Heterozygous Pathogenic Variants in SERPINB7 Potentially Associated With Concomitant Moyamoya Angiopathy and Nagashima-Type Palmoplantar Keratoderma
Funding: The authors received no specific funding for this work.
ABSTRACT
The authors present the first documented case of concomitant Nagashima-type palmoplantar keratoderma and moyamoya angiopathy, identifying a novel gene as a potential link between rare dermatologic and cerebrovascular diseases. The subject of this case report was identified and clinically evaluated at the Neurological Institute of Children's Hospital Los Angeles. Written informed consent for clinical care, genetic testing, and participation in this case study was obtained. The patient initially presented with a history of several dermatologic conditions, including eczema, vitiligo, and Nagashima-type palmoplantar keratoderma. Neurological examination and diagnostic imaging were strongly suggestive of moyamoya angiopathy, prompting a bilateral encephaloduroarteriomyosynangiosis. Singleton Clinical Exome Sequencing was subsequently performed, revealing pathogenic heterozygous variants in SERPINB7. This study identifies SERPINB7 as a possible link between Nagashima-type palmoplantar keratoderma and moyamoya angiopathy, indicating the pleomorphism of SERPINB7-mediated changes in human disease. Further studies are warranted to investigate the function of SERPINB7 in endovascular tissue. Furthermore, the increasingly recognized association between autoimmune dermatologic disease and moyamoya may be mediated through genetic mechanisms, highlighting the importance of genetic testing in individuals with rare dermatologic and cerebrovascular disorders.
1 Introduction
Moyamoya angiopathy (MA) is a rare cerebrovascular disease characterized by stenosis of the internal carotid arteries, prompting compensatory branching of vessels and increasing the risk of ischemic or hemorrhagic stroke (Li et al. 2022; Mejia-Munne et al. 2017; Mitri et al. 2021; Scott and Smith 2009). MA is more frequently reported in populations of Asian descent, and it is the most common cerebrovascular disease affecting the pediatric population in Japan, with approximately 10% of cases arising by familial inheritance (Scott and Smith 2009). Given the ethnic distribution and high familial rate of MA, it is not unlikely that the pathogenesis of MA is genetic in nature (Mitri et al. 2021; Scott and Smith 2009). Several genetic loci have been discovered and implicated in the pathogenesis of moyamoya, including variations in the RING finger 213 gene (RNF213) that encodes for a protein with important immune functions (Asselman et al. 2022; Li et al. 2022; Mitri et al. 2021). Low penetrance of RNF213, however, suggests the importance of additional environmental, genetic, and/or immune risk factors to trigger the onset of moyamoya (Asselman et al. 2022).
The etiology of MA currently remains unknown. Multiple studies have found an association between moyamoya and autoimmune disease, suggesting that immunologic and inflammatory conditions may contribute to the development of MA (Asselman et al. 2022; Li et al. 2022; Mejia-Munne et al. 2017). Interestingly, multiple genetic- and autoimmune-based dermatologic conditions have been strongly associated with MA, further indicating a potential association between the immune system and MA through dermatologic comorbidities (Mitri et al. 2021).
Here the authors present a case of heterozygous pathogenic variants in the SERPINB7 gene in a patient with MA and several co-occurring dermatologic conditions, including Nagashima-type palmoplantar keratoderma (NPPK). NPPK is a diffuse hereditary palmoplantar keratoderma known to be caused by variants in the SERPINB7 gene (Kubo et al. 2013). Given the key immune and inflammatory roles of the proteins in the serpin superfamily (Kelly-Robinson et al. 2021), SERPINB7-associated protein abnormalities may serve as a potential link between NPPK and MA, supporting emerging evidence of the association between the immune system and MA. Furthermore, as a novel case of MA and NPPK possibly linked by SEPINB7 gene variants, these findings contribute to the literature supporting an immunologic basis for the pathophysiology of MA and offer both future research directions and clinical considerations for rare dermatologic and cerebrovascular diseases.
