No association between tagging SNPs of SNARE complex genes (STX1A, VAMP2 and SNAP25) and schizophrenia in a Japanese population†‡
Please cite this article as follows: Kawashima K, Kishi T, Ikeda M, Kitajima T, Yamanouchi Y, Kinoshita Y, Takahashi N, Saito S, Ohi K, Yasuda Y, Hashimoto R, Takeda M, Inada T, Ozaki N, Iwata N. 2008. No Association Between Tagging SNPs of SNARE Complex Genes (STX1A, VAMP2 and SNAP25) and Schizophrenia in a Japanese Population. Am J Med Genet Part B 147B:1327–1331.
Kunihiro Kawashima and Taro Kishi contributed equally to this work.
Abstract
Abnormalities in neural connections and the neurotransmitter system appear to be involved in the pathophysiology of schizophrenia. The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, which consists of Syntaxin1A, vesicle-associated membrane protein 2 (VAMP2) and synaptosomal-associated protein 25 kDa (SNAP25), plays an important role in the neurotransmitter system, and is therefore an attractive place to search for candidate genes for schizophrenia. We conducted a two-stage genetic association analysis of Syntaxin1A (STX1A), VAMP2 and SNAP25 genes with schizophrenia (first-set screening samples: 377 cases and 377 controls, second-set confirmation samples: 657 cases and 527 controls). Based on the linkage disequilibrium, 40 SNPs (STX1A, 8 SNPs; VAMP2, 3 SNPs; SNAP25, 29 SNPs) were selected as ‘tagging SNPs’. Only nominally significant associations of an SNP (rs12626080) and haplotype (rs363014 and rs12626080) in SNAP25 were detected in the first-set screening scan. To validate this significance, we carried out a replication analysis of these SNP and haplotype associations in second-set samples with a denser set of markers (including five additional SNPs). However, these associations could not be confirmed in the second-set analysis. These results suggest that the SNARE complex-related genes do not play a major role in susceptibility to schizophrenia in the Japanese population. © 2008 Wiley-Liss, Inc.
There is growing evidence that the presynapse is involved with the pathophysiology of schizophrenia. Within the presynaptic area, neurotransmitters are released by synaptic vesicle exocytosis, and the regulation of this release is critical for neural function. The machinery for this release consists of several groups of proteins that work together as a functional unit, the soluble N-ethylmaleimide sensitive factor attachments receptor (SNARE) complex [Montecucco et al., 2005].
The SNARE complex consists of Syntaxin1A, vesicle-associated membrane protein 2 (VAMP2) and synaptosomal-associated protein 25 kDa (SNAP25) [Marz and Hanson, 2002], and it has been reported that alterations in the components in the SNARE complex may underlie the pathophysiology of schizophrenia. First, postmortem studies measuring the level of SNARE complex protein or its mRNA revealed specific brain region alternations in schizophrenia [Gabriel et al., 1997; Thompson et al., 1998; Young et al., 1998; Karson et al., 1999; Sokolov et al., 2000; Hemby et al., 2002; Honer et al., 2002; Halim et al., 2003; Thompson et al., 2003]. Second, genetic association studies showed a significant association between SNPs in the Syntaxin1A gene (STX1A) and schizophrenic patients from Portugal and Toronto [Wong et al., 2004]. In addition, a very recent report showed that SNPs in SNAP25 were associated with schizophrenia in Irish high-density families [Fanous et al., 2007].
In this study, we investigated whether genetic polymorphisms within STX1A (7p11.23: OMIM *186590), VAMP2 (17p13.1: OMIM *185881) and SNAP25 (20p12-p11.2: OMIM *600322) were associated with schizophrenia in a Japanese population.
A first-set screening analysis was conducted with 377 schizophrenic patients (196 males and 181 females; mean age ± standard deviation (SD) 42.4 ± 14.8 years) and 377 healthy controls (212 males and 172 females; 35.9 ± 14.7 years). In a confirmation analysis a different panel of samples was used, consisting of 657 patients with schizophrenia (350 male and 307 female; 50.1 ± 14.4 years) and 527 controls (303 male and 224 female; 40.8 ± 15.3 years).
