Biallelic Variant in LYSET Associated With Mucolipidosis II-Like Phenotype
ABSTRACT
Dysostosis multiplex is a skeletal dysplasia often associated with lysosomal storage disorders (LSDs) such as mucopolysaccharidoses (MPS) and mucolipidoses (ML). Recently, pathogenic variants in the LYSET gene have been linked to a novel disorder resembling mucolipidosis types II/III (MLII/III). We report two Iranian brothers with homozygous pathogenic variants in LYSET (c.197dupA) who exhibit clinical, enzymatic, and radiographic features strikingly similar to MLII. Our findings reinforce the similarity between LYSET-related phenotypes and MLII, aligning with previously described cases. We propose the term “LYSET-related mucolipidosis” to describe this disorder and emphasize the importance of including LYSET in the genetic diagnostic panel for MLII/III-like presentations.
Dysostosis multiplex encompasses a group of characteristic skeletal dysplasias found in lysosomal storage disorders (LSDs) such as mucopolysaccharidoses (MPS), mucolipidoses (ML), and related conditions. These skeletal abnormalities arise due to lysosomal dysfunction, leading to impaired degradation of macromolecules essential for normal cartilage and bone development. Dysostosis multiplex is characterized by hallmark radiographic features, including paddle-shaped ribs, vertebral flattening with anterior protrusions, and metaphyseal widening of long bones.
MLs, a subset of LSDs, are caused by defective lysosomal enzyme trafficking, leading to intracellular substrate accumulation. Mucolipidosis type II (MLII) is a severe, early-onset condition characterized by coarse facies, developmental delay, skeletal dysplasia, and elevated lysosomal enzyme activity in plasma and decreased activity in fibroblasts. In contrast, mucolipidosis type III (MLIII) presents later with milder symptoms. Both conditions result from variants in GNPTAB or GNPTG, which encode components of the GlcNAc-1-phosphotransferase enzyme responsible for lysosomal enzyme targeting via mannose-6-phosphate (M6P) modification.
Pathogenic variants in LYSET, also historically referred to as TMEM251 (transmembrane protein 251) or GCAF (golgi-associated coiled-coil domain-containing factor), have recently been associated with a phenotype closely resembling MLII/III. The gene was originally annotated as TMEM251, reflecting its role as a transmembrane protein. Subsequent studies elucidated its critical role in lysosomal enzyme trafficking, leading to the alternative designation LYSET (lysosomal enzyme trafficking factor), which highlights its biological function (Richards et al. 2022). The term GCAF was also proposed based on its localization and structural features (Zhang et al. 2022). Interestingly, they showed LYSET knock-out mice exhibited an MLII-like phenotype. Despite these nomenclature variations, LYSET has emerged as the preferred name because of its functional significance.
Ain et al. (2021) reported six patients with homozygous LYSET variants who exhibited features typical of MLII, including skeletal dysplasia, developmental delay, and elevated lysosomal enzyme activity in dried blood spots. These findings suggest that LYSET may play a critical role in lysosomal enzyme trafficking similar to GNPTAB and GNPTG.
In this report, we describe two additional patients with a homozygous LYSET variant and clinical features highly reminiscent of MLII. Our findings corroborate the phenotype described by Ain et al. and further establish the role of LYSET in MLII-like disorders.
The two patients, siblings from an Iranian family with consanguineous parents, presented with clinical features strikingly similar to MLII.
1 Patient 1
The proband was the first child of the family, born full-term via cesarean section. His birth parameters were within the normal range, but developmental milestones were significantly delayed. He achieved head control at 6 months, began walking at 2 years, and spoke his first words at 2.5 years.
At 2 years of age, he exhibited coarse facial features, including a narrow forehead, puffy eyelids, epicanthal folds, a low nasal bridge, prominent lips, and macroglossia (Figure 1B). His abdomen was protuberant, and hepatomegaly was noted. Joint contractures were present, involving the elbows, wrists, fingers, hips, knees, and ankles, along with thickened and tight skin (Figure 1). The radiographs have been described as showing dysostosis multiplex including scapular hypoplasia, paddle-shaped ribs, ovoid vertebrae, and metaphyseal widening of long bones, but were not available for our personal evaluation. Enzyme analysis demonstrated a pattern consistent with MLII (Table S1). Urinary mucopolysaccharides were normal. The patient succumbed to cardiac arrest at the age of 6 years.