2 Methods
2.1 Ethical Approval and Data Collection
The subject of this case report was identified and clinically evaluated at the Neurological Institute of Children's Hospital Los Angeles (CHLA). Patient data was retrospectively extracted from the CHLA electronic medical records system. Written informed consents for clinical care and genetic testing were obtained from the participant's adoptive parents and approved under CHLA protocol. The signed Patient Consent Form for participation in this case report and its inclusion of de-identified photographic images was obtained from both the participant and their legally authorized representative as the participant was identified for this case study at 17 years of age and has since turned 18.
2.2 Exome Sequencing and Analysis
Exome sequencing of the index case was performed in the CHLA Center for Personalized Medicine (CPM) Clinical Genomics Laboratory, a CAP/CLIA-certified laboratory within the Division of Genomic Medicine, Department of Pathology and Laboratory Medicine at CHLA. DNA was extracted from the peripheral blood using a commercially available kit (Promega Maxwell RSC DNA Extraction Kit). The Clinical Exome Sequencing (CES) library was generated using the Agilent SureSelect Human All Exon V6 plus a custom mitochondrial genome capture kit, and the subsequent sequencing of the isolated DNA fragments used the Illumina Nextseq 500 or HiSeq 4000 sequencing system, with 2 × 100 basepair (bp) paired-end reads. Single nucleotide variants (SNVs) and small insertions and deletions (< 10 bp) were detected by mapping and comparing the DNA sequences with the human reference genome (GRCh38). To identify the potential disease-causing variants, a primary gene list was generated based on the phenotype-related keywords for prioritization of variant analysis and interpretation. The rare nuclear DNA variants (minor allele frequency < 1%) within protein-coding regions and splice-site junctions (5 bp into introns) and any rare mitochondrial DNA variants with < 0.5% MitoMap GB frequency were further annotated and analyzed using a commercial tool (Agilent Alissa Interpret). Sequence variant classification and interpretation were performed based on American College of Medical Genetics (ACMG) and Association for Molecular Pathology (AMP) standards by a certified medical genomics specialist at CPM. Variants were additionally analyzed using the widely accepted online databases Varsome and Franklin as they align with ACMG/AMP variant classification guidelines (Kopanos, 2019; Franklin).
3 Results
3.1 Clinical Evaluation
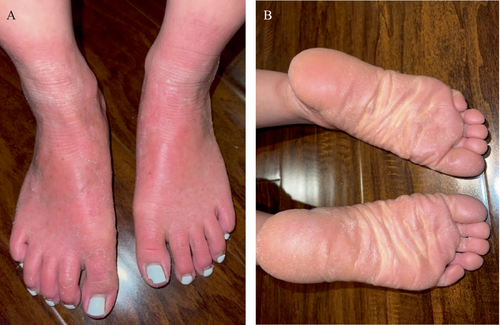
A female patient of Vietnamese descent initially presented at age 9 with chronic daily headaches in which three episodes included left-sided hemiplegia concerning for transient ischemic attacks (TIAs). Her medical history included severe eczema. Her neurologic examination at presentation was normal, and dermatologic evaluation revealed mild vitiligo and diffuse Nagashima-type palmoplantar keratoderma (NPPK) to the wrists and ankles bilaterally (Figure 1).
3.2 Imaging Results and Surgical Intervention
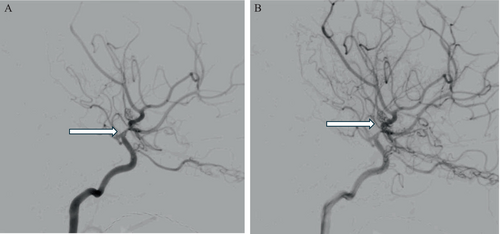
MRI revealed stenosis of both supraclinoid carotid arteries and M1 segments of the middle cerebral arteries with developing collateral vessels, a result strongly suggestive of Suzuki grade three moyamoya angiopathy (MA) (Figure 2). She underwent a successful bilateral encephaloduroarteriomyosynangiosis (EDAMS) to treat her MA. She had intermittent migraines afterwards but no further TIAs.