The patients were diagnosed according to DSM-IV criteria with the consensus of at least two experienced psychiatrists on the basis of unstructured interviews and a review of medical records. All healthy control subjects were also psychiatrically screened based on unstructured interviews. None of the subjects was known to be related to each other, and all were ethnically Japanese.
Written informed consent was obtained from each subject. This study was approved by the ethics committees at Fujita Health University, Nagoya University Graduate School of Medicine, Osaka University Graduate School of Medicine and Teikyo University School of Medicine.
After consulting the HapMap database (release#16.c.1, June 2005, www.hapmap.org, population: Japanese Tokyo: minor allele frequencies (MAFs) of more than 0.05 for STX1A and VAMP2, and 0.1 for SNAP25), 39 SNPs (STX1A, 7 SNPs; VAMP2, 3 SNPs; SNAP25, 29 SNPs) were selected as ‘tagging SNPs’ based on the criterion of an r2 threshold greater than 0.8 in ‘pair-wise tagging only’ mode using the ‘Tagger’ program (Paul de Bakker, http://www/broad.mit.edu/mpg/tagger). For STX1A, since a previous report showed the positive association of an SNP in intron 7 [Wong et al., 2004], we included this SNP with the aforementioned ‘tagging SNPs’ for the association analysis. Overall, 40 SNPs were examined in this study (Supplementary Figures 1–3).
For denser mapping in the confirmation analysis, we added five SNPs around nominally significant SNPs or haplotypes detected in the first-set screening scan (rs610457, rs363013, rs363015, rs6039792 and rs363050).
For genotyping of these SNPs, a TaqMan assay (Applied Biosystems, CA), PCR-RFLP assay, and direct sequencing techniques were used. Detailed information is available in Supplementary Table 1. Genotype deviation from the Hardy–Weinberg equilibrium (HWE) was evaluated by chi-square test (SAS/Genetics, release 8.2, SAS Japan Inc., Tokyo, Japan). Marker-trait association was evaluated by a likelihood ratio test (allele-wise and haplotype-wise analyses) and χ2-test (genotype-wise analysis). For exhaustive screening, we tested all one-marker (by conventional allele-wise analysis), two-marker, and three-marker haplotypes (and seven-marker haplotypes for second-set confirmation analysis) using the COCAPHASE 2.403 program [Dudbridge, 2003].
The power and sample size calculations were performed with a statistical program (http://biostat.mc.vanderbilt.edu/twiki/bin/view/main/powersamplesize). This significance threshold for all statistical tests was 0.05.
All genotype frequencies of each group were in HWE (data not shown). The LD structures examined in our control samples were almost the same as the one shown in HapMap database (Supplementary Figure 1–3).
The SNP (rs12626080: SNAP25-M8: P = 0.0236, uncorrected) in SNAP25 and the haplotype constructed by M7 (rs363014) and M8 in SNAP25 showed a nominally significant association with schizophrenia in the first-set screening samples (global P = 0.0215, uncorrected), although no association was detected with any tagging SNP in STX1A and VAMP2, including the SNP reported to be associated with schizophrenia in Caucasian samples [Wong et al., 2003] (Tables I and II and Supplementary Table 2).
