Differential dopamine receptor D4 allele association with ADHD dependent of proband season of birth†
Please cite this article as follows: Brookes KJ, Neale B, Xu X, Thapar A, Gill M, Langley K, Hawi Z, Mill J, Taylor E, Franke B, Chen W, Ebstein R, Buitelaar J, Banaschewski T, Sonuga-Barke E, Eisenberg J, Manor I, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Faraone SV, Asherson P. 2007. Differential Dopamine Receptor D4 Allele Association With ADHD Dependent of Proband Season of Birth. Am J Med Genet Part B 147B:94–99.
Abstract
Season of birth (SOB) has been associated with attention deficit hyperactivity disorder (ADHD) in two existing studies. One further study reported an interaction between SOB and genotypes of the dopamine D4 receptor (DRD4) gene. It is important that these findings are further investigated to confirm or refute the findings. In this study, we investigated the SOB association with ADHD in four independent samples collected for molecular genetic studies of ADHD and found a small but significant increase in summer births compared to a large population control dataset. We also observed a significant association with the 7-repeat allele of the DRD4 gene variable number tandem repeat polymorphism in exon three with probands born in the winter season, with no significant differential transmission of this allele between summer and winter seasons. Preferential transmission of the 2-repeat allele to ADHD probands occurred in those who were born during the summer season, but did not surpass significance for association, even though the difference in transmission between the two seasons was nominally significant. However, following adjustment for multiple testing of alleles none of the SOB effects remained significant. We conclude that the DRD4 7-repeat allele is associated with ADHD but there is no association or interaction with SOB for increased risk for ADHD. Our findings suggest that we can refute a possible effect of SOB for ADHD. © 2007 Wiley-Liss, Inc.
INTRODUCTION
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most prevalent and heritable childhood behavioral disorders. The disorder is characterized by an onset of age-inappropriate hyperactivity, impulsivity and inattentiveness before the age of 7 years [American Psychiatric Association, 1994]. Familial risk is established with an estimated sibling risk ratio (λs = risk to siblings of ADHD probands/population risk) for broadly defined ADHD of around threefold to fourfold [Faraone and Doyle, 2000]. Twin studies support the view that genetic factors are the major influence on familial risk with heritability estimates for ADHD symptom scores consistently reported to be in the region of 60–90% [Thapar et al., 1999]. In general these studies find little evidence of shared environmental influences on familiarity, although the role of environment may still be pivotal acting through mechanisms of gene–environment interaction. Progress in identifying some of the genes involved in ADHD susceptibility has been relatively fruitful over the past decade by screening genetic variants that lie within or close to genes that regulate neurotransmitter systems, particularly dopamine pathways.
One of the first genetic markers reported to be associated with ADHD was the 7-repeat allele of a variable number tandem repeat (VNTR) polymorphism located within exon 3 of the Dopamine D4 Receptor gene (DRD4) [LaHoste et al., 1996]. Subsequent studies replicated this finding although several investigations have also reported negative findings [Faraone et al., 2005]. A recent meta-analysis of available data concluded that there was a small but significant effect of the DRD4 polymorphism on risk for ADHD, with a pooled odds ratio of 1.34 (95% CI 1.23–1.45, P = 2 × 10−12) [Li et al., 2006].
Although, genetic risk factors are prominent in the development of ADHD, environmental risks are also thought to be important, acting through gene–environmental interactions. Associated environmental risks for ADHD include low birth weight and maternal use of alcohol and tobacco during pregnancy [Mick et al., 2002a,b]. More recently specific gene–environment interactions have been reported between genotypes of the dopamine transporter gene and maternal use of tobacco during pregnancy on levels of hyperactive-impulsive behavior [Kahn et al., 2003] and maternal use of alcohol on risk for ADHD [Brookes et al., 2006b]. Other research suggests that gene–environment interactions may increase the rates of antisocial behavior among ADHD probands, rather than having a main effect on risk for ADHD. For example the effects of a catechol-O-methyltransferase (COMT) gene variant and birth weight on the risk of early-onset antisocial behavior in children with ADHD [Thapar et al., 2005].
Another environmental measure that has been investigated is the effect of season of birth (SOB). This association is not well established, with the two studies reporting on this variable in relation to ADHD giving contradictory findings. Mick et al. [1996] concluded that winter birth was associated with ADHD in individuals with learning difficulties, ADHD without psychiatric comorbidities, and ADHD with family history of the disorder. In contrast, an earlier study concluded that spring and summer births increased risk for neurodevelopment disorders, including ADHD [Liederman and Flannery, 1994].