3.3 Genetic Findings
The patient continued to return to the Neurological Institute at CHLA for frequent follow-up visits, during which a genetic work-up was recommended to investigate risk factors for inflammatory disorders given a prior medical history of eczema and MA.
At age 17, singleton CES was obtained, revealing several pathogenic variants: two heterozygous pathogenic variations in the SERPINB7 gene (NM_003784.4: c.650_653del, p.Ser217Leufs*7; NM_003784.4: c.796C>T, p.Arg266*) and one heterozygous pathogenic variant in the HBB gene (NM_000518.5: c.79G>A, p.Glu27Lys). Biallelic pathogenic variants in SERPINB7 are associated with autosomal recessive NPPK, and the c.796C>T variant has been recognized as a founder mutation in select ethnic groups (Kubo et al. 2013; Mitri et al. 2021; Mizuno et al. 2014; Songsantiphap et al. 2020). The SERPINB7 presented variants are likely loss of function variants given that the first variant (SERPINB7: c.650_del) results in a frameshift mutation and the second variant (SERPINB7: c.796C>T) results in a premature stop codon; there is a possibility that they may act as dominant negatives and perturb other protein interactions (Kubo et al. 2013). Parental testing was not available in this case as the patient was adopted. Thus, the singleton exome data could not distinguish whether the two variants were in cis or in trans. However, given that the patient is clinically affected, the two variants are likely in trans.
The heterozygous HBB missense variant is a common pathogenic variant in the south and east Asian population. It is associated with autosomal recessive beta thalassemia and mild hemolytic anemia in the homozygous state (Jayasree et al. 2016; Tubsuwan et al. 2011). Given the heterozygous status, the patient is considered a carrier for beta thalassemia.
4 Discussion
4.1 The Role of the SERPINB7 Gene and the Vasculopathy Hypothesis
Nagashima-type palmoplantar keratoderma (NPPK) is a diffuse hereditary palmoplantar keratoderma characterized by hyperkeratosis and well-demarcated erythema, primarily affecting the palms of the hands and soles of the feet (Kubo et al. 2013). However, it may extend proximally beyond the palmar/plantar margins, affecting the dorsal surfaces of the hands and feet, inner wrist, and ankle joint (Kubo et al. 2013). NPPK is caused by biallelic pathogenic variants in the SERPINB7 gene (Kubo et al. 2013; Mitri et al. 2021; Mizuno et al. 2014; Songsantiphap et al. 2020). This gene encodes SERPINB7, which is part of the large superfamily of serpins (SERine Protease INhibitor) (Bouton et al. 2012; Kubo et al. 2013). Members of the serpin superfamily share a conserved tertiary structure and are primarily responsible for regulating proteases that play key roles in hemostasis, tissue remodeling, and inflammatory/immune responses (Kelly-Robinson et al. 2021). Some serpins are produced by the liver and are circulated in the bloodstream, while others are synthesized by peripheral cells, such as mesenchymal stromal cells of the vascular wall (Bouton et al. 2012). They have been found to protect cells from damage and injury caused by proteolytic degradation of the extracellular matrix (Bouton et al. 2012).
Notable members of the serpin superfamily include SERPINC1, also known as antithrombin, which is the primary inhibitor of blood coagulation proteases (O'Reilly et al. 1999), and SERPINE1, or plasminogen activator inhibitor-1, an inhibitor of tissue plasminogen activator (tPA) produced by various circulating and stromal cells within the cardiovascular system (Kubo et al. 2013). Protease NEXIN-1, or SERPINE2, is another member of this protein superfamily that has been implicated in vascular disease (Bouton et al. 2012). Its affinity for glycosaminoglycans on the cellular membrane places SERPINE2 at the blood-endothelium interface, where it plays a critical role in the protection of vessel walls from the development of lesions, cellular degradation, or thrombosis (Bouton et al. 2012). SERPINB5, SERPINC1, and SERPINF1 have also been shown to strongly inhibit angiogenesis in various tissues via their expression in fibroblasts, keratinocytes, prostate and breast cancer cells, and retinal cells (Dawson et al. 1999; O'Reilly et al. 1999; Zhang et al. 2000). Given the diverse implications of serpins in vascular disease, SERPINB7 may serve as a link between NPPK and moyamoya angiopathy (MA) via endovascular change. Of note, SERPINB7 is predominantly expressed in the epidermis of the skin, specifically in keratinocytes of the stratum corneum and stratum granulosum (Kubo et al. 2013; Songsantiphap et al. 2020). Additionally, SERPINB7 was originally observed in kidney mesangial cells (Kubo et al. 2013; Nangaku et al. 2001), and it is also expressed in lymphoid tissues (Uhlén et al. 2015), suggesting the potential for a more widespread presence in the perivascular connective matrix. However, its role and implications in vascular diseases remain unclear. Further studies are warranted to elucidate the function of SERPINB7 in endovascular tissue.