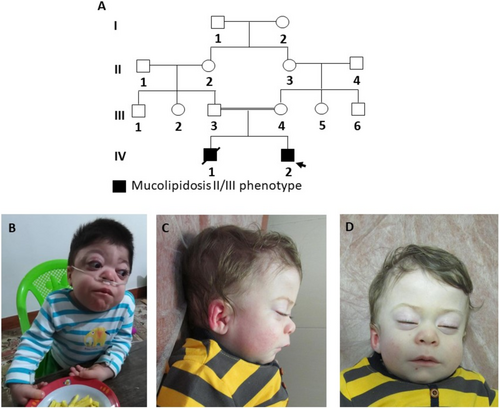
2 Patient 2
The proband's younger brother had similar birth parameters and a less severe developmental delay. He began walking at 16 months and speaking his first words at 13 months. By 18 months, his physical examination revealed mildly coarse facial features (puffy eyelids, low nasal bridge, anteverted nostrils), hepatomegaly, joint contractures, and thickened skin (Figure 1C,D). Although enzyme analysis was not performed, his clinical findings mirrored those of his elder brother.
Clinical features of our patients and those previously reported with biallelic variants in LYSET are shown in Table 1.
| Ain et al. (2021) | Present cases | |||||||
|---|---|---|---|---|---|---|---|---|
| Family 1 | Family 2 | Family 1 | ||||||
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | |
| Sex | F | F | M | F | M | F | M | M |
| Onset | 1 year | 1 year | 1 year | 1 year | 1 year | 6 months | Birth | Birth |
| Birth weight | NA | NA | NA | NA | NA | NA | 3700 | 3420 g |
| Birth length | NA | NA | NA | NA | NA | NA | NA | 51 cm |
| Birth head circumference | NA | NA | NA | NA | NA | NA | NA | 35 cm |
| Age at last examination | 24 years | 20 years | 17 years | 15 years | 13 years | 3 years | 18 months | 18 months |
| Height/SD score | −11.1 SD | −11.6 SD | −11.9 SD | −8.8 SD | −8.5 SD | NA | 76 cm at 18 months/−2 SD | 74 cm at 18 months/−2.67 SD |
| Hypotonia at birth | NA | NA | NA | NA | NA | NA | + | + |
| Progressive failure to thrive | NA | NA | NA | NA | NA | NA | + | + |
| Coarse features | + | + | + | + | + | + | + | + |
| Abdominal protruberance | + | + | + | + | + | − | + | + |
| Hepatomegaly | NA | NA | NA | NA | NA | NA | + | NA |
| Joint contractures | + | + | NA | NA | NA | NA | + | + |
| Delayed motor milestones | NA | NA | NA | NA | NA | NA | + | + |
| Cognition | Normal | Normal | Normal | Normal | Normal | NA | ID | Mild ID |
| Speech | Normal | Normal | Normal | Normal | Normal | 2–3 word | 2–3 words | 2–3 words |
| Skeletal dysplasia, multiplex dysostosis | + | + | + | + | + | + | + a | NA |
| Additional skeletal features | NA | NA | Long femoral neck, enlarged distal femoral epiphyses hemivertebrae, osteolysis | NA | Long femoral neck, enlarged distal femoral epiphyses, hemivertebrae, osteolysis | Hypoplastic glenoid fossa | − | NA |
| Recurrent respiratory infection | NA | NA | NA | NA | NA | + | + | − |
| Recurrent otitis media | NA | NA | NA | NA | NA | NA | + | − |
| Heart | NA | NA | NA | NA | NA | Small VSD | Cardiomegaly | NA |
| Skin | NA | NA | NA | NA | NA | NA | Thick and tight | Thick and tight |
| Death | Early 20s | Early 20s | − | − | − | − | 6 years | − |
| Other | − | − | − | − | − | Thinning of corpus callosum and ventriculomegaly | − | − |
| Variant in LYSET | c.133C>T homozygous | c.133C>T homozygous | c.133C>T homozygous | c.133C>T homozygous | c.133C>T homozygous | c.215dupA; p.(Tyr72Ter) homozygous b | c.197dupA; p.(Tyr72Ter) homozygous | c.197dupA; p.(Tyr72Ter) homozygous |
- Abbreviations: F, female; M, male; NA; not available.