More recently, a report on a potential interaction between SOB and the DRD4 exon 3 polymorphism was published [Seeger et al., 2004]. In a sample of 64 children with comorbid hyperkinetic disorder and conduct disorder (HD + CD) and a matched control sample of 163 children, no main affects of the DRD4 polymorphism or SOB were observed. However, it was found that children with HD + CD born in the winter, had significantly fewer 7-repeat alleles (12.5%) compared to those born in the summer (50%, P = 0.001, OR = 7). This suggested that the 7-repeat allele might be a risk factor for ADHD only for those born in the summer months. The control population exhibited the opposite relationship between SOB and the 7-repeat allele, with those born in winter having a higher allele frequency of 7-repeat alleles (43.7%) in comparison to those being born in the summer (26.1%, P = 0.019, OR 2.2).
Discrepancies between the various studies on SOB and ADHD mean that no firm conclusions can be reached at this time. We therefore set out to establish whether in a large collaborative set of clinical ADHD samples there was any evidence for the association of SOB with ADHD, and whether SOB interacts with the DRD4 exon 3 VNTR polymorphism in the risk for the disorder. Allowing the confirmation or rejection of the hypothesis that SOB may be a risk factor for ADHD.
In the course of this research, we also considered whether plausible biological arguments could be made for the association between ADHD and SOB. For example SOB might be a proxy for risk factors such as viral infections or amount of daylight exposure during gestation or birth weight [Liederman and Flannery, 1994; Mick et al., 1996]. Those born in the winter spend most of their gestation period in the summer months while conversely those born in the summer have the majority of their gestational time in the winter months. Maternal disorders such as seasonal affective disorder, which might confer prenatal risk, show seasonal variation [Chotai et al., 2003; McGrath et al., 2005; Amons et al., 2006]. In relation to the DRD4, the 7-repeat allele could influence mating behavior in mammals and the associated pattern of mating may be part of a natural cycle with seasonal variation observed in the general population. The dopamine system has been highly implicated in the development of ADHD and it has been discussed that this system is influenced by exogenous factors, such as hours of sunlight, in creating an endogenous daily rhythm of dopamine receptor binding, therefore giving credence that hours of daylight could impact on the dopamine system [Naber et al., 1981]. Furthermore, the hormone melatonin is secreted from the pineal gland, in a cyclic rhythm. This rhythm is entrained by the length of daylight the individual is exposed to, and alters the timing of mammalian circadian rhythms [Brzezinski, 1997]. Melatonin is synthesized from serotonin by the enzyme N-acetyl-transferase, which is entrained by the day length cycle, and is more active during dark periods. Therefore, melatonin production is highest during the night and lowest during the day [Reppert and Weaver, 1995]. Melatonin is known to inhibit dopamine release in numerous brain regions including the striatum and dopamine is thought to inhibit the production of melatonin via the DRD4 [Zisapel and Laudon, 1983; Zisapel et al., 1983; Zawilska and Nowak, 1994; Tosini and Dirden, 2000; Zisapel, 2001]. Finally, melatonin can also pass from the mother via the placenta to the fetus, entraining the fetus' circadian rhythm [Goldman, 2003].
We can therefore see that is not difficult to derive biologically plausible explanations for the possible influence of SOB on risk for ADHD and interaction with components of the dopamine system. However, on the basis of the data presented here, we conclude that it is far more likely that there is no effect of SOB on risk for ADHD.
METHODS
Four independent samples were used, collected by groups in London, Cardiff, Dublin, and the International Multi-centre ADHD Gene (IMAGE) project. The IMAGE project is a multi-site site with samples collected in Belgium, England, Germany, Holland, Ireland, Israel, Spain, and Switzerland. Children taking part in these studies were all of white European origin and consisted predominantly of male children with combined subtype ADHD and with DNA available from both parents (Table I). The individual groups gathered DRD4 exon 3 VNTR genotypes and date of birth information separately and data was sent for this analysis to Keeley Brookes in London. The association findings with DRD4 for these groups have previously been reported [Hawi et al., 2000; Holmes et al., 2000; Mill et al., 2001] with only the large IMAGE sample exhibiting a trend for excess in transmission of the 7-repeat allele from heterozygote parents to their affected offspring [Brookes et al., 2006a]. Clinical procedures for making research diagnoses of ADHD across the different studies used comparable approaches since probands were all ascertained from specialist ADHD clinics and research interviews were the main source of data capture. DSM-IV operational criteria were applied in each case however no direct comparisons were made to check reliability of diagnosis between the different sites and slightly different protocols applied. Detailed descriptions of the sample ascertainment and assessment procedures can be found in the original articles for the DRD4 VNTR [Hawi et al., 2000; Holmes et al., 2000; Mill et al., 2001; Brookes et al., 2006a] and the measures used in each study are listed in Table I.