4.2 Immune Dysregulation Hypothesis
Evidence additionally suggests that disruption in SERPINB7 may contribute to immune dysregulation. Disruption of SERPINB7 has been reported in association with several autoimmune conditions such as psoriasis (Zheng et al. 2022). One study found that SERPINB7 deficiency exacerbated skin inflammation and increased expression of inflammatory cytokines and chemokines (TNF-α, IL-1b, IL-23, IFN-γ, and CXCL2) in a psoriasis mouse model (Zheng et al. 2022). SERPINB7 was found to be highly expressed in psoriatic keratinocytes derived from patients (Zheng et al. 2022). Furthermore, a genome-wide association study identified the SERPINB gene cluster as a susceptibility locus for food allergy (Marenholz et al. 2017). This study identified a lead SNP (rs12964116), located in an intron of SERPINB7, affecting a binding site for several transcription factors including STAT3, which is involved in maturation of immune cells, and CEBPB, which regulates Th2 cytokines such as IL-13, IL-4, and IL-5 (Marenholz et al. 2017). As such, it is possible that NPPK is linked to MA by immune dysregulation mediated through SERPINB7-associated protein abnormalities. This aligns with emerging evidence suggesting that an immune-related angiopathy may serve as an underlying etiology for MA in some cases (Asselman et al. 2022).
4.3 Connections Between NPPK and MA
As this case presents a novel identification of SERPINB7 as a potential link between MA and NPPK, it is relevant to mention the additional conditions with cutaneous abnormalities that occur in association with moyamoya. These include neurofibromatosis type 1, Hypomelanosis of Ito, livedo racemosa, and Raynaud's phenomenon (associated with Sneddon syndrome), PHACES syndrome, phakomatosis pigmentovascularis, Noonan syndrome, and incontinentia pigmenti (Mitri et al. 2021). Of these dermatologic conditions associated with MA, many have vascular components to their etiology. For instance, livedo racemosa and Raynaud's phenomenon present with distinct patterns and discoloration visible on the skin, respectively, caused by underlying vascular pathologies. The high penetrance of these phenomena in MA suggests microvascular dysfunction in the pathophysiology of MA when associated with such dermatologic comorbidities (Mitri et al. 2021). Furthermore, certain dermatologic manifestations such as Raynaud's phenomenon and chilblains have an autoimmune/inflammatory component to their vasculopathy. Collectively, the above findings support the hypothesis that MA is linked to dermatologic disease via a vascular mechanism and/or a cellular immune role. As such, SERPINB7-associated protein abnormalities may contribute to the pathophysiology of moyamoya in the case of concomitant NPPK and MA through mechanisms of endovascular change and/or immune dysregulation.
5 Conclusions: Clinical Implications and Future Investigative Directions
The patient presented has a unique combination of inflammatory dermatologic disease and MA associated with heterozygous pathogenic variants in SERPINB7. This gene has been heavily associated with NPPK and may potentially have a co-morbid association with MA. Thus, the increasingly recognized association between autoimmune dermatologic disease and MA may be partially mediated through genetic mechanisms. Although this report is limited in its narrow nature, it highlights the importance of genetic testing in individuals with rare dermatologic and