- a Based on radiograph reports.
- b c.215dupA; p.(Tyr72Ter) is based on reference sequence NM_001098621.1 and is the same as c.197dupA; p.(Tyr72Ter) based on reference sequence NM_001098621.4.
In Patient 1, enzymatic analysis revealed elevated lysosomal hydrolase activity in plasma and marked reductions of lysosomal hydrolase activity in fibroblasts, a profile consistent with MLII (Table S1). Genetic analysis identified a homozygous c.197dupA frameshift variant (also known as c.215dupA) in LYSET (NM_001098621.4). This sequence change results in a premature translational stop signal in the LYSET gene (p.(Tyr66Ter)). Although this is not anticipated to result in nonsense-mediated decay, it is expected to disrupt the last 97 amino acids of the LYSET protein. This variant is absent from population databases, indicating it is not a common benign variant. Furthermore, it has been listed in ClinVar as pathogenic (variation ID: 1120021 as of May 29, 2021) though without assertion criteria provided. Based on its predicted impact on protein function and absence in general populations, this variant is classified as likely pathogenic.
We subsequently performed Sanger sequencing of the LYSET gene using DNA extracted from stored fibroblasts of the older brother (Patient 1). The primers were 5′-TGGAGATAAAGCAAGTACATTTGG-3′ and 5′-CCTGAGATCTGTGAAGAAACTGC-3′. This analysis confirmed the presence of the homozygous c.197dupA variant of the LYSET gene (NM_001098621.4) (Figure 1).
This variant has previously been reported in an Iranian patient with severe skeletal dysplasia and short stature as described by Ain et al., suggesting a potential founder effect.
Our report highlights two additional cases of homozygous LYSET pathogenic variants leading to a phenotype highly similar to MLII. The clinical features in our patients—including dysostosis multiplex, coarse facial features, hepatomegaly, joint contractures, developmental delay, and elevated lysosomal enzyme activity in plasma and decreased activity in fibroblasts—are consistent with the cases described by Ain et al. This striking similarity reinforces the hypothesis that LYSET variants disrupt lysosomal enzyme trafficking, producing a phenotype indistinguishable from MLII.
Ain et al. suggested that LYSET encodes an essential cofactor for GlcNAc-1-phosphotransferase, the enzyme disrupted in MLII/III. This mechanistic overlap explains why LYSET mutations result in such a closely related phenotype. Given the phenotypic overlap with MLII, we propose that LYSET-related disorders be categorized as “LYSET-related mucolipidosis.” Furthermore, our findings, along with the cases reported by Ain et al., suggest that LYSET should be routinely included in genetic diagnostic panels for MLII/III-like presentations.
Ain et al. described their patients as having a dysostosis; however, we think the phenotype of the disorder caused by LYSET variants is more widespread and is better described as mucolipidosis. The designation of “Dysostosis Multiplex Ain-Naz Type” by the authors reflects the skeletal findings but underemphasizes the significant biochemical and clinical overlap with MLII. This connection strengthens our proposition to classify this disorder as a subtype of mucolipidosis.
In conclusion, our study provides additional evidence linking LYSET pathogenic variants to a distinct mucolipidosis phenotype closely resembling MLII. The clinical, enzymatic, and radiographic similarities between our patients and those described by Ain et al. emphasize the critical role of LYSET in lysosomal enzyme trafficking. This work further establishes the importance of reevaluating the classification of “Dysostosis Multiplex Ain-Naz Type” as LYSET-related mucolipidosis and highlights the need for expanded genetic testing in suspected MLII/III cases.
Author Contributions
Ariana Kariminejad contributed to conceptualization, methodology, investigation, writing, supervision, and visualization. Farzaneh Pouya contributed to methodology and writing. Fatemeh Ahangari provided formal analysis and resources. Saeed Talebi, Fariba Afroozan, and Frans W. Verheijen provided resources. Hossein Najmabadi provided supervision. Edwin H. Jacobs contributed to writing and supervision.
Acknowledgments
We thank Paul van den Berg, Marijke Boer, Jaccqueline Boonman, Guido Breedveld, Heidi de Gruyter, Ana Tripic, and Marianne Hoogeveen-Westerveld for performing the enzymatic and genetic analyses in materials of Patient 1.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