| Sample | N trios | % Males | Age range (mean; SD) | Clinical procedure | Diagnosis | Comorbidity (ODD and CD) (%) |
|---|---|---|---|---|---|---|
| London | 137 | 90 | 5–15 (10.41; 2.34) | Conners, CAPA, HYPESCHEME | DSM IV | 53.3 |
| Cardiff | 128 | 92 | 6–12 (9.3; 1.8) | CAPA | DSM IV | 88 |
| Dublin | 174 | 85 | 4–14 (11.73; 3.9) | Conners, CBCL, ACTeRS | DSM IV | 80 |
| IMAGE | 671 | 89 | 5–15 (11.2; 2.7) | Conners, SDQ, PACS | DSM IV | 78.7 |
In this study, we investigated each sample separately before combining the data into a single set of 1,110 ADHD-parent trios. Each cohort was stratified into two subsets dependent on the date of birth of the proband. Following the seasonal definitions used by Seeger et al. [2004] those born between the 22nd March and 22nd September were classified as the summer season group, whereas those born between the 23rd September and the 21st March were classified as the winter season group. Each seasonal subset was analyzed using the transmission disequilibrium test (TDT) implemented in the UNPHASED program [Dudbridge, 2003; http://portal.litbio.org/Registered/Menu/]. Allele-specific tests of association were calculated from the number of transmissions and non-transmission of the 2-, 4-, and 7-repeat alleles from heterozygote parents to their affected offspring for the two seasonal groups.
Significant differences between the seasonal groups were tested using the Chi-square test on the number of transmitted (T) and un-transmitted (NT) transmissions for each allele. Since there are several alleles that could show transmission ratio differences between the two seasons, we adjusted for the number of tests by permuting the data to derive an empirical distribution of P-values in the following way. The SOB group status for each family was permuted a total 10,000 times and the T/NT ratio and significance re-calculated for each allele. We then took the most significant Chi-square value from the analysis of the various alleles, to derive the empirical distribution of maximum Chi-square values. This enabled us to determine how frequently the most significant Chi-square values occur by chance in our sample.
For the combined dataset, 95% confidence intervals were derived using the t-test statistic (T-statistic [Mitchell et al., 2003]). The T-statistic represents the TDT information in terms of the proportion of transmitted alleles to the total number of transmissions from heterozygote parents. Under the null hypothesis of no association the proportion of the transmitted alleles to the total number of transmissions is expected to be 0.5. The general formula for confidence intervals is then applied: 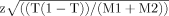 .
.
RESULTS
Season of Birth Effect
UK Census data over the last decade (www.statistics.gov.uk/statbase/Product.asp?vlnk=5768) of 6,919,604 live births during the period 1994–2004 suggests that there is no bias in the SOB for babies born in the UK. Close to 50% of live births occurs in the summer months (March to August = 50.72%) and in the winter months (September to February = 49.28%). Since the ADHD samples are predominantly male (>95%), we further investigated whether males were predominantly born in either the summer months or the winter months. The UK census data showed no difference in the proportions of male birth between seasons with near 50% of males being born in the summer and winter seasons.
The IMAGE sample consists of a combination of ADHD probands ascertained from around Europe and a subset from Israel, however we did not have access to census data from each of the different countries involved. Never-the-less, we reasoned that the Irish and UK samples would be closely similar due to geographic proximity, and this would also be the case for the majority of the IMAGE samples that were derived from Northern European countries. We therefore, looked to see if there was evidence of heterogeneity for SOB between the various countries. Since no evidence of heterogeneity for the percentage of births in each season was observed between sites (P = 0.27), we considered that the UK census data would be sufficient to establish the control SOB rates for this study.
Therefore, the proportions of ADHD children born in the different seasonal groups in the four independent samples were assessed to see if they deviated from the census data (Table II). In all four samples a numerically higher proportion of ADHD probands were born during the summer months compared to the winter months, although this difference when compared to the control census data was only significant for the London sample (P = 0.05). Combining all four datasets together we found that 54.1% of cases were born in the summer months compared to 50.72% in the census data, a small but nominally significant difference of 3.3% (P = 0.026, OR = 1.07, 95% CI 1.10–1.13).
| Sample | Summer births | Winter births | Chi-square P-value |
|---|---|---|---|
| London | 59.1% (n = 81) | 40.9% (n = 56) | 0.049 |
| Cardiff | 55.5% (n = 71) | 44.5% (n = 57) | NS |
| Dublin | 54.0% (n = 94) | 46.0% (n = 80) | NS |
| IMAGE | 52.8% (n = 354) | 47.2% (n = 317) | NS |
| Combined | 54.1% (n = 600) | 45.9% (n = 510) | 0.026 |
- Significance values compared to UK census data.
The number of births across all months between the UK census data and the combined ADHD datasets were inspected (Fig. 1). Overall there appears to be no clear pattern, with a small increase in the percentage of births in the ADHD sample observed for the months of May, June, and August and a small decrease during April, September, and December. In order to derive a better estimate of the significance of the SOB association with ADHD and adjust for the effect of outliers, we performed 10,000 permutations of the month of birth status for each proband and reconstituted SOB group membership based on the permutated datasets to derive an empirical significance for the association. The result was similar to the nominal observation and did not alter our conclusions (P = 0.028). While a significant difference is observed for SOB and case/control status, the P-value is not hugely significant. This Paucity of significance points to an extremely marginal effect considering the total sample size in this analysis (6,920,716).
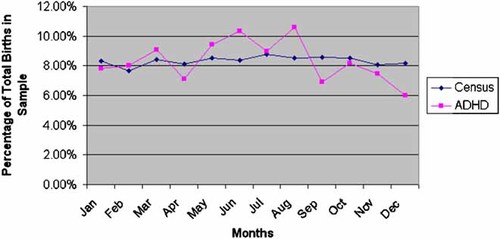
Percentage of births within each calendar month, starting with January, in each of the control samples and ADHD combined sample. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Association Between ADHD and the 7-Repeat Allele of DRD4
We re-analyzed the four datasets and the combined dataset for association between ADHD and the DRD4 VNTR (Table III). The dataset used in this analysis had slightly lower numbers of probands than the original published reports due to the lack of date of birth information in a few cases, explaining minor differences from the original reports. The data show a small but non-significant excess transmission of the 7-repeat allele in the London, Dublin, and IMAGE datasets, which was significant in the combined dataset (OR = 1.18, P < 0.04). Overall there was no global significance for the association between the DRD4 VNTR and ADHD taking all alleles into account.
| Dataset (global P-value) | Allele | T | NT | OR | P-value |
|---|---|---|---|---|---|
| London (P = 0.20) | 2 | 18 | 17 | 1.08 | 0.87 |
| 4 | 36 | 44 | 0.9 | 0.37 | |
| 7 | 29 | 22 | 1.2 | 0.33 | |
| Cardiff (P = 0.34) | 2 | 13 | 19 | 0.68 | 0.29 |
| 4 | 56 | 44 | 1.27 | 0.23 | |
| 7 | 38 | 39 | 0.97 | 0.91 | |
| Dublin (P = 0.12) | 2 | 25 | 15 | 1.6 | 0.11 |
| 4 | 54 | 77 | 0.74 | 0.04 | |
| 7 | 50 | 38 | 1.24 | 0.20 | |
| IMAGE (P = 0.22) | 2 | 88 | 96 | 0.92 | 0.56 |
| 4 | 271 | 293 | 0.92 | 0.35 | |
| 7 | 198 | 166 | 1.19 | 0.09 | |
| Combined (P = 0.53) | 2 | 144 | 147 | 0.97 | 0.86 |
| 4 | 417 | 458 | 0.92 | 0.17 | |
| 7 | 315 | 265 | 1.18 | 0.04 |
- No association of the marker is found in any of the samples; only when the samples are combined does the over-transmission of the hypothesized risk 7-repeat allele reaches significance (P = 0.04) with an odds ratio of 1.18.
Interaction Between SOB and DRD4
Table IV lists the TDT transmission ratios for each of the DRD4 alleles grouped by SOB. In addition the table displays nominal significance values of the difference in allele transmissions between the two SOB groups and the global significance values for each SOB group. In the combined datasets the overall association between the VNTR and ADHD was significant in the winter (P = 0.05) but not in the summer.
| Allele | Summer | Winter | Summer (global-P) OR | Winter (global-P) OR | Nominal P-values | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | NT | T | NT | Summer | Winter | Differences | ||||
| London | (P = 0.48) | (P = 0.068) | ||||||||
| 2 | 15 | 7 | 3 | 10 | 2.14 | 0.30 | 0.09 | 0.05 | 0.009 | |
| 4 | 19 | 30 | 17 | 14 | 0.60 | 1.20 | 0.12 | 0.59 | 0.16 | |
| 7 | 18 | 17 | 11 | 5 | 1.06 | 2.20 | 0.87 | 0.13 | 0.25 | |
| Cardiff | (P = 0.05) | (P = 0.29) | ||||||||
| 2 | 7 | 5 | 6 | 14 | 1.4 | 0.43 | 0.56 | 0.07 | 0.11 | |
| 4 | 33 | 24 | 23 | 20 | 1.8 | 1.15 | 0.23 | 0.65 | 0.66 | |
| 7 | 19 | 24 | 19 | 15 | 0.79 | 1.27 | 0.45 | 0.49 | 0.31 | |
| Dublin | (P = 0.2) | (P = 0.13) | ||||||||
| 2 | 11 | 6 | 14 | 9 | 1.8 | 1.56 | 0.23 | 0.30 | 0.8 | |
| 4 | 32 | 38 | 22 | 39 | 0.84 | 0.56 | 0.47 | 0.03 | 0.26 | |
| 7 | 27 | 22 | 23 | 16 | 1.23 | 1.44 | 0.48 | 0.26 | 0.7 | |
| IMAGE | (P = 0.21) | (P = 0.27) | ||||||||
| 2 | 56 | 51 | 32 | 45 | 1.1 | 0.71 | 0.63 | 0.14 | 0.15 | |
| 4 | 146 | 157 | 125 | 136 | 0.9 | 0.92 | 0.53 | 0.50 | 0.95 | |
| 7 | 97 | 86 | 101 | 80 | 1.13 | 1.26 | 0.42 | 0.12 | 0.59 | |
| Combined | (P = 0.28) | (P = 0.05) | ||||||||
| 2 | 89 | 69 | 55 | 78 | 1.29 | 0.71 | 0.11 | 0.05 | 0.01 | |
| 4 | 230 | 249 | 187 | 209 | 0.92 | 0.89 | 0.39 | 0.27 | 0.82 | |
| 7 | 161 | 149 | 154 | 116 | 1.08 | 1.33 | 0.50 | 0.02 | 0.22 | |
In each sample the transmission of the 2-repeat allele was found to be numerically higher in the summer group compared to the winter group, with over-transmission in the summer and under-transmission in the winter for all samples apart from the Dublin dataset. Under-transmission of the 2-repeat allele in the winter showed nominal significance in the London (OR = 0.3, P = 0.05) and combined (OR = 0.71, P = 0.05) datasets, however over-transmission in the summer was not significant.
The difference in transmission ratios between the summer and winter groups was nominally significant for the London sample (P = 0.01) and for the combination of all four data sets (χ2 = 6.48, P = 0.01). However, the difference statistic for the 2-repeat allele is one of three Chi-square values, since we also examine transmission of the common 4- and 7-repeat alleles representing somewhere between two to three independent tests, and we therefore need to take into account the distribution of maximum Chi-square values under the null hypothesis of no association. Using empirical methods, we determined that the significance of an observed P-value of 0.0149 as the most extreme value, occurs approximately 19% of the time and therefore conclude that this is likely to be a chance observation.
In contrast, the 7-repeat allele showed numerically higher transmission in the winter group compared to the summer group in each of the independent samples. The over-transmission of the 7-repeat allele in the winter, but not in the summer, reached nominal significance in the combined dataset (OR = 1.33, P = 0.02). The difference in transmission ratios for the 7-repeat allele between the two SOB groups was not however significant.
The results of the analysis of the combined dataset are shown in Figure 2, illustrating the difference in transmission of the 2- and 7-repeat alleles in the SOB groups using the T-statistic, the proportion of transmitted alleles. These data show that there is a significant over-transmission of the 7-repeat allele and under transmission of the 2-repeat allele in the winter months. In contrast, in the summer months none of the alleles show a significant association with ADHD; confidence intervals on the T-statistic overlapping with the null hypothesis of 0.5.