| Genes | Marker IDs | Distance to next SNP (bp) | Na | MAFb | P-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCZ | CON | SCZ | CON | Genotype | (1) Windowc | (2) Window | (3) Window | ||||
| STX1A (minus strand) | SNP1 | rs867500 | 0 | 375 | 375 | 0.204 | 0.217 | 0.754 | 0.527 | ||
| SNP2 | Intron7 SNP | 3592 | 373 | 372 | 0.260 | 0.261 | 0.767 | 0.978 | 0.947 | ||
| SNP3 | rs4363087 | 164 | 373 | 370 | 0.326 | 0.311 | 0.424 | 0.537 | 0.859 | 0.946 | |
| SNP4 | rs3793243 | 3151 | 375 | 377 | 0.419 | 0.386 | 0.304 | 0.196 | 0.592 | 0.896 | |
| SNP5 | rs875342 | 5751 | 375 | 370 | 0.228 | 0.238 | 0.211 | 0.653 | 0.553 | 0.775 | |
| SNP6 | rs6951030 | 6143 | 373 | 372 | 0.0563 | 0.0565 | 0.826 | 0.594 | 0.781 | 0.698 | |
| SNP7 | rs9654749 | 7145 | 376 | 374 | 0.483 | 0.469 | 0.460 | 0.602 | 0.593 | 0.924 | |
| SNP8 | rs2030921 | 785 | 376 | 377 | 0.249 | 0.240 | 0.728 | 0.696 | 0.869 | 0.872 | |
| VAMP2 (minus strand) | m1 | rs2278637 | 0 | 372 | 369 | 0.430 | 0.436 | 0.348 | 0.724 | ||
| m2 | rs1061032 | 1981 | 377 | 377 | 0.399 | 0.403 | 0.750 | 0.975 | 0.732 | 0.802 | |
| m3 | rs8067606 | 2800 | 375 | 371 | 0.425 | 0.431 | 0.490 | 0.765 | 0.694 | ||
- a N, number; SCZ, schizophrenia; CON, control.
- b MAF, minor allele frequency.
- c Identical as conventional allele-wise analysis.
| Genes | Marker IDs | Distance to next SNP (bp) | Na | MAFb | P-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCZ | CON | SCZ | CON | Genotype | (1) Windowc | (2) Window | (3) Window | ||||
| SNAP25 | M1 | rs6104567 | 0 | 377 | 370 | 0.263 | 0.242 | 0.298 | 0.341 | ||
| M2 | rs1889189 | 1,653 | 377 | 370 | 0.236 | 0.223 | 0.675 | 0.550 | 0.493 | ||
| M3 | rs3787303 | 11,662 | 377 | 376 | 0.312 | 0.300 | 0.306 | 0.630 | 0.830 | 0.825 | |
| M4 | rs2423487 | 4,347 | 374 | 368 | 0.171 | 0.171 | 0.146 | 0.978 | 0.874 | 0.721 | |
| M5 | rs363012 | 6,704 | 377 | 377 | 0.308 | 0.295 | 0.364 | 0.612 | 0.403 | 0.795 | |
| M6 | rs363039 | 697 | 375 | 368 | 0.432 | 0.397 | 0.128 | 0.173 | 0.552 | 0.364 | |
| M7 | rs363014 | 8,198 | 377 | 377 | 0.460 | 0.453 | 0.197 | 0.767 | 0.328 | 0.660 | |
| M8 | rs12626080 | 4,358 | 375 | 367 | 0.208 | 0.162 | 0.073 | 0.0236d | 0.0215d | 0.126 | |
| M9 | rs363052 | 2,374 | 375 | 369 | 0.163 | 0.159 | 0.177 | 0.866 | 0.0747 | 0.0882 | |
| M10 | rs363053 | 159 | 374 | 370 | 0.298 | 0.291 | 0.918 | 0.748 | 0.774 | 0.411 | |
| M11 | rs4813024 | 2,231 | 377 | 377 | 0.222 | 0.235 | 0.650 | 0.544 | 0.719 | 0.865 | |
| M12 | rs6074113 | 4,195 | 372 | 369 | 0.337 | 0.341 | 0.615 | 0.868 | 0.690 | 0.855 | |
| M13 | rs363022 | 383 | 374 | 369 | 0.394 | 0.413 | 0.950 | 0.457 | 0.509 | 0.643 | |
| M14 | rs362564 | 2,232 | 376 | 372 | 0.455 | 0.425 | 0.725 | 0.265 | 0.579 | 0.739 | |
| M15 | rs362547 | 513 | 372 | 376 | 0.222 | 0.184 | 0.380 | 0.0712 | 0.254 | 0.524 | |
| M16 | rs362567 | 952 | 377 | 376 | 0.144 | 0.129 | 0.259 | 0.379 | 0.0883 | 0.375 | |
| M17 | rs362570 | 773 | 375 | 368 | 0.351 | 0.331 | 0.572 | 0.414 | 0.615 | 0.134 | |
| M18 | rs362584 | 7,611 | 372 | 370 | 0.209 | 0.212 | 0.716 | 0.877 | 0.489 | 0.617 | |
| M19 | rs16991334 | 7,442 | 372 | 367 | 0.0970 | 0.0989 | 0.574 | 0.903 | 0.999 | 0.716 | |
| M20 | rs6039807 | 1,659 | 372 | 367 | 0.451 | 0.434 | 0.580 | 0.544 | 0.401 | 0.737 | |
| M21 | rs362995 | 13,463 | 377 | 377 | 0.269 | 0.248 | 0.764 | 0.361 | 0.601 | 0.891 | |
| M22 | rs363006 | 3,044 | 373 | 370 | 0.0134 | 0.0082 | 0.873 | 1 | 0.208 | 0.587 | |
| M23 | rs6108463 | 422 | 374 | 368 | 0.182 | 0.192 | 0.893 | 0.647 | 0.732 | 0.510 | |
| M24 | rs362988 | 865 | 374 | 370 | 0.379 | 0.401 | 0.325 | 0.391 | 0.679 | 0.689 | |
| M25 | rs6039820 | 657 | 377 | 376 | 0.400 | 0.373 | 0.253 | 0.286 | 0.526 | 0.788 | |
| M26 | rs6108464 | 1,923 | 377 | 376 | 0.401 | 0.405 | 0.892 | 889 | 0.612 | 0.807 | |
| M27 | rs3787283 | 468 | 376 | 375 | 0.460 | 0.465 | 0.614 | 0.850 | 0.942 | 0.548 | |
| M28 | rs3746544 | 2,666 | 372 | 367 | 0.260 | 0.249 | 0.746 | 0.634 | 0.642 | 0.862 | |
| M29 | rs6133852 | 3,876 | 377 | 377 | 0.237 | 0.206 | 0.176 | 0.156 | 0.437 | 0.676 | |
- a N, number; SCZ, schizophrenia; CON, control.
- b MAF, minor allele frequency.
- c Identical as conventional allele-wise analysis.
- d Bold numbers represent significant P-values.
To validate this nominal significance, we carried out a replication analysis using an independent set of samples. In this analysis, five additional SNPs were further included for denser mapping around M7 and M8 (rs610457, rs363013, rs363015, rs6039792 and rs363050: Supplementary Figure 4). However, this second-set confirmation analysis showed no evidence of the significance of these markers (P-values for M7-M8 combination: 0.541: Supplementary Table 3). To increase the power, the genotypes of these five new SNPs in the first-set samples were determined and we then combined the samples (first-set and second-set samples), but again we could not detect an association in this explorative analysis (P-values for M7–M8 combination; 0.280: Table III and Supplementary Table 4).
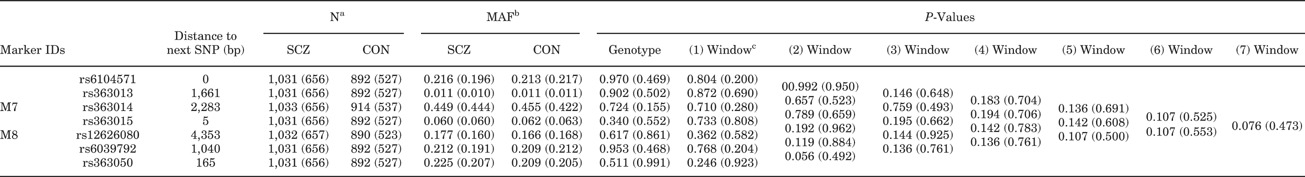
- aN, number; SCZ, schizophrenia; CON, control.
- bMAF, minor allele frequency.
- cIdentical as conventional allele-wise analysis.
- Numbers in parentheses indicate results from second-set samples.
This genetic two-stage case–control association study revealed no association between SNARE complex-related genes (STX1A, VAMP2 and SNAP25) and schizophrenia in the Japanese population. Because postmortem studies showed a change in expression of SNARE complex genes (see Introduction), the most interesting variants of these genes are SNPs located in the promoter regions that might affect gene expression. To cover such regions, particularly the 5′ region of each gene, we applied the recently recommended ‘gene-based’ approach [Neale and Sham, 2004], in which it is important to include both the exon region and the flanking region. There is also emphasis on selecting genetic variants that adequately reflect the LD background in the targeted population (e.g., tagging SNPs). Our selection of tagging SNPs represented the all regions of these genes in the Japanese population, significantly reducing genotyping effort without much loss of power.
Moreover, we included confirmation analysis using an independent set of samples to check for Type I error, after significance was obtained in the screening samples. For SNAP25, an SNP and a two-marker haplotype were associated with schizophrenia in the first-set screening samples, but no significance could be seen in the larger second set, suggesting that the significance in the screening samples may have resulted from Type I error due to multiple testing or small sample size. We carried out power calculations and determined that our sample had sufficient power in the second-set analysis to detect association of 0.999 at P < 0.05, assuming an odds ratio of 1.69, which was shown in the first-set analysis of SNAP25-M8.
In addition, our sample size in the first-set screening analysis was large enough to deny Type II error in replicating the previous positive association of an SNP in STX1A intron 7 with schizophrenia in Caucasian samples [Wong et al., 2004]. The power was more than 0.997 at P < 0.05 when the odds ratio was set at 2.1, which is the estimated odds ratio of TDT in Wong's report [Wong et al., 2004]. One explanation for the different outcomes may be that STX1A susceptibility alleles were present in the Caucasian samples, but not in the Japanese population.
Although our sample size was large enough for replication of Wong's study, in general the odds ratios of common variants found to be associated with schizophrenia so far are less than 1.5. In this regard, a larger sample size might be required for conclusive results, since our sample size showed power surpassing 0.8 only when we set the odds ratio at more than 1.62.
With this statistical methodology, it is generally accepted that gene–gene interactions should be examined when a number of related genes are analyzed. We included explorative analysis to evaluate the interaction among these genes by multiple dimensionality reduction (MDR) [Hahn et al., 2003], but no interaction was detected (data not shown). In addition, we conducted MDR analysis for other genes related to SNARE complex genes, Complexin I and II (CPLX I and CPLXII), for which we previously found no association to schizophrenia [Kishi et al., 2006]. Again, no interaction could be detected in this analysis (data not shown).
There are numerous molecules related to the SNARE complex besides CPLX genes [Wang and Tang, 2006]. The most interesting molecule is dysbindin (DTNBP1: dustrobrevin-binding protein 1), for which there is evidence of an association with schizophrenia, since recent studies showed that dysbindin regulates the expression of SNAP25 [Numakawa et al., 2004]. Therefore, it will be essential to evaluate the other candidate genes related to SNARE complex genes for conclusive results.
With regard to interpretation of the results from this study, several limitations should be mentioned. Firstly, we did not perform mutation screening of these genes. Secondly, our samples were un-matched for age and gender between cases and controls, and were not assessed with the use of a standard structured interview. Therefore, detailed association analysis with mutation search in well-phenotyped samples will be essential in future study.
To conclude, our results provide no evidence that SNARE complex genes play a major role in susceptibility for schizophrenia in the Japanese population. Our results also imply that caution is needed in drawing conclusions about positive associations from small-sample case–control studies. We strongly suggest that two-stage genetic association analysis be conducted when positive results are found in screening samples.
Acknowledgements
This work was supported in part by research grants from the Japan Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare, and the Health Sciences Foundation (Research on Health Sciences focusing on Drug Innovation).