions of this work. Special thanks go to Ms Sue Pearl of TSA for her encouragement over nearly two decades. We would also like to acknowledge the National Institute for Neurological Disorders and Stroke, the National Library of Medicine, and the National Cancer Institute for grant support of this research—NINDS grant U01NS040024 (David Pauls, PI), the NLM training grant T15 LM07124 (support for Stacey Knight) and NCI grant K07 CA98364 (Nicola Camp, PI). We would also like to thank the University of Utah Center for Clinical and Translational Sciences, which is supported by the Public Health Service research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources.




