Ordered subsets linkage analysis of antisocial behavior in substance use disorder among participants in the collaborative study on the genetics of alcoholism†‡
Please cite this article as follows: Jacobson KC, Beseler CL, Lasky-Su J, Faraone SV, Glatt SJ, Kremen WS, Lyons MJ, Tsuang MT. 2008. Ordered Subsets Linkage Analysis of Antisocial Behavior in Substance Use Disorder Among Participants in the Collaborative Study on the Genetics of Alcoholism. Am J Med Genet Part B 147B:1258–1269.
Jacobson and Beseler contributed equally to this work.
Abstract
Heterogeneity in complex diseases such as Substance Use Disorder (SUD) reduces the power to detect linkage and makes replication of findings in other populations unlikely. It is therefore critical to refine the phenotype and use methods that account for genetic heterogeneity between families. SUD was operationalized as diagnosis of abuse or dependence to alcohol and/or any one of five illicit substances. Whole-genome linkage analysis of 241 extended pedigree families from the Collaborative Study on the Genetics of Alcoholism was performed in Merlin using an affected sibship design. An Ordered Subsets Analysis (OSA) using FLOSS sought to increase the homogeneity of the sample by ranking families by their density of childhood and adult antisocial behaviors, producing new maximum Nonparametric Lod (NPL) scores on each chromosome for each subset of families. Prior to OSA, modest evidence for linkage was found on chromosomes 8 and 17. Although changes in NPL scores were not statistically significant, OSA revealed possible evidence of linkages on chromosome 7, near markers D7S1795 and D7S821. NPL scores >3.0 were also observed on chromosomes 2, 3, 5, 9, and 14. However, the number of families used in these latter subsets for linkage may be too small to be meaningful. Results provide some evidence for the ability of OSA to reduce genetic heterogeneity, and add further support to chromosome 7 as a possible location to search for genes related to various SUD related processes. Nonetheless, replication of these results in other samples is essential. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Heterogeneity in complex diseases such as substance use disorder (SUD) reduces the power to detect linkage and makes replication of a linkage finding in other populations unlikely. It is therefore critical to correctly define the SUD phenotype and use methods that account for genetic heterogeneity between families, as different families may have different susceptibility loci [Tsuang et al., 1993].
Typologies such as Cloninger's Type I and Type II alcoholism [Cloninger, 1987] and Babor's Type A and Type B alcoholism [Babor et al., 1992] emphasize the role of externalizing behaviors in differentiating subtypes of alcoholics. Numerous empirical studies have demonstrated the validity of these subtypes, suggesting that alcoholic individuals with a history of antisocial behavior (i.e., Type II or Type B alcoholics) have a more severe disorder [Galen et al., 2000; Goldstein et al., 2007], earlier onset of problems [Goldstein et al., 2007], and are more resistant to treatment than alcoholics without a history of antisocial behavior [Galen et al., 2000; Hunter et al., 2000]. In addition, alcoholic individuals with a history of antisocial behavior show different patterns of social, neuropsychological, and psychophysiological correlates compared with non-antisocial alcoholics, suggesting distinct etiological pathways [McGue et al., 1997]. At least one study suggests that alcoholism accompanied by antisocial behavior is more heritable than alcoholism without a history of antisocial behavior [van den Bree et al., 1998]. More recently, similar typologies have also been proposed for categories of illicit substance disorders [Feingold et al., 1996].
Both cross-sectional and longitudinal studies using epidemiological samples demonstrate a strong correlation between substance use and antisocial behavior. Studies of adolescents find that a history of delinquent or conduct-disordered behavior predicts onset of both alcohol and marijuana use [Loeber, 1988; Van Kammen et al., 1991; Henry et al., 1993; Grilo et al., 1996; Biederman et al., 2000]. Studies of adults also show high rates of comorbidity of antisocial behavior and substance use. For example, the estimated rate of adult antisocial personality disorder (ASPD) in the National Comorbidity Sample among alcohol-dependent men was 17% and in alcohol-dependent women was 8% [Kessler et al., 1997], compared to a general population rate of 3.6% in non-alcohol dependent individuals [Grant et al., 2004].
Family, twin, and adoption studies suggest a genetic basis for the comorbidity of antisocial behavior and SUD. Relatives of alcohol-dependent probands showed significant familial aggregation of ASPD in the Collaborative Study on the Genetics of Alcoholism (COGA) [Nurnberger et al., 2004]. A positive family history of alcoholism is associated with symptoms of ASPD [Grande et al., 1984; Alterman and Cacciola, 1998; Slutske et al., 1998; Jang et al., 2001] and evidence from family and twin studies supports a genetic contribution to the overlap in substance abuse and dependence, conduct disorder (CD), and ASPD [Stallings et al., 1997; Blonigen et al., 2005]. A number of large, epidemiologically based twin studies have shown that common genetic effects account for a large part of the comorbidity between antisocial behavior and SUD. Among adult male twins from the Vietnam Era Twin Registry, genetic effects on ASPD accounted for 50% of the variance in alcohol dependence and 58% of the variance in marijuana dependence [Fu et al., 2002]. CD, ASPD, and alcohol and drug dependence shared a common vulnerability that was highly heritable among both male and female adolescent twins in the Minnesota Twin Family Study [Hicks et al., 2004] and male and female adults from the Mid-Atlantic Twin Registry [Agrawal et al., 2004]. Similar results have been obtained for the comorbidity between CD and substance use vulnerability in adolescence and young adulthood [Miles et al., 2002; Malone et al., 2004; Button et al., 2007]. Notably, despite the consistent evidence for a common genetic vulnerability to antisocial behavior and SUD, the majority of previous twin studies have also found evidence for substance-specific genetic effects, supporting the notion of two distinct, genetically based etiological pathways to SUD.
Behavioral genetic evidence supporting two genetic pathways to alcohol and illicit substance use was further found in the Iowa adoption study. Genetic factors influenced alcohol use among 95 male adoptees through a direct transmission of biological parent risk for SUD to adoptee risk [Cadoret et al., 1995]. A second path, however, was also genetically mediated, and began with biological risk for ASPD, and involved circuitous mediational pathways through intervening variables of adoptee aggressivity, CD, ASPD, and, eventually, drug abuse/dependency [Cadoret et al., 1995]. These results were more recently replicated in females [Cadoret et al., 1996] and for illicit SUDs [Langbehn et al., 2003].
Although a tremendous amount of evidence exists from family, twin, and adoption studies suggesting a common underlying genetic vulnerability to ASPD and SUD, molecular genetic studies have just begun to examine individual genes that may account for some of the genetic overlap between antisocial behavior and substance misuse. In the COGA sample, linkage at the same region of chromosome 2p has been reported for both alcohol dependence [Foroud et al., 2000] and history of childhood conduct disorder [Dick et al., 2004]. In other samples, the antisocial alcoholism phenotype has been linked to the genes involved in the serotonin pathway [Hill et al., 1999]. For example, linkage and association studies of individuals with both ASPD and alcoholism have identified the serotonin 5-HT1B receptor gene 2 in two populations [Lappalainen et al., 1998; Soyka et al., 2004]. In adolescents, individuals diagnosed with CD and substance dependence showed linkage to 9q34, 3q24-3q25, and 17q12 [Stallings et al., 2005]. The region on 17q12 is within 5 Mb of SLC6A4, the serotonin transporter gene [Stallings et al., 2005]. These linkage results provide emerging and encouraging evidence for shared genetic vulnerability to SUD and antisocial behavior that may be accounted for by specific genetic loci.
In the present study we searched for genetic linkage to SUD by using an ordered subsets analysis (OSA) to account for potential genetic heterogeneity due to presence or absence of antisocial behavior measured among family members. In contrast to prior linkage strategies, which have attempted to create a more genetically homogeneous population by restricting analysis of linkage to SUD among individuals with a history of antisocial behavior, OSA attempts to reduce the genetic heterogeneity of SUD within a given sample by identifying more homogeneous subsets of families based on family history of antisocial behavior. OSA identifies the family subset that maximizes the LOD score through the use of a covariate that rank-orders families. In this study, we used alcohol or drug dependence/abuse as the phenotype in a genome-wide linkage analysis, and we used multiple indices of child and adult antisocial behavior as our subsetting variables. The present study extends previous research in at least two important ways. First, the study includes both alcohol and illicit drug use disorders in the outcome phenotype, rather than considering alcohol use disorders alone. Only recently have studies using the COGA sample begun to perform linkage and association analyses on combined alcohol and illicit substance use phenotypes [Agrawal et al., 2006, 2008; Dick et al., 2007a]. Second, to our knowledge this study is the first to apply an OSA based on family history of antisocial behavior in order to reduce potential genetic heterogeneity in linkage studies of SUD using the COGA sample.
METHODS
Study Population
The present study uses data from the Collaborative Study on the Genetics of Alcoholism [Reich et al., 1998; Foroud et al., 2000]. The COGA data is publicly available via the NIAAA website at www.niaaa.nih.gov/researchinformation/extramuralresearch/sharedresources/projcoga.htm. In 1989 COGA began recruiting probands and their families at the Indiana University School of Medicine, University of Iowa, University of Connecticut, State University of New York Health Sciences Center at Brooklyn, University of California San Diego, and Washington University. The Institutional Review Board at each site approved the study. Probands who had been diagnosed with DSM-III-R alcohol dependence and also met the Feighner alcoholism criteria [Feighner et al., 1972] were recruited through inpatient and outpatient alcohol treatment facilities. Included were probands who were at least 18 years of age, had at least three first-degree relatives available for participation in the study and had two relatives who lived within a catchment area. Probands and their relatives had to be English-speaking and could not be intravenous drug users, HIV positive or have a terminal illness. The total sample consists of 262 extended pedigrees combined from the first and second waves of COGA, and, after deleting four monozygotic twins, a total of 2,174 individuals. Detailed information on the COGA sample has been published elsewhere [Reich et al., 1998; Foroud et al., 2000]. The present study uses publicly available data from release 2.0 of the Alcohol Dependence Dataset.
Genotyping, Allele Frequencies, and Maps
All genotyping, calculation of allele frequencies, and the creation of genetic marker maps were conducted by the COGA investigators. Details on these procedures have been published elsewhere [Reich et al., 1998; Foroud et al., 2000]. Briefly, genotyping was performed using 390 markers spaced an average of 10 cM apart. The Gene Master database and programs CRIMAP [Green, 1990] and USERM13 [Boehnke, 1991] were used to check for non-Mendelian inheritance and CRIMAP was used to generate recombination-based marker maps. USERM13 was used to generate maximum likelihood estimates of marker allele frequencies from the data.
Measures
Outcome measure
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) [Bucholz et al., 1994] was used to assess DSM-III-R, Feighner and ICD-10 abuse and dependence of alcohol and of five illicit drugs. DSM-IV diagnosis was available only for alcohol dependence at the time of COGA data collection; thus, affected status for the present study was defined using a DSM-III-R diagnosis of dependence or abuse for six substances: alcohol, opioids, sedatives, stimulants, cocaine, and marijuana. Individuals with one or more of any of these diagnoses were classified as affected.
Subsetting covariates
We used four different measures of antisocial behavior as our subsetting covariates in the OSA, based on items assessed using the SSAGA. Two of the variables were simply DSM-III-R diagnosis of CD and ASPD. The family-level covariates from these diagnoses were generated by taking the mean number of individuals in a family with a diagnosis of CD or ASPD. Compared with the presence or absence of a diagnosis, a more quantitative approach to assessing antisocial behavior may provide additional power to detect linkage. Thus, we also generated two quantitative composite variables reflecting continuous measures of child and adult antisocial behavior. To avoid the diagnostic hierarchy implicit in the DSM-based CD and ASPD diagnoses (i.e., a diagnosis of ASPD includes a diagnosis of CD), we created two quantitative subsetting variables by separately factor-analyzing 18 child (before age 15) antisocial behavior items and 28 adult (age 15 and older) antisocial behavior items (see Table I). The items selected for the factor analysis were similar to the items used in a previous latent class analysis of antisocial behavior using the COGA sample [Bucholz et al., 2000]. In the SSAGA interview, a skip pattern was introduced so that a number of the antisocial behavior items were only asked if the respondent endorsed at least two of 11 items for antisocial behavior prior to age 18, or showed evidence of a SUD from previous questions (see Table I). Responses for these behaviors in the skip pattern were necessarily missing, and were consequently coded as not present (0) in creating the quantitative measures for these analyses. We note, however, that given the clinical severity of the COGA sample, only 17.6% (383 of 2,174) of the sample were missing data on these behaviors due to these skip patterns. “True” missing data (i.e., missing data not due to the skip pattern) on any one item occurred for fewer than 15 cases. Because the missing data in these latter instances appeared random across individuals, these cases were also coded as zero for the given item.
| Prevalence (%) | Factor loadings | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Child antisocial behavior (prior to age 15) | ||||
| Played hooky from school | 35.3 | 18.4 | 0.58 | 0.62 |
| Suspended or expelleda | 21.2 | 9.2 | 0.66 | 0.67 |
| Runaway from home | 10.6 | 6.4 | 0.59 | 0.63 |
| Told a lot of lies | 12.1 | 5.4 | 0.64 | 0.73 |
| Theftb | 41.0 | 20.8 | 0.61 | 0.61 |
| Damaged property | 13.5 | 3.0 | 0.64 | 0.66 |
| Started physical fights | 21.2 | 5.4 | 0.62 | 0.72 |
| Use a weapon in a fight | 3.8 | 0.9 | 0.74 | 0.66 |
| Physically injured someone | 1.8 | 0.5 | 0.55 | 0.57 |
| Been arrested | 4.3 | 1.2 | 0.61 | 0.77 |
| §Often challenged authoritya | 24.0 | 17.3 | 0.74 | 0.73 |
| §Often threw temper tantrumsa | 13.0 | 11.8 | 0.44 | 0.61 |
| §Often bullied other childrena | 7.1 | 4.7 | 0.55 | 0.74 |
| §Hurt animals on purpose | 9.8 | 1.6 | 0.41 | 0.60 |
| §Deliberately set fires | 19.4 | 3.3 | 0.41 | 0.35 |
| §Enjoyed outsmarting others | 11.1 | 3.8 | 0.60 | 0.69 |
| §Breaking and entering | 11.1 | 1.6 | 0.72 | 0.73 |
| §Taken items by force | 0.9 | 0.2 | 0.82 | 0.76 |
| Adult antisocial behavior (age 15 and older) | ||||
| Told a lot of lies | 23.7 | 11.1 | 0.75 | 0.72 |
| Theftb | 40.2 | 19.8 | 0.68 | 0.61 |
| Damaged property | 25.9 | 9.4 | 0.61 | 0.63 |
| Started physical fights | 45.3 | 16.3 | 0.65 | 0.75 |
| Use a weapon in a fight | 11.1 | 4.9 | 0.74 | 0.68 |
| Physically injured someone | 5.9 | 2.4 | 0.58 | 0.45 |
| Had at least four tickets for reckless driving | 43.1 | 12.7 | 0.31 | 0.17 |
| Been arrested | 42.7 | 12.8 | 0.68 | 0.67 |
| §Hurt animals on purpose | 7.3 | 0.9 | 0.53 | 0.71 |
| §Deliberately set fires | 4.4 | 0.7 | 0.48 | 0.51 |
| §Enjoyed outsmarting others | 19.3 | 5.9 | 0.62 | 0.74 |
| §Breaking and entering | 17.8 | 2.8 | 0.69 | 0.63 |
| §Taken items by force | 4.7 | 0.3 | 0.73 | 0.71 |
| §Engaged in illegal activityc | 46.1 | 15.8 | 0.79 | 0.78 |
| §Chronic infidelityd | 5.8 | 1.6 | 0.29 | 0.54 |
| §Forced sexe | 1.8 | 0.2 | 0.51 | 0.51 |
| §Child abuse | 3.2 | 4.4 | 0.29 | 0.53 |
| §Often violent | 10.1 | 9.0 | 0.56 | 0.73 |
| §Quit three or more jobs without a plan | 25.2 | 12.0 | 0.67 | 0.76 |
| §Chronically late/absent from work/school | 27.6 | 16.6 | 0.66 | 0.59 |
| §Unemployed for six or more months | 16.0 | 8.6 | 0.49 | 0.68 |
| §No place to live for more than a month | 15.0 | 5.1 | 0.65 | 0.69 |
| §Often failed to pay debts | 26.5 | 14.1 | 0.67 | 0.74 |
| §Child neglectf | 11.7 | 3.4 | 0.47 | 0.72 |
| §Often ignored feelings of othersa | 33.6 | 12.4 | 0.69 | 0.83 |
| §Frequently lost tempera | 37.2 | 26.1 | 0.65 | 0.77 |
| §Often felt others were to blamea | 18.8 | 15.4 | 0.65 | 0.67 |
| §Lack of guilt or remorse | 22.7 | 7.4 | 0.20 | 0.40 |
- §Question was not asked for 383 individuals (17.6% of sample) who did not meet any alcohol or drug use criteria and did not endorse at least two other antisocial behavior items prior to age 18.
- a Item does not correspond to a symptom in DSM-3R diagnosis of conduct disorder or adult antisocial behavior disorder.
- b Symptom was created by combining information from three different items regarding theft behavior.
- c Symptom was created by combining information from six different items regarding illegal activity.
- d Seventy-seven individuals (3.5% of sample) reported never having been in a committed relationship and were given scores of zero for this item.
- e Includes two male cases who report forced sex prior to age 18.
- f Symptom was created by combining information from five different items regarding child neglect. Nearly one-quarter (23.1%) of the sample reported never having been a parent and were given scores of zero for this item.
Factor analysis was performed separately for males and females, using least squares estimation of binary items in MPLUS (Mplus, Version 4.2, Muthen & Muthen, Los Angeles, CA). Because we were using a linear combination of factor-weighted scores to create our composite measures of child and adult antisocial behavior, factor loadings from one-factor model solutions were used.1 To create the family-level, quantitative measures of child and adult antisocial behavior, we used the gender-specific factor loadings (see Results section and Table I). These loadings were used in a multiplicative term with each binary behavior item (0 = not present; 1 = present) and all items were summed to obtain an overall factor-weighted sum score specific for each disorder for each individual. These composite scores were then averaged across all members in a given family to create the family-wide covariates used in the OSA analyses.
Statistical Analyses
Preliminary linkage analysis
A genome-wide multipoint linkage analysis was done in Merlin on all 22 autosomes using the SUD phenotype described above. An affected sib pair analysis with the nonparametric LOD score (NPL) and “per family” options was used to generate input for the OSA. Only sibling pairs with genotyped parents were included in the linkage analyses.
Ordered subsets analysis (OSA)
The purpose of OSA is to evaluate evidence for linkage even when genetic heterogeneity is present in a data set, a common problem in the genetic analysis of complex traits. In OSA, families are ranked by covariate values in order to test for linkage among more homogeneous subsets of families [Hauser et al., 2004]. OSA uses family level NPL scores generated from a preliminary linkage analysis. A subsetting covariate is used to rank-order families on the basis of their similarity on this subsetting variable. An iterative process is then used to recalculate the NPL score based on all possible ordered subsets of families. OSA is conducted beginning with the family that scored highest on the covariate (designated by a positive number of families, see Tables III and IV), as well as beginning with the family that scored lowest on the covariate (designated by a negative number of families, see Tables III and IV). The subset of families that produced the maximum NPL score is then identified, and permutation tests are used to determine whether the maximum NPL score based on the best subset of the families resulted in a significant improvement over the initial linkage findings.
Because this is a relatively new approach to linkage, we also considered any new peak NPL scores of greater than 3.0 as a potential linkage finding, regardless of the statistical significance of the increase. Other output from OSA includes the location and score of the new peak NPL on each chromosome, and a listing of the families that were included in the best subset analysis. We used FLOSS for the OSA because it is formatted to use Merlin files for input [Browning, 2006]. As detailed above, we used four separate covariates to subset these results in the OSA: (1) proportion of family members with DSM-III-R diagnosis of CD; (2) proportion of family members with DSM-III-R diagnosis of ASPD; (3) a quantitative variable for family-level severity of child antisocial behavior; and (4) quantitative variable for family-level severity of adult antisocial behavior.
RESULTS
Factor Analysis of Antisocial Behavior Items
The factor analysis was done using all 2,174 COGA participants for whom data on antisocial behavior was available. This sample included 1,148 females and 1,026 males. Table I shows the prevalence of each item and the factor loadings from a one-factor solution, separately for males and females. The magnitude of the factor loadings was reasonably similar across sex, suggesting that the items in our antisocial behavior composites represented an underlying latent antisocial behavior phenotype similarly for males and females. However, males had a higher mean factor score than females (child behaviors: males, M = 1.56, SD = 1.65; females, M = 0.75, SD = 1.21, P < 0.001; adult behaviors: males M = 3.64, SD = 3.10; females 1.67, SD = 2.40, P < 0.001), consistent with well-established gender differences in the prevalence of antisocial behavior [Moffitt et al., 2001]. The phenotypic correlation between the child and adult antisocial behavior composites in males was 0.66 (P < 0.0001) and in females it was 0.64 (P < 0.0001). Individuals with a diagnosis of CD had higher antisocial behavior factor scores for both child and adult behaviors (child: M = 3.76 (SD = 1.56) vs. 0.66 (SD = 0.84); adult: 6.21 (SD = 3.11) vs. 1.95 (SD = 2.35), both P-values <0.0001), and individuals with a diagnosis of ASPD also had higher antisocial behavior factor scores for both child and adult behaviors (child: 4.00 (SD = 1.56) vs. 0.79 (SD = 1.05); adult: 7.58 (SD = 2.50) vs. 2.01 (SD = 2.34), both P-values <0.0001).
Descriptive Results
The total COGA genotyped sample consists of 262 extended pedigree families containing 2,024 individuals with both phenotype and genotype data. The average age at assessment of the study sample was 40.6 (SD = 14.6). The sample was 47.5% male, 78.8% white, 52.3% married, and 73.8% were high school graduates.
A DSM-III-R diagnosis of CD was found among 27.0% of males and 5.4% of females in the study sample. Diagnosis of ASPD occurred in 19.8% of males and 2.9% of females. In addition, a broad range of alcohol and drug abuse and dependence diagnoses was represented in the sample: alcohol (53.8%, n = 1,089), marijuana (21.4%, n = 432), cocaine (16.7%, n = 337), stimulants (9.7%, n = 197), sedatives (5.6%, n = 113), and opiates (5.1%, n = 103). Our outcome SUD measure of alcohol use disorder, illicit drug use disorder, or both occurred in 57.7% (n = 1,167) of the sample. Of these 1,167 individuals who had any SUD, 48.0% had only an alcohol use disorder (n = 560), 6.7% had only an illicit drug use disorder (n = 78), and 45.3% had both an both an alcohol and an illicit drug use disorder (n = 529). The 1,167 individuals with any SUD were more likely to be male than female (62.5% vs. 37.5%, P < 0.0001) and to be unmarried than married (55.4% vs. 44.6%, P < 0.0001). Those with a CD diagnosis were much more likely to have an SUD than those without a CD diagnosis (SUD prevalence with CD = 88.3%, SUD prevalence without CD = 52.0%, P < 0.0001). The same was true for ASPD (SUD prevalence with ASPD = 95.5%, SUD prevalence without ASPD = 53.1%, P < 0.0001).
Preliminary Linkage Analyses
The present study used all affected sib pairs for whom genotype data from both parents was available. The original COGA sample consists of 262 extended pedigrees. Seven pedigrees had to be broken down into smaller families to conduct the linkage analyses, and the “trim” function was used to remove uninformative individuals. This resulted in a final sample consisting of 241 pedigrees, containing 282 nuclear families, and 818 affected siblings with sibship sizes varying from 2 to 7 siblings per family. Most nuclear families contributed two affected siblings (n = 122 families, 43.3%), 3 affected siblings (n = 95 families, 33.7%) or 4 affected siblings (n = 46 families, 15.9%). Only 20 families contributed from 5 to 7 affected siblings (7.1%). All possible affected sibling pairs were used in these analyses.
Table II presents the maximum NPL scores on each chromosome from the preliminary MERLIN linkage analyses. After running a linkage analysis on all 241 families, no NPL scores above 3.0 were observed. An NPL score of 2.10 (LOD score of 1.22) was found on chromosome 8, 1.00 cM, marker D8S1109, and an NPL score of 2.02 (LOD score of 1.32) was found at chromosome 17, 102.9 cM, marker D17S1531.
| Chromosome | Location of Max NPL | Max NPL |
|---|---|---|
| 1 | 93.6 | 0.47 |
| 2 | 278.1 | 1.78 |
| 3 | 55.2 | 0.78 |
| 4 | 51.0 | 0.82 |
| 5 | 233.0 | 0.80 |
| 6 | 55.7 | 1.63 |
| 7 | 0.0 | 1.88 |
| 8 | 1.0 | 2.10 |
| 9 | 181.8 | 1.26 |
| 10 | 29.9 | 1.68 |
| 11 | 135.6 | 1.02 |
| 12 | 177.3 | 1.52 |
| 13 | 97.3 | 0.10 |
| 14 | 71.2 | 1.92 |
| 15 | 165.0 | 0.73 |
| 16 | 7.3 | 0.50 |
| 17 | 102.9 | 2.02 |
| 18 | 82.6 | 0.19 |
| 19 | 56.1 | 0.10 |
| 20 | 0.0 | −0.55 |
| 21 | 88.2 | 1.05 |
| 22 | 57.0 | 1.22 |
Ordered Subsets Analysis
Results of the OSA using the diagnosis of CD and ASPD are shown in Table III. The table presents the location and NPL score for the maximum linkage signal on each chromosome from the OSA analysis, as well as the original linkage score from analysis of the whole sample for that particular location. The P-value refers to the statistical test of whether the change in NPL score is statistically significant. The final column shows the number of families used to generate the new maximum peak NPL. A positive number of families (e.g., +111/241 for chromosome 4 using diagnosis of CD, see Table III) indicates that the maximum NPL score was obtained by iteratively adding in families beginning with the family with the highest level of antisocial behavior. Thus, the +110 refers to the 110 most antisocial families in the sample. In contrast, a negative number of families (e.g., −165/241 for chromosome 2 using diagnosis of CD) indicates that the OSA was done starting with the full sample and sequentially eliminating families, beginning with the most antisocial families. Thus, negative numbers indicate that the NPL was found among the least antisocial families.
| Chromosome number | Location of new Max NPL | MERLIN NPL before subsettinga | OSA NPL after subsetting | P-value | No. of families |
|---|---|---|---|---|---|
| Diagnosis of CD | |||||
| 1 | 253.3 | 1.05 | 1.95 | 0.35 | +19/241 |
| 2 | 278.1b | 1.78 | 2.17 | 0.97 | −165/241 |
| 3 | 156.9 | −0.05 | 2.03 | 0.67 | −72/241 |
| 4 | 146.4 | 0.40 | 2.19 | 0.55 | +111/241 |
| 5 | 154.1 | −0.14 | 2.41 | 0.62 | +4/241 |
| 6 | 95.3 | −0.21 | 2.45 | 0.54 | +19/241 |
| 7 | 127.5 | 1.66 | 2.49 | 0.66 | −96/241 |
| 8 | 91.1 | 0.42 | 2.40 | 0.84 | +4/241 |
| 9 | 145.1 | −0.20 | 1.95 | 0.70 | +6/241 |
| 10 | 135.7 | 0.82 | 2.35 | 0.43 | −150/240 |
| 11 | 78.9 | 0.82 | 1.98 | 0.60 | +19/241 |
| 12 | 177.3b | 1.52 | 2.29 | 0.71 | −110/241 |
| 13 | 30.2 | −1.32 | 0.96 | 0.94 | +19/241 |
| 14 | 71.2b | 1.92 | 2.66 | 0.63 | −108/241 |
| 15 | 88.8 | −1.09 | 2.16 | 0.73 | +11/241 |
| 16 | 162.4 | 0.19 | 2.37 | 0.20 | +6/241 |
| 17 | 102.9b | 2.02 | 2.50 | 0.71 | +157/241 |
| 18 | 82.6b | 0.19 | 1.66 | 0.44 | −96/241 |
| 19 | 80.8 | −0.47 | 2.05 | 0.12 | +10/241 |
| 20 | 41.5 | −2.20 | 1.41 | 0.31 | +1/241 |
| 21 | 33.0 | 0.22 | 1.83 | 0.80 | +8/241 |
| 22 | 26.6 | 0.19 | 1.65 | 0.84 | −133/241 |
| Diagnosis of ASPD | |||||
| 1 | 253.3 | 1.05 | 1.73 | 0.44 | +11/241 |
| 2 | 278.1b | 1.78 | 3.08 | 0.19 | +2/241 |
| 3 | 182.8 | −1.39 | 2.88 | 0.09 | +3/241 |
| 4 | 171.5 | −1.20 | 2.31 | 0.38 | +1/241 |
| 5 | 154.1 | −0.14 | 2.30 | 0.66 | +1/241 |
| 6 | 140.0 | 0.98 | 2.44 | 0.52 | +1/241 |
| 7 | 127.5 | 1.66 | 2.62 | 0.63 | −100/241 |
| 8 | 89.6 | 0.05 | 2.43 | 0.87 | +1/241 |
| 9 | 181.8b | 1.26 | 1.75 | 0.85 | +139/241 |
| 10 | 29.9b | 1.68 | 2.24 | 0.65 | −161/241 |
| 11 | 112.1 | 0.62 | 1.98 | 0.53 | +3/241 |
| 12 | 177.3b | 1.52 | 2.17 | 0.84 | −110/241 |
| 13 | 30.2 | −1.32 | 1.02 | 0.90 | +11/241 |
| 14 | 71.2b | 1.92 | 2.63 | 0.57 | −142/241 |
| 15 | 71.0 | −0.29 | 1.86 | 0.93 | +11/241 |
| 16 | 162.4 | 0.19 | 1.88 | 0.51 | +47/241 |
| 17 | 12.9 | 0.75 | 2.52 | 0.56 | +11/241 |
| 18 | 82.6b | 0.19 | 1.31 | 0.70 | −102/240 |
| 19 | 80.8 | −0.47 | 1.43 | 0.47 | +68/240 |
| 20 | 31.1 | −1.90 | 0.71 | 0.89 | +1/241 |
| 21 | 0.0 | 0.49 | 1.83 | 0.76 | +110/241 |
| 22 | 45.8 | 1.04 | 2.24 | 0.35 | +1/241 |
- a Merlin NPL at same location of maximized NPL from OSA.
- b Chromosomal location is the same as the location of the peak NPL in the initial MERLIN analyses (Table III).
Using the proportion of family members with CD as a subsetting variable, none of the changes in NPL scores reached statistical significance, and none of the new peak NPL scores was greater than 3.0. For analyses using the proportion of family members with ASPD diagnosis, a trend towards a significant increase in NPL was observed in chromosome 3, position 182.3 (NPL = 2.88, P = 0.09), based on a subset of the three families with the highest rates of ASPD. In addition, a potential linkage peak (NPL = 3.08) was found on chromosome 2, near marker location 278.1 cM. However, the change in NPL was not statistically significant, and the peak NPL was obtained using only the two most severely antisocial families, suggesting that this finding may be spurious.
Results from the analyses using the quantitative subsetting variables of child and adult antisocial behaviors are shown in Table IV. In these analyses, four regions reached an NPL of at least 3.0 after subsetting on childhood antisocial behavior (chromosomes 5, 7, 9, and 14) and one region reached an NPL score >3.0 in the analysis using adult antisocial behavior (chromosome 5). However, none of the increases in NPL were statistically significant, and all but one of the locations was based on a subset of less than 10 families. The one location that showed an NPL score of greater than 3.0 based on a sizeable proportion of the sample was found on chromosome 7, location 27.3 cM, marker D7S1795, among a subset of the 70 most antisocial families (NPL = 3.52, P = 0.17). This location was not identified as the location of peak NPL in the initial linkage analysis or in any of the other OSA analyses, indicating that it could be a region of interest specific to SUD accompanied by a history of early conduct problems.
| Chromosome number | Location of new Max NPL | MERLIN NPL before subsettinga | OSA NPL after subsetting | P-value | No. of families |
|---|---|---|---|---|---|
| Childhood antisocial behavior | |||||
| 1 | 177.1 | 0.86 | 2.55 | 0.86 | −80/241 |
| 2 | 278.1b | 1.78 | 2.41 | 0.94 | −148/241 |
| 3 | 207.1 | 0.37 | 2.37 | 0.61 | +7/241 |
| 4 | 56.5 | 0.51 | 2.64 | 0.52 | −12/241 |
| 5 | 88.0 | 0.33 | 3.15 | 0.43 | −7/241 |
| 6 | 55.7b | 1.63 | 2.41 | 0.76 | +135/241 |
| 7 | 27.3 | 0.77 | 3.52 | 0.17 | +70/241 |
| 8 | 1.0b | 2.10 | 2.35 | 0.98 | +161/241 |
| 9 | 145.1 | −0.20 | 3.39 | 0.13 | +8/241 |
| 10 | 123.9 | −0.25 | 2.58 | 0.52 | −68/240 |
| 11 | 122.5 | 0.84 | 2.49 | 0.40 | −11/241 |
| 12 | 177.3b | 1.52 | 2.49 | 0.70 | −37/241 |
| 13 | 113.5 | −0.50 | 1.35 | 0.94 | −17/241 |
| 14 | 36.5 | −0.02 | 3.23 | 0.35 | −3/241 |
| 15 | 71.0 | −0.29 | 2.73 | 0.53 | −7/241 |
| 16 | 0.0 | 0.41 | 2.18 | 0.69 | +71/241 |
| 17 | 69.8 | 1.24 | 2.51 | 0.86 | +78/241 |
| 18 | 82.6b | 0.19 | 1.57 | 0.75 | −54/241 |
| 19 | 40.8 | −0.99 | 2.19 | 0.25 | +12/240 |
| 20 | 41.5 | −2.20 | 1.41 | 0.63 | +1/240 |
| 21 | 0.0 | 0.49 | 1.60 | 0.96 | +108/241 |
| 22 | 26.6 | 0.19 | 2.82 | 0.17 | −28/241 |
| Adult antisocial behavior | |||||
| 1 | 125.5 | 0.53 | 2.95 | 0.63 | +15/241 |
| 2 | 280.2 | 1.58 | 2.36 | 0.95 | −86/241 |
| 3 | 224.4 | 0.37 | 2.18 | 0.76 | −4/241 |
| 4 | 0.0 | −0.13 | 2.11 | 0.88 | −64/241 |
| 5 | 88.0 | 0.33 | 3.44 | 0.38 | −6/241 |
| 6 | 140.0 | 0.98 | 2.64 | 0.55 | +37/241 |
| 7 | 127.5 | 1.66 | 2.95 | 0.60 | −78/241 |
| 8 | 0.0 | 1.81 | 2.47 | 0.92 | +42/241 |
| 9 | 149.1 | −0.32 | 1.74 | 0.99 | +2/241 |
| 10 | 135.7 | 0.82 | 2.48 | 0.55 | −21/241 |
| 11 | 86.7 | 0.92 | 2.31 | 0.51 | +44/241 |
| 12 | 42.9 | 0.97 | 2.81 | 0.51 | +141/241 |
| 13 | 113.5 | −0.50 | 2.31 | 0.34 | −7/241 |
| 14 | 82.9 | 1.58 | 2.76 | 0.69 | −143/241 |
| 15 | 165.0b | 0.73 | 2.05 | 0.94 | −29/241 |
| 16 | 39.1 | 0.02 | 2.49 | 0.34 | +48/241 |
| 17 | 12.9 | 0.75 | 2.76 | 0.54 | +17/241 |
| 18 | 53.9 | −0.27 | 1.81 | 0.61 | +31/241 |
| 19 | 97.2 | 0.04 | 1.52 | 0.73 | +79/240 |
| 20 | 41.5 | −2.20 | 1.16 | 0.79 | −14/241 |
| 21 | 88.2b | 1.05 | 1.80 | 0.90 | +124/241 |
| 22 | 45.8 | 1.04 | 2.65 | 0.29 | −35/241 |
- a Merlin NPL at same location of maximized NPL from OSA.
- b Chromosomal location is the same as the location of the peak NPL in the initial MERLIN analyses (Table III).
Comparison of Linkage Findings With and Without OSA
Because OSA is a relatively new approach to reducing genetic heterogeneity in linkage analyses, it may be informative to compare results from Tables II–IV. Our preliminary linkage analyses in MERLIN identified two regions of suggestive linkage on chromosomes 8 (NPL = 2.10) and 17 (NPL = 2.02) (Table II). The same region on chromosome 8 was also identified in the OSA analysis using the continuous measure of child antisocial behavior (OSA NPL = 2.35, see Table IV), and the same region on chromosome 17 was also identified in the OSA analysis using diagnosis of CD (OSA NPL = 2.50, see Table III).
Figure 1 displays the maximum NPL scores on each chromosome for the initial linkage analysis and for the four OSA analyses, regardless of the specific location on the chromosome. For child behavior, five out of 22 chromosomal locations were the same for the diagnostic and quantitative OSA (chromosomes 2, 12, 14, 17, and 18, see Tables III and IV). For adult antisocial behavior OSA analyses, four chromosomes had peak NPL scores at the same location (chromosomes 6, 7, 17, and 22, see Tables III and IV). In all but one of these nine instances, the NPL score generated by the OSA using the quantitative measure was higher than the NPL based on the OSA using the diagnostic measure. For the 9th location (chromosome 20 for child antisocial behavior), the NPL score was identical in the diagnostic and quantitative OSA. This pattern suggests that OSA analyses using quantitative measures of child and adult antisocial behavior tended to result in increased NPL scores relative to the diagnostic measures of CD and ASPD. Thus, OSA using quantitative traits may increase power to detect novel areas of linkage.
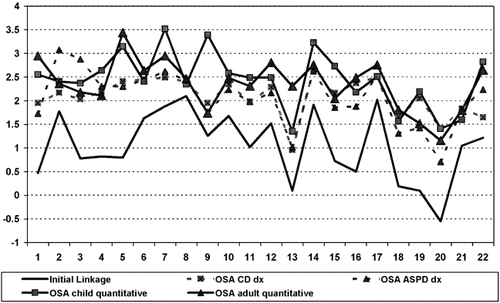
Max NPL scores on each chromosome before and after OSA analysis of quantitative antisocial behavior measures.
Comparison of Results With Previous Linkage Studies of SUD Using COGA
As will be discussed in more detail below, a number of previous studies using the COGA samples have conducted linkage analyses related to various alcohol-related phenotypes. Although results vary widely across studies, the most replicated linkage findings have occurred on chromosomes 1, 4, and 7. In the present study, there was no evidence for linkage to chromosome 1 when antisocial behavior was not considered as a covariate (max NPL = 0.47, Table II), and the maximum NPL score of 2.95 that occurred when subsetting on the continuous measure of adult antisocial behavior occurred in only a small number of families (N = 15 families, Table IV). Similarly, there was no evidence of linkage on chromosome 4 in the initial Merlin analyses (NPL = 0.80), and the locations of maximum NPL scores during the OSA analyses varied widely across the various subsetting variables, and resulted in a maximum NPL of 2.1–2.6 (Tables III and IV). For chromosome 7, our initial linkage analysis revealed a maximum NPL score of only 1.88 when family history of antisocial behavior was not considered. However, as discussed above, our strongest evidence for linkage from the genome-wide OSA analyses occurred on chromosome 7, position 27.3 (NPL = 3.52), when subsetting on the continuous measure of child antisocial behavior (see Table IV). To our knowledge, this specific location, near marker D7S1795, has not been identified in any previous COGA studies. Interestingly, however, all three of the other OSA analyses revealed maximum NPL scores of 2.49–2.95 at the same location on chromosome 7, position 127.5. This location, near marker D7S821, has been found in previous COGA studies of alcohol dependence.
DISCUSSION
The present study examined linkage to an SUD trait defined by alcohol and/or illicit substance abuse or dependence. To our knowledge, this is one of the first published linkage studies of the COGA data to include both alcohol and illicit drug use disorders in the outcome phenotype, and to also combine dependence and abuse diagnoses. This study is also the first to use an ordered subsets approach to identify genes related to comorbidity of substance use and antisocial behavior. In our preliminary MERLIN analyses of affected sib pairs without considering antisocial behavior as a covariate, suggestive linkage was found on chromosomes 8 (position 1.00, marker D8S1109, NPL = 2.10) and 17 (position 102.9, marker D17S1531, NPL = 2.02). In the OSA analyses, a slightly higher NPL score for D17S1531 was observed among a subset of 157 families using CD diagnosis (NPL = 2.50), and a slightly higher NPL score for D8S1109 was observed in 161 families using continuous measure of child antisocial behavior (2.35), although these increases in NPL scores were not statistically significant.
Linkage peaks have not been identified at D8S1109 or D17S1531 in previous COGA studies, possibly because many of these prior studies focused on strict criteria for alcohol dependence, and did not combine alcohol and illicit drugs using abuse or dependence as the phenotype. Assuming these newly identified peaks are not false positives, it is possible that these areas point to genes that represent a common vulnerability underlying SUDS, rather than genes representing physiological effects of alcohol on the brain. Few known genes lie near D8S1109, although we note that a prior study investigating linkage to a nested alcohol dependence phenotype in the combined COGA sample did report a LOD score of 2.21 at D8S549, which is located <10 cM away from the D8S1109 marker [Corbett et al., 2005].
In contrast, the D17S1531 locus is in the vicinity of a number of interesting genes, including the serotonin transporter gene at 17q12 (SLC6A4), which has been shown to play a role in alcohol abuse in animal models and humans [Sellers et al., 1992]. The short allele of the 5-HTTLPR gene product of SLC6A4 has been associated with alcohol dependence in both a population-based association study [Hammoumi et al., 1999] and a family-based association study [Lichtermann et al., 2000], although we note that the SLC6A4 has been genotyped in the COGA sample and no association with alcohol dependence was found [Edenberg et al., 1998; Dick et al., 2007b]. Nevertheless, the serotonin pathway has been increasingly linked to comorbidity between substance use and antisocial behavior [Lappalainen et al., 1998; Soyka et al., 2004], and the region on 17q12 near SLC6A4 has been identified in linkage and association studies of CD and substance dependence in adolescent samples [Stallings et al., 2005]. This is consistent with findings in the present study showing an increase in maximum NPL at the DS17S1531 locus for the OSA using proportion of family members with CD as the subsetting variable. Polymorphisms in SLC6A4 have more frequently been associated with alcohol dependence in the presence of comorbidities such as ASPD [Sander et al., 1998], early onset alcoholism with impulsivity and violent behavior [Hallikainen et al., 1999], behavioral disinhibition and negative affect in children of alcoholics [Twitchell et al., 2001], and depression and suicide [Gorwood et al., 2000]. SLC6A4 may be related to alcohol consumption and craving, and possibly, impulsivity and novelty-seeking [Sander et al., 1998; Hammoumi et al., 1999], which may increase the risk of SUD indirectly. Thus, further work examining this region at 17q12 for a common vulnerability to both substance use and antisocial behavior is warranted.
In our initial linkage analysis of affected siblings from the combined wave 1 and wave 2 samples, the present study did not replicate previous reports of linkage to alcohol related phenotypes using the COGA sample that have been reported at specific locations on chromosomes 1 [Reich et al., 1998; Nurnberger et al., 2001; Corbett et al., 2005], 2 [Reich et al., 1998; Dick et al., 2002], 3 [Foroud et al., 2000; Dick et al., 2002], 7 [Reich et al., 1998; Foroud et al., 2000; Bierut et al., 2004], and 8 [Corbett et al., 2005], or the relatively well-replicated findings on chromosome 4 near the alcohol dehydrogenase and GABAA receptor gene clusters [Reich et al., 1998; Williams et al., 1999; Saccone et al., 2000; Covault et al., 2004; Corbett et al., 2005; Schuckit et al., 2005; Dick et al., 2006]. Nor did we replicate findings on chromosomes 2 and 10 recently reported for a combined quantitative measure of average alcohol and illicit substance dependence criteria [Agrawal et al., 2008]. The most likely reason for our failure to replicate these prior results is that we used a different measure of SUD than other studies. For example, although a number of studies using different alcohol-related phenotypes have identified linkage regions on chromosome 1, the locations of the linkage peaks can vary from study to study, depending on the phenotype used in the analysis [Reich et al., 1998; Foroud et al., 2000; Schuckit et al., 2001; Bierut et al., 2004; Corbett et al., 2005]. While studies using a strict criteria for alcohol dependence found evidence for linkage on chromosome 7 at markers D7S1793 and D7S821 [Reich et al., 1998; Foroud et al., 2000], a study combining alcohol dependence with comorbid heavy nicotine use failed to find LOD scores >1.0 at these same locations [Bierut et al., 2004], and a different study using a model of nested alcohol dependence criteria failed to find any linkage on chromosome 7 at all [Corbett et al., 2005]. In the present study, we did observe an initial NPL score of 1.88 at D7S1790 (0 cM) from the Merlin linkage analysis, which replicates a previous finding at this marker and location showing a LOD score of 1.31 for alcohol dependence in the COGA sample [Williams et al., 2005]. Moreover, a region near D7S821 was identified as a possible linkage site in three of the four OSA (see below).
On the other hand, we saw no evidence of linkage for the alcohol dehydrogenase and GABAA clusters on chromosome 4 using our combined alcohol or drug dependence phenotype. Although lack of linkage to alcohol dehydrogenase may be due to the combined alcohol and illicit SUD phenotype, GABRA2 has been previously linked to both illicit substance dependence and alcohol dependence in the COGA sample (although the latter finding occurred only among individuals with comorbid illicit drug dependence) [Agrawal et al., 2006]. Our results are consistent, however, with results from a subsequent analysis of COGA data based on quantitative SUD phenotypes, including a definition of SUD that combined the average number of DSM-IV dependence criteria across both alcohol and drug dependence [Agrawal et al., 2008], which also failed to find linkage on chromosome 4. This latter study did, however, find LOD scores >2.0 on chromosomes 2 and 10 for their combined SUD phenotype, which we have not replicated in the present study. It is possible that our analyses, which were based on binary coding of dependence to alcohol or any illicit substance using only affected sib pairs, was less powerful than Agrawal's quantitative approach [Agrawal et al., 2008], which also allowed for the inclusion of more family members in the analyses.
We further note that our analyses were conducted on the combined wave 1 and wave 2 samples. In a number of prior instances (e.g., linkage on chromosomes 3 and 4), significant peaks are found either in the wave 1 (screening) or wave 2 (replication) samples, but not both [Reich et al., 1998; Foroud et al., 2000]. In a previous COGA study using maximum number of drinks as the phenotype, whole-genome linkage analyses revealed that only chromosome 4, around areas related to the alcohol dehydrogenase gene cluster, showed significant linkage in both the wave 1 and wave 2 samples [Saccone et al., 2000]. If we had analyzed each wave independently, we might have uncovered different suggestive linkage peaks. However, finding suggestive linkage in one sample but not another would only have increased our chances of spurious results. Moreover, although the sampling strategy differed slightly across waves, few meaningful differences across the two samples have been observed with respect to rates of SUD, other psychopathology, or comorbidity of SUD [Bierut et al., 2004], so the reason for different patterns of results across waves in previous studies is not well understood. Finally, prior studies have also varied in terms of the sibling pairs selected (i.e., all sibling pairs vs. sibling pairs in which both parents have been genotyped; independent vs. non-independent pairs), as well as in the choice of statistical analysis (e.g., parametric vs. non-parametric; two-point vs. multipoint linkage), both of which have contributed to inconsistencies both across studies, and sometimes within a given study.
An Ordered Subsets Approach to Controlling Genetic Heterogeneity
In addition to performing standard linkage analysis with a new measure of SUD, this study also sought to determine whether an OSA approach to linkage analysis would reduce genetic heterogeneity and allow us to discover novel regions of interest. Although the OSA analyses failed to detect any statistically significant changes in NPL scores, the results from these analyses do suggest that using an ordered subsets approach may increase signals for linkage, as a number of peak NPL scores were greater than 3.0. The strongest evidence for potential linkage in our study (NPL = 3.52) was detected on chromosome 7 near marker D7S1795 (27.3 cM), among a subset of 70 of the 241 families with the highest scores on the quantitative measure of child antisocial behavior. There are numerous genes in the vicinity of this marker, including most immediately DNAH11 (a dynein chain subunit), CDCA7L (which is associated with cell division and the cell cycle), and RAPGEF5 (a rap guanine nucleotide exchange factor). However, given that none of the other OSA analyses identified this location as the location of peak NPL, despite the fact that the four measures of antisocial behavior we used as subsetting variables in the OSA analyses were strongly related, we caution that this may be a spurious result. Thus, replication of this finding is warranted.
However, we note that an interesting trend in this study is that OSA analysis of chromosome 7 using the other three measures of antisocial behavior as subsetting variables all identified max NPL scores at position 127.5 cM (new max NPL scores range from 2.49 to 2.95). This location is near marker D7S821, which has been implicated as a potential site for linkage in a prior study of alcohol dependence using both the wave 1 and combined wave 1 and wave 2 samples [Foroud et al., 2000]. Additionally, this location is between markers D7S1870 (112 cM) and D7S1799 (145 cM), which have also been linked to alcohol dependence in other COGA studies (LOD scores = 1.15 and 1.13, respectively) [Williams et al., 2005]. Interestingly, in the present analyses, the max NPL at the D7S821 location was obtained using an OSA that began with the full sample and systematically deleted the most antisocial families. This suggests that this location might be near a gene related to “pure” SUD, that is SUD that is unaccompanied by antisocial behavior. Other regions of chromosome 7 that we did not identify in this study have also been linked to alcohol-related phenotypes in previous studies [Reich et al., 1998; Foroud et al., 2000; Bierut et al., 2004]. Thus, overall, the results from this study, combined with prior linkage studies of the COGA sample, suggest that chromosome 7 may have multiple genes related to different aspects of SUD.
Limitations
The NPL scores generated using family-based composites of quantitative measures of antisocial behavior as the subsetting variables were higher than the respective NPLs using the proportion of family members with CD or ASPD diagnoses as the subsetting variable, suggesting that the power of OSA may be increased when antisocial behavior is considered on a continuum. However, we caution that many of the chromosomal locations for the new peak NPL scores did not replicate across analyses, and a number of the newly identified locations were based on small numbers of families. In addition, it is unclear how well these newly identified peaks from the OSA would replicate in different statistical analyses. For example, Reck et al. 2005 compared different covariate-based analyses of alcohol dependence in Wave 1 COGA using age of onset, cigarette use, and two electrophysiological measurements as the subsetting covariates and found little agreement among covariate-based linkage statistics across the various statistical programs, although the OSA program we used (FLOSS) was not tested. FLOSS estimates the statistical significance of the change in NPL score from the OSA based on bootstrapping techniques. However, FLOSS does not provide a statistical estimate of the significance of the new NPL score itself. In addition, FLOSS only produces output from the highest NPL score identified across the chromosome, which may occur by chance in a small number of families at any location of the chromosome. Thus, it is not currently possible to use FLOSS to calculate a new NPL score based on reduced genetic heterogeneity at a specific region of a priori interest.
In addition to potential limitations of the current OSA approach, there are other limitations of study design, which may have impacted our results. First, our analyses were based on a phenotype that combined both abuse and dependence criteria as well as alcohol and illicit substances. Not only may this have lead to an inconsistency of our results with prior studies, the combining of both alcohol and illicit substances may have inadvertently increased the heterogeneity of our outcome phenotype, which may have reduced the ability of the OSA to control for genetic heterogeneity. Unfortunately, the number of individuals in the COGA data set who met criteria for illicit substance use disorder without a history of alcohol use disorder (N = 78) was too small to analyze independently. However, future studies could examine whether OSA using antisocial behavior as the ordering covariate reveals new linkage peaks for a more stringent definition of alcohol dependence. Second, the exclusion criteria for COGA included omitting individuals who were current or habitual IV drug users. This may have limited the numbers of individuals meeting criteria for certain illicit substances, which may have reduced statistical power. Third, the COGA sample itself has a high rate of probands drawn from psychiatric and substance-abuse clinics, as a method of maximizing linkage signals to SUD, especially alcohol use disorders. Thus, different results may occur for individuals drawn from largely community-based samples. Finally, the present study was based on an OSA using linkage analysis. Association analyses can be more powerful than linkage strategies, and may have revealed a different pattern of results. However, whole-genome wide association studies require larger samples to be truly informative, and may provide spurious positive results due to ethnic stratification, a drawback that does not exist in our family-based linkage analyses. In summary, the present study is one of the first published studies using an OSA approach to determine whether accounting for family history of antisocial behavior could reveal novel locations of possible linkage to general liability to SUD.
Acknowledgements
Work for the present study was supported by a National Institute on Drug Abuse grant to Dr. Tsuang (5R01DA018662). The COGA study is the result of an NIH initiative and was funded by the National Institute on Alcohol Abuse and Alcoholism (U10AA0503 and AA00231). The Collaborative Study on the Genetics of Alcoholism (COGA) (Co-Principal Investigators: L. Beirut, H. Edenberg, V. Hesselbrock, B. Porjesz) includes nine different centers where data collection, analysis, and storage take place. The nine sites and Principal Investigators and Co-Investigators are: Howard University (R. Taylor); Indiana University (H. Edenberg, J. Nurnberger Jr, P.M. Conneallly, T. Foroud); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasay); State University of New York Health Sciences Center at Brooklyn (B. Porjesz, H. Begleiter); University of California at San Diego (M. Schuckit); University of Connecticut (V. Hesselbrock); University of Iowa (R. Crowe, S. Kuperman); Washington University in St. Louis (T. Reich, C.R. Cloninger, J. Rice, A. Goate). Zhaoxia Ren serves as the NIAAA Staff Collaborator. We would also like to acknowledge the feedback on earlier drafts of this manuscript by B. Kia-Keating, EdD, at the University of California, San Diego.