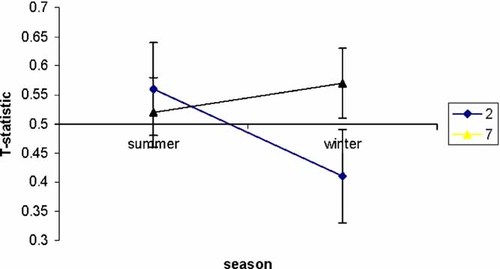
Line diagram of the T-statistic, with 95% confidence intervals of the 2- and 7-repeat alleles between seasonal subsets for the combined dataset. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Examination of the combined dataset month by month shows that the evidence for increased transmission of the 2-repeat allele during summer months can be attributed to a single finding in June. Similarly, there is no clear pattern of effect for the 7-repeat allele. Due to the small number of samples in each month, the findings are “noisy” and are therefore likely to represent random sampling error rather than a true effect of SOB.
DISCUSSION
The motivation for this article came from the current interest in gene by environment interactions on risk for psychiatric disorders [Moffitt et al., 2005]. Previous articles have suggested that SOB might be associated with ADHD, indicating the influence of environmental risks showing seasonal variation, such as viral infections or number of hours exposed to sunlight [Liederman and Flannery, 1994; Mick et al., 1996]. In addition, one article reported the possible interaction between SOB with genotype of DRD4 on risk for ADHD [Seeger et al., 2004]. These previous analyses failed to reach firm conclusions due to the limited amount of data reported and discrepancies in the findings between various studies, therefore we set out to confirm or challenge these previous findings.
We have now investigated SOB variation in four independent ADHD family samples consisting of 1,110 clinically ascertained ADHD probands. We observed a small 3.3% but significant increase in the number of ADHD probands born in the summer months compared to a large control sample of live births born in the UK over the decade 1994–2004. Inspection of the month-by-month data did not however suggest a consistent pattern and the mismatch in size of the control data to the case data means that the study is over-powered (i.e. even a small percent difference would be significant). We therefore conclude that the effect is likely to represent the effect of random sampling error and is unlikely to represent a biological mechanism.
We further investigated the interaction between SOB and DRD4 alleles and risk for ADHD. Overall the combined dataset confirms the allele-specific hypothesis established by the results of meta-analyses of world data, for a small but significant association of the 7-repeat allele in the exon 3 VNTR with ADHD [Faraone et al., 2005]. The average odds ratio we observed is in keeping with previous published estimates. We were very interested in the pattern of findings we observed with increase transmission of the 2-repeat allele in the summer and decreased transmission in the winter months, which gave a nominally significant association in transmission ratios between the two SOB groups. However, since this was not an a priori hypothesis, it is appropriate to adjust for the transmission ratio differences for the three common alleles that we investigated. Inspection of the Chi-square distribution for maximum Chi-square indicated that the true significance level is around 0.19 and is therefore not worth further investigation. For the 7-repeat allele we observed significant allele-specific association in the winter but not the summer months. However this difference did not reach even nominal significance. We therefore conclude that the main observation is the previously identified over-transmission of the 7-repeat allele with no evidence for an interaction with SOB. Our data proposes the rejection of an effect of SOB for increased risk for ADHD.
This conclusion seems highly plausible. These data show the opposite trend to that observed by Seeger et al. [2004] who concluded that the 7-repeat allele confers risk for ADHD in those born in the summer months, whereas our data shows increased risk from the 7-repeat allele in the winter months. Furthermore, the two studies that only considered the association with SOB found opposite trends [Liederman and Flannery, 1994; Mick et al., 1996]. One limitation of this study is that we have not been able to examine the specific clinical sub-groups of ADHD described in the previous research leaving open the possibility, although unlikely, that SOB effects are restricted to clinical sub-groups. The current study utilized DSM-IV combined subtype ADHD subjects, whereas to fully replicate the findings presented in the Seeger et al. [2004] study a sample of hyperkinetic probands with comorbid conduct disorder would have to be investigated.
Acknowledgements
We thank all the families who kindly participated in this research. Research was funded by the MRC, Wellcome Trust and ACTION research in the UK, the Health Research Board and Molecular Medicine Centre in Dublin. The IMAGE project is supported by NIH grant R01MH62873 to S.V. Faraone.