The effect of smoking on MAOA promoter methylation in DNA prepared from lymphoblasts and whole blood†
How to Cite this Article: Philibert RA, Beach SRH, Gunter TD, Brody GH, Madan A, Gerrard M. 2009. The Effect of Smoking on MAOA Promoter Methylation in DNA Prepared From Lymphoblasts and Whole Blood. Am J Med Genet Part B 153B:619–628.
Abstract
Prior work using lymphoblast DNA prepared from 192 subjects from the Iowa Adoption Studies (IAS) demonstrated that decreased MAOA promoter methylation was associated with lifetime symptom count for nicotine dependence (ND) and provided suggestive evidence that the amount of methylation is genotype dependent. In the current investigation, we replicate and extend these prior findings in three ways using another 289 IAS subjects and the same methodologies. First, we show that methylation is dependent on current smoking status. Second, we introduce a factor analytic approach to DNA methylation, highlighting three distinct regions of the promoter that may function in somewhat different ways for males and females. Third, we directly compare the methylation signatures in DNA prepared from whole blood and lymphoblasts from a subset of these subjects and provide suggestive evidence favoring the use of lymphoblast DNA. We conclude that smoking reliably decreases MAOA methylation, but exact characterization of effects on level of methylation depend on genotype, smoking history, current smoking status, gender, and region of the promoter-associated CpG Island examined. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Monoamine Oxidase A (MAOA) plays a key role in modulating monoaminergic neurotransmission through its catabolism of dopamine, norepinephrine, epinephrine, serotonin, and related neurotransmitter catabolism byproducts. The MAOA gene is located on Xp11 and consists of 15 exons that are transcribed to 4.1 kb mRNA and translated into a 527 amino acid protein [Chen et al., 1991]. Two regulatory motifs for the gene have been previously described. The first is a 44 bp variable nucleotide repeat (VNTR) that is found approximately 1,200 bp upstream of the transcription start site (TSS) [Hotamisligil and Breakefield, 1991]. The second is a set of two promoter-associated CpG islands that flank either side of the VNTR [Philibert et al., 2008a].
In previous work that used clinical and biomaterial from 192 Iowa Adoption Studies (IAS) subjects, we demonstrated that increased lifetime symptom counts for Alcohol (AD) and nicotine dependence (ND) were associated with decreased MAOA methylation, with the effects being most prominent in women in the region of the gene surrounding the TSS. Furthermore, we provided suggestive evidence that the 3 repeat (3R) allele of the VNTR was associated with increased methylation at this locus. Despite the overall strength of the findings, they raised several questions. First, were these findings simply a type I error due to multiple tests across CpG island loci? Second, given the direct pharmacological effects of nicotine consumption, is decreased methylation associated with current smoking only, or is there an effect of history of smoking as well? Third, are some regions of the promoter more important in characterizing this process? Finally, do lymphoblasts provide better or worse resolution than alternative media, such as whole blood, for the examination of epigenetic effects in substance use research.
These are important concerns because MAOA is hypothesized to play a key role in ND and other complex behavioral illnesses. MAOA inhibitors are used in the treatment of ND as well as other frequently co-morbid syndromes, such as major depression (MD) [George and Weinberger, 2008]. Furthermore, MAOA VNTR gene–environment (GxE) interaction specific to the 3 repeat allele (3R) may be important in the etiology of antisocial conduct [Caspi et al., 2002; Frazzetto et al., 2007]. Finally, our group has recently confirmed earlier findings that a similar GxE effect specific to the 4R allele may moderate vulnerability to MD [Beach et al., in press]. Therefore, the development of a detailed understanding of the molecular underpinnings of genetic and epigenetic effects at this locus may be beneficial to the understanding and treatment of complex behavioral illness.
To help accomplish this goal and more finely hone our understanding of genetic and epigenetic effects at this locus, we recently re-examined our original findings using the insights derived from our prior study and the resources provided by 289 additional participants in the IAS.
METHODS
The study design and clinical measures in the IAS have been described in detail elsewhere [Yates et al., 1996]. The behavioral and demographic data were obtained from subjects participating in the last two waves of the IAS (1997–2003; 2004–2009). In each wave, subjects were interviewed with a version of the Semi-Structured Assessment for the Genetics of Alcoholism, version 2 (SSAGA-II) [Bucholz et al., 1994]. In addition, in the last wave subjects were phlebotomized to provide biomaterial for the preparation of DNA and lymphoblast cell lines. All these procedures were approved by the University of Iowa Institutional Review Board.
The clinical and laboratory methods used in this study are very similar to those used previously and are available in detail upon request. With respect to the behavioral data, symptom counts and categorical diagnoses for ND were derived from SSAGA-II data using criteria from DSM-IV [American Psychiatric Association, 1994]. The highest total symptom count from these two interviews was defined as the lifetime symptom count. Smoking status was also determined using SSAGA data. Lifetime Fagerstrom Test for Nicotine Dependence (FTND) scores were compiled as described using data from the SSAGA [Heatherton et al., 1991]. Those who denied a history of daily smoking at both interviews were classified as “non-smokers.” Those subjects who were daily smokers at the time of the first interview, but had totally quit at time two were classified as “quitters.” Those who smoked daily at the time of both interviews were classified as “continuous smokers.”
DNA from two different cellular sources was used in this study. The lymphoblast (LB) DNA for all 289 subjects was prepared from cell lines using blood contributed by the participants. These cell lines were derived using standard EBV transfection techniques [Klaus, 1987] and the DNA was harvested using the method of Lahiri and Schnabel 1993. For a subset of the female subjects (n = 78), we also analyzed DNA that was prepared from the whole blood sample (WB DNA) drawn at the same time as the specimen used to prepare the cell lines. This DNA was also extracted using the method of Lahiri and Schnabel 1993, and the methylation signatures of both types of DNA were determined at the same time.
Genotyping of the MAOA VNTR polymorphism was conducted as previously described [Philibert et al., 2008b]. Quantitatative methylation determination was performed under contract by Sequenom, Inc. (San Diego, CA) using the same methods previously described [Philibert et al., 1998]. First, aliquots of purified DNA underwent bisulfite modification. The modified DNA samples were then used as a template for the PCR amplification of three contigs covering the MAOA promoter islands using standard touchdown conditions [Philibert et al., 2007]. Amplicon A stretches from BP 43398925 to 43399181, covers CpG residues 1–18, and uses the following primers: F-TAAAGAATGAAAGTATTAGGTTGAGAGTT and R-ATACCCACTCTTAAAAACCAACCCC. Amplicon B stretches from BP 43399430 to 43399858, covers CpG residues 19–45, and uses the following primers: F-GGGTGTTGAATTTTGAGGAGAAG and R-AAACACAACTACCCAAATCCC; Amplicon C stretches from BP 43400453 to 43400805, covers CpG residues 46–74, and uses the following primers: F-GGGGAGTTGATAGAAGGGTTTTTTTTAT and R-TATATCTACCTCCCCCAATCACACC. A fourth contig that covered CpG 75–88 was not used in this study because the residues in this amplicon were neither correlated with methylation in other amplicons nor with substance use in our prior communication [Philibert et al., 2008a].
After amplification, the methylation ratios for each of the CpG residues (methyl CpG/total CpG) in these contigs were then determined by Sequenom using a MassARRAY™ mass spectrometer. These data were then analyzed with proprietary peak picking and spectra interpretation tools to generate the methyl CpG/total CpG ratios [Ehrich et al., 2005, 2007]. The peak for some residues could not be de-convoluted by the spectral interpretation tools. In those cases (CpG 5–7, 8–9, 11–12, 19–20, 30–31, 61–62, 67–68, 72–73), the value for each residue is presented as an average of the aggregated values. In addition, no signal could be reliably observed for CpG residues 24, 26, and 28.
All methylation data were Z-transformed within gender before comparison to genotype or clinical data. All data were analyzed using the JMP (version 7; SAS Institute, Cary, SC) using Pearson's correlation coefficients, regression, analysis of variance (ANOVA), T tests, and ordinal logistic regression (OLR)] as indicated in the text [Fleiss, 1981]. Factor analyses were conducted using SAS Version 9.1 (SAS Institute). For analyses of VNTR genotype data, genotypes that contained uncommon alleles (i.e., 2, 3.5, and 5 repeats) were excluded and the remaining genotype data were analyzed using an additive model. All tests were two-tailed.
RESULTS
The basic behavioral and demographic characteristics of this cohort of 289 IAS subjects are given in Table I. As with the prior cohort, most of the subjects are White and well into adulthood. The male subjects do not differ from the female subjects with respect to age nor ethnicity. Consistent with the study design of the IAS, the sample is enriched for behavioral illness with 100 subjects reporting 3 or more lifetime criteria for ND.
| Male | Female | |
|---|---|---|
| N | 125 | 164 |
| Age (years ± SD) | 41.1 ± 7.7 | 40.9 ± 7.7 |
| Ethnicity | ||
| White | 117 | 155 |
| African American | 3 | 2 |
| White of Hispanic origin | 4 | 4 |
| Other | 1 | 3 |
| No. of symptoms | Males | Females |
|---|---|---|
| DSM IV symptom counts for ND | ||
| 0 | 55 | 84 |
| 1 | 9 | 12 |
| 2 | 8 | 11 |
| 3 | 12 | 9 |
| 4 | 20 | 18 |
| 5 | 9 | 19 |
| 6 | 11 | 9 |
| 7 | 1 | 2 |
The genotype distribution of the subjects is given in Table II. No relationship emerged between the MAOA VNTR genotype and lifetime symptom count for ND for males (P < 0.98, OLR) or females (P < 0.19, OLR).
| Genotypea | Female subjects (n = 164) | Male subjects (n = 125) |
|---|---|---|
| 2, 2 | 0 | 1 |
| 2, 4 | 0 | — |
| 3, 3 | 21 | 43 |
| 3, 3.5 | 1 | — |
| 3, 4 | 64 | — |
| 3, 5 | 1 | — |
| 3.5, 3.5 | 0 | 4 |
| 3.5, 4 | 2 | — |
| 4, 4 | 71 | 76 |
| 4, 5 | 2 | — |
| 5, 5 | 0 | 1 |
| Unknown | 2 | — |
- a Male subjects are hemizygous with respect to this X-chromosome locus.
The untransformed sex averaged methyl CpG/total CpG ratio for each residue is given in Figure 1. The first CpG island contains 18 CpG residues and begins approximately ∼1200 bp before the TSS of MAOA. The VNTR lies between the two CpG islands. The second island consists of 70 CpG residues, the first 56 residues of which were measured in this study. The TSS is located between CpG residues 64 and 65. Overall, males have an average methylation ratio (methyl CpG/total CpG) of 7.2% and females have an average methylation ratio of 34.8%.
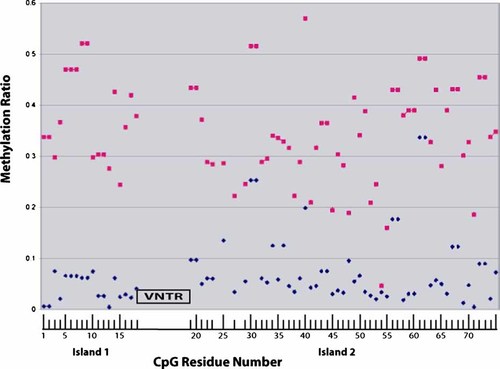
The average methylation (methyl CpG/total CpG) at each CpG residue for each sex. The first island consists of 18 CpG residues while the second larger island consists of 70 residues, of which only the first 56 were analyzed in this study. Tic marks at the positions corresponding to CpG 24, 26, and 28 are missing because average methylation could not be reliably determined at those residues. The average methylation value for females at each residue is depicted by a pink square while the corresponding value for males is depicted by a blue diamond. The overall average methylation value is depicted by the value corresponding at position 75 (34.8% and 7.2% for females and males, respectively). The exact position of the transcription start site is between CpG 65 and 66.
Not surprisingly, because MAOA is an X-chromosome gene that is subject to random X-inactivation [Gartler and Riggs, 1983], females consistently had a higher average methylation ratio at every CpG residue. In addition there was a trend for increasing age to be associated with increasing methylation in females (P < 0.07; ANOVA) but not males (P < 0.30; ANOVA).
Average and Locus Specific Methylation
The relationship between the Z-transformed average methylation ratios across the 74 residues examined and VNTR genotype is shown in Figure 2. The average Z-transformed methylation ratio was greater in DNA from heterozygous females (3R,4R) than in DNA from 4R homozygotes (P < 0.04; T-test). Although the directionality of differential methylation was consistent with prior findings, hemizygous males and homozygous females for the 3R allele did not have significantly higher average amounts of methylation than did their 4R counterparts (P < 0.24 and P < 0.20, respectively).
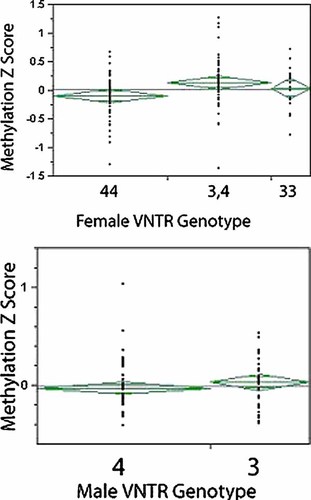
The relationship of MAOA VNTR genotype to methylation in females (above) and males (below). The genotype for each group is displayed on the X-axis while the average methylation Z-score for each individual is given on the Y-axis There was no significant difference between males with the 3R (n = 35; mean Z-score −0.03, non-transformed average methylation (NTWAM) is 7.1%), and those with a 4R allele (n = 61; mean Z-score −0.03, NTWAM is 7.2%). Female 4R homozygotes (Z = −0.101, NTWAM 33.6%) had significantly lower methylation than 3,4 heterozygotes (Z = 0.137, NTWA 36.2%; P < 0.01). The difference between 4R and 3R homozygotes (Z = 0.007, NTWAM, 34.7%) was not statistically different (P < 0.39).
Next, we examined the relationship between global or TSS region specific methylation, which we defined as being the average of Z-transformed values for residues CpG 61–70, and lifetime ND symptom count for all 289 subjects. Although the pattern of relationships was similar to our prior findings, the relationship between global methylation and lifetime ND symptom count was not statistically significant for males (P < 0.19) or females (P < 0.12). There also was not a significant relationship between methylation and lifetime FTND score for males (P < 0.46, OLR) or females (P < 0.52, OLR). However, before correction for multiple comparisons, eight individual CpG residues (CpG 22, 25, 32, 36, 39, 64, 65, and 69), including three in the TSS region, were nominally associated (P < 0.05) with ND symptom for the male subjects but no such relationships emerged in the female subjects. Please see supplemental Table I for the individual P values for each analysis for both sexes.
Because we noted that a substantial number of subjects had quit smoking, yet were still counted as affected using the lifetime symptom count criterion, we next examined current smoking status for 274 subjects whose smoking status could be easily classified. First, for these analyses of current smoking status, those who denied a history of smoking one or more days per week were designated non-smokers (male n = 59 and female n = 83). “Daily smokers” were defined as those who smoked 7 days per week at the times of both the first and second interviews (male n = 42 and female n = 45). Finally, “quitters” were defined as those subjects who smoked daily at the time of the first interview, but denied smoking regular smoking (1 or more days per week) at the time of the second interview (male n = 20 and female n = 27). The 15 subjects excluded from these three groups were removed because either they were never truly daily smokers at both interviews (i.e., did not smoke every day; n = 10), did not fully quit smoking (n = 4), or started smoking after the first interview (n = 1).
Using these definitions of smoking status, we examined the relationship between global and site-specific methylation and current daily smoking status. The distribution of the differential methylation at each residue for male and female “lifetime daily smokers,” “quitters” and non-smokers” is illustrated in Figure 3. The results are most marked for the male subjects. As compared to non-smokers, smokers had lower amounts of methylation globally (P < 0.02; T-test) and at the TSS (P < 0.009; T-test) with seven residues meeting nominal significance level before correction for multiple comparisons. As Figure 3 demonstrates, smoking is associated with a pervasive decrease in methylation across the second larger CpG island with particular consistency in two areas. The first is from CpG 19 to 32. The second is from CpG 55 to CpG 69, a region that includes the TSS. In contrast, the methylation pattern in those male subjects who quit in the 5 years prior to the blood draw is decidedly mixed across both islands, with both elevated and decreased methylation at particular residues. Finally, in those male subjects without a history of daily smoking, the net methylation is pervasively increased across the larger CpG island, but somewhat mixed and perhaps decreased overall in the first CpG island.
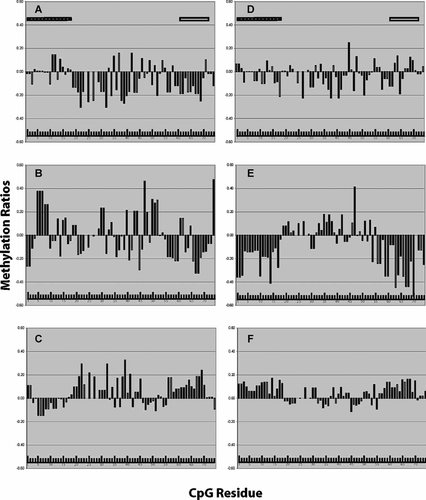
A plot of average methylation Z-score at each residue in LB DNA for each grouping of smoking status that illustrates the effect of the timing of smoking to the methylation signature. CpG residues are in order from left to right. The hatched bar indicates the residues in the first promoter island. The open bar indicates the TSS region. Group A: Current daily male smokers (n = 42). Group B: Males who have quit smoking (n = 20). Group C: Males who have never smoked daily (n = 59). Group D: Current daily female smokers (n = 45). Group E: Females who have quit (n = 27). Group F: Females who have never smoked daily (n = 83).
The methylation pattern in LB DNA from female smokers is similar to that of the male smokers but less intense and consistent. A clear contrast is seen between the amount and pattern of methylation observed in those females who quit smoking as compared to those who never smoked, with a trend for reduced overall methylation (P < 0.08; T-test) and a significant reduction of methylation at the TSS (P < 0.04; T-test) in those who quit.
Factor Analytic Results
To identify separate regions of the CpG island in which values covaried we used the FACTOR procedure in SAS [SAS, 2002] to factor analyze the set of CpG residues for which >95% of both male and female participants had scores. This approach provided a stable three-dimensional factor structure as described below, accounting for 39% of the reliable variance. There were an additional nine factors with Eigen values greater than one which would have been retained by the Kaiser criteria. However, because it has been shown that retaining all factors with Eigen values greater than one substantially overestimates the number of true factors [Lance et al., 2006], we used the Cattell scree plot criterion to eliminate potentially unreliable factors. Accordingly, because there was a clear elbow in the scree plot (8.8, 5.3, 3.9, 2.1, 1.7, 1.6, 1.5, 1.3, 1.2, 1.1, 1.0) we retained only the first three factors for subsequent analysis. We then used a varimax rotation to better identify the three regions of covariation in methylation. The varimax rotation is an orthogonal rotation of the extracted factor axes which make it as easy as possible to identify factors. Biological plausibility suggested the importance of looking for contiguous sets of CpG residues, that is, sets of CpG values that identified a physical region of the CpG island. To the extent that physical regions are identified by the factor analysis, use of factor scores (or the average value across the regions identified in the factor analysis) has the advantage of summarizing the reliable signal across a potentially biologically meaningful region of the CpG island while minimizing the number of separate contrasts required to capture distinct effects that may vary by location.
The three regions identified by the factor analysis were: Factor 1 (CpG 19–45), with factor loadings ranging from 0.42 to 0.80 and an Eigen value of 8.9; Factor 2 (CpG 58–74) with factor loadings ranging from 0.45 to 0.82 and an Eigen value of 5.3; and Factor 3 (CpG 1–18) with factor loadings ranging from 0.34 to 0.78 and an Eigen value of 3.9. Use of average scores across each identified region provided a similar pattern of results as use of factor scores. Factor scores were used in all analyses reported below. However, factor analytic results should be considered preliminary until the factor structure of the CpG island has been replicated in independent samples. In addition, there may be additional meaningful variation in CpG values that is not captured by the first three factors.
Replicating and extending the analyses reported above for genotype, we found that methylation was greater for heterozygous (3R,4R) than for homozygous (4R) females, but the effect was confined to Factor 3 (i.e., CpG 1–18), F(1,137) = 4.50, P < 0.05. The average factor scores for the three groups across CpG 1–18 were (−0.17 vs. 0.23 vs. −0.10) for homozygous 4R, heterozygous 3R,4R and homozygous 3R females, respectively. We also found a significant effect of genotype for males, but in this case the effect was confined to factor 1 (CpG 19–45), F(1,122) = 5.25, P < 0.03. The average factor scores for the two groups across CpG 19–45 were (−0.11 vs. 0.20) for the hemizygous 4R versus 3R males, respectively. For both males and females, the 4R allele was associated with significantly less methylation.
Replicating and extending the analysis of global methylation effects we found a significant association between methylation in the region of CpG 19–45 and days smoking at time 1 (P < 0.002) and time 2 (P < 0.02) for males. A significant association also emerged between days smoking and methylation in the region around the TSS (i.e., Factor 2; CpG 56–74) for males, but only at time 1 (P < 0.02). For females, the only significant association emerged between factor 3 (CpG 1–18) and smoking at time 1 (P < 0.04). For ND symptom count, we found trends for males P < 0.07, for factor 1 (CpG 19–45) and P < 0.1 for factor 2 (CpG 56–74), but no significant associations for females.
We next replicated and extended the analyses contrasting continuous smokers, quitters, and non-smokers. We found significant group differences for males in methylation of factor 1 (CpG 19–45), F(2,117) = 5.46, P < 0.01, and factor 2 (CpG 56–74), F(2,117) = 3.91, P < 0.05. The average factor scores for the three groups across factor 1, that is, CpG 19–45 were (−0.19 vs. −0.25 vs. −0.15) for non-smokers, continuous smokers, and quitters, respectively. Males who never smoked had the highest level of methylation whereas continuous smokers had the least, and quitters were intermediate. For Factor 2 (CpG 56–74), those who never smoked also had the highest methylation, but the quitters had the least. The average factors scores for the three groups were (0.15 vs. −0.15 vs. −0.29) for non-smokers, continuous smokers, and quitters, respectively. For females, only Factor 3 (CpG 1–18) reliably differentiated the groups F(2,150) = 3.04, P = 0.05. Females who never smoked had the highest methylation and those who had quit had the lowest. The average factors scores for the three groups were (0.15 vs. −0.15 vs. −0.41) for non-smokers, continuous smokers, and quitters, respectively.
Comparison of Lymphoblasts to Whole Blood
Finally, because there is considerable controversy in the field as to which source(s) of DNA can or should be used in methylation studies, we next compared the relationship of smoking status to ND in 78 of the female subjects included in the above analyses using DNA prepared from whole blood (WB) or from the lymphoblast line (LB) derived from the same sample of blood. Each set of samples had a similar amount of overall methylation (LB 33.3% vs. WB 34.0%, P < 0.45; T-test). The distribution with respect to VNTR allele status was virtually identical (data not shown). With respect to smoking status, there was a trend for decreased overall methylation in DNA of smokers (n = 24) as compared to that from non-smoking females (n = 38) when the DNA was derived from the lymphoblasts (P < 0.09; T-test). However, there was no difference when the same comparison was performed using DNA prepared from whole blood (P < 0.89; T-test). To gain a better understanding of this, we once again plotted the methylation signatures at each residue for those who wear daily smokers, recently quit, or who had never smoked. Although the same patterns are present in the DNA from both sources, visual inspection of the methylation plots demonstrates greater consistency and intensity of the differential methylation patterns in the DNA derived from lymphoblasts as compared to that from whole blood (Fig. 4).
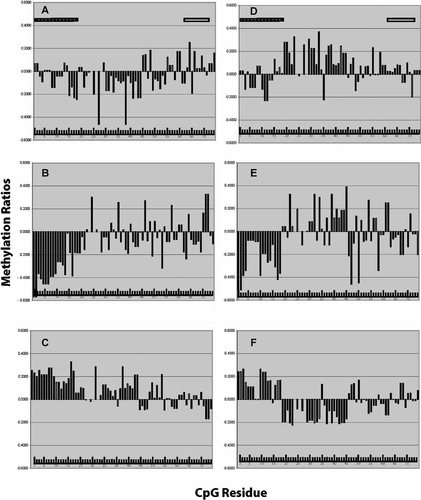
Plot of average methylation Z-score at each residue in LB (A–C) or WB (D–F) DNA from 77 female subjects of function of smoking status. The CpG residues are in order from left to right. The hatched bar indicates the residues from the first promoter island. The open bar indicates the TSS region. Group A and D: Female daily smokers (n = 24). Group B and E: Females who have quit smoking (n = 15). Group C and F: Females who have never smoked daily (n = 38).
To compare the results of the methylation results from the two sources of DNA in a more quantitative manner, we next examined average methylation in the three regions identified in the factor analysis using a three (smoking status) by two (LB vs. WB DNA) ANOVA for each region. As before, only the region identified by Factor 3 (CpG 1–18) reliably differentiated the three smoking status groups F(2,74) = 4.61, P < 0.02. There was no interaction with type of assessment (WB or LB DNA) for this region, suggesting that, given enough observations, a method using either source of DNA would have identified the pattern—even though the spread of the distribution of means was slightly more pronounced for LB than for WB samples (0.21, −0.09, −0.38 vs. 0.15, −0.03, −0.34 for non-smokers, continuous smokers, and quitters, respectively, for LB vs. WB samples). There was, however, a trend toward significance for the interaction of smoking status with assessment method for Factor 1 (CpG 19–45) F(2,74) = 2.45, P < 0.1, suggesting that the two approaches might lead to somewhat different conclusions for that region of the CpG island. In particular, the pattern of means for the three smoking status groups was (0.10, −0.11, −0.03 vs. −0.14, 0.16, 0.11) for non-smokers, continuous smokers, and quitters, respectively, for LB versus WB samples, indicating a reversal of the relative positions of never smokers and quitters in average level of methylation in this region depending on which assessment method was used.
DISCUSSION
In summary, using another sample of subjects from the IAS, we replicated and extended our previous findings to show that a significant portion of the methylation signature status at MAOA is associated with current smoking status, that quitting has an effect on methylation status, and that gender and region of the CpG island examined are also important for accurate specification of associations between smoking and level of methylation. Furthermore, we added to the previous body of evidence suggesting that methylation at this island is dependent on VNTR status. Finally, we examined an important methodological issue by using methylation data on the same subjects using two different sources of DNA, and by examining relationships using a factor analytic approach to reduce the number of dimensions required to describe the methylation results.
The current data provide compelling evidence that the methylation status of the two CpG islands associated with the MAOA promoter is dependent upon smoking status. The real question is “why?” Previous work by others has shown that acute exposure to tobacco smoke decreased human brain MAOA activity [Fowler et al., 1996] and that this decrease in protein activity may be a direct pharmacological/toxicological effect of substances in tobacco smoke [Berlin and Anthenelli, 2001; Fowler et al., 2003]. Since promoter methylation, particularly at the TSS generally decreases mRNA transcription, it seems plausible that the association of decreased methylation with increasing ND symptom count could result from the attempt of the cell to up-regulate MAOA RNA production in the face of increased MAOA protein turnover or inhibition caused by smoking.
Whereas this appears to readily explain the contrast in methylation between current smokers and non-smokers, this rationale does not fully explain the effect of “quitting” on MAOA methylation. Specifically, “quitting” does not appear to lead to a return to methylation levels similar to those who never smoked. Indeed, on most indices the quitters were as different from non-smokers as the continuous smokers, albeit more variable in their methylation profiles. However, for the time being we suggest caution in the interpretation of this portion of our findings. The window of time for “quitting” for these subjects used in this study was rather large and it is highly likely that the subjects differed significantly between one another with respect to total time of smoking abstinence. Therefore, aggregating all “quitters” together in analyses may be insensitive to important heterogeneity in this group. Still, taken at face value, these data suggest that, at a minimum, the process of returning to non-smoking methylation status may be a lengthy one and that the process may be dynamic at the molecular level as well as at the clinical level.
Our finding that female 4R homozygotes have significantly lower methylation than 3R,4R heterozygotes and arithmetically lower methylation than 3R homozygotes is consistent with our prior article in which we showed a trend for the 4R homozygotes to have lower average methylation than 3R homozygotes (40.9% vs. 43.3%; P < 0.10). In unpublished data from that analysis, the average methylation of the 3,4 heterozygotes was only slightly less than that of the 3R homozygotes (42.9%). Hence, when the data is pooled, it is clear that the average methylation of the 4R homozygotes is significantly lower than that of both 3,4 heterozygotes as well as the 3R homozygotes. In addition, this pattern was found for males when we examined factor scores, albeit only for CpG residues in the region from 19 to 45. Unfortunately, we do not have a good explanation for the observation that the “low activity” 3R allele is associated with greater average methylation overall, and in particular, in the region of the first CpG island. Our expectation going into these studies was that the 4R allele would have greater methylation than the 3R allele in order to compensate for the greater amount of gene transcription that has been shown in most, but not all, transfection studies [Sabol et al., 1998; Cirulli and Goldstein, 2007; Guo et al., 2008; Beach et al., in press]. But this is not the case, suggesting that more complex regulatory processes may be at work or that transfections of these MAOA alleles do not fully capture the transcriptional complexity present at this locus.
The finding that LB DNA appears to provide a clearer signal than WB DNA is not unexpected. Lymphoblast cultures are homogenous cell lines that are derived from long-lived peripheral β-lymphocyte populations and are relatively unaffected by acute changes in the health status of the host [Tough and Sprent, 1995; Hao and Rajewsky, 2001]. Others have demonstrated that the epigenetic signature is preserved in lymphoblasts [Monks et al., 2004; Morello et al., 2004]. Our observation of nearly identical amounts of total methylation and allele specific methylation in the WB and LB samples further supports this supposition. In contrast, there are several reasons to believe that the methylation signatures in WB DNA may be more variable. Peripheral white blood cells are a varying mixture of neutrophils, lymphocytes, eosinophils, basophils, and monocytes, each of which probably has a slightly different methylation signature. The composition of this cell mix can change suddenly. In particular, the neutrophil portion of this mixture is subject to marked swings in population secondary to margination of these cells to the blood stream in response to processes such as stress, infection, or drug ingestion (e.g., lithium). Because these processes are associated with changes in neutrophil protein and gene expression signatures [Bussiere et al., 2002; Macdonald et al., 2003], it is likely that as part of these processes, changes in methylation signatures also occur, leading to greater variability in WB than LB DNA. In light of this source of variability in the constituent elements of WB DNA and the likelihood that the various cell types in blood differ slightly in their methylation signatures, it is reasonable to assume that WB DNA may have greater variability in its methylation signature. However, this does not mean it should not be used in these types of studies. Careful review of Figure 4 demonstrates that the same patterns are evident in both sources of DNA. Furthermore, though unlikely, it is possible that some of our observations could be secondary to lymphoblast cell culture artifacts. Therefore, further comparisons of methylation patterns at other loci are needed before any definitive conclusions can be made.
The apparent differences in the methylation profiles with respect to smoking status are intriguing. Although we initially analyzed only overall and TSS specific methylation, one advantage of using factor analytic scores is that they provide a potentially useful way of defining and then summarizing methylation for all regions of the CpG island, allowing better specification of possible differences between groups and between genders. For example, average factor analytic scores for males show an orderly transition from decreased to increased methylation as a function of smoking status that is most apparent for Factor 1 comprising the region from CpG 19 to 45. For females, non-smokers also demonstrate the highest methylation, but this is most evident on Factor 3 comprising the region from CpG 1 to 18. Both male and female quitters demonstrated lower levels of methylation than did non-smokers on Factor 2 (i.e., the region containing the TSS) with continuous smokers being intermediate (−0.29 vs. −0.14 vs. 0.14 for males; −0.26, −0.02, 0.13 for females), suggesting that effects of smoking status at the TSS may be more similar than different for males and females, and that quitting smoking may be associated with lowered methylation for both.
Because it is clear that smoking status has a prominent effect on the MAOA methylation signature, we have elected not to analyze the current methylation data with respect to AD symptom counts or consumption until we have a firmer understanding of the effects of cigarette consumption and time of smoking cessation on methylation at this locus. As in many high-risk populations, AD and ND are frequently co-morbid in the IAS. Fortunately, however, in our IAS pool of 560 subjects, there are a number of subjects who smoke, but do not drink and vice versa. This may allow us to dissect out the relative contributions of drinking and smoking to methylation at this locus. However, it may be that there are a number of other co-morbid factors that also need to be considered such as cannabis smoking. Hence, whereas the current findings are very stimulating, they should be viewed only as a first approximation to our understanding of the relationship between smoking and methylation. In order to generate a better understanding of MAOA methylation, it is apparent that we will need to develop fairly sophisticated methods for incorporating temporally specific consumption data for tobacco and other substances, as well as possibly co-morbid medical and treatment information, into our analyses.
In summary, in this communication we refine and extend our previous findings and suggest that further examination of the relationship of smoking and VNTR genotype to MAOA methylation is in order. The factor analytic results also suggest that there is good reason to continue to focus attention on the region containing the TSS, but careful specification of earlier portions of this CpG rich motif may help illuminate gender differences and provide a more accurate picture of the overall association of smoking to methylation.
Acknowledgements
This work was generously supported by grants to Dr. Philibert (DA015789) and to Dr. Gene Brody and Dr. Steven Beach (DA010923 and DA02173603). The authors wish to express their continuing gratitude to the late Dr. Remi Cadoret, founder of the Iowa Adoption Studies, whose pioneering efforts led to these and other studies of gene–environment interactions in behavioral illness. The University of Iowa has filed intellectual property claims on material described in this manuscript on behalf of Dr. Philibert and Dr. Madan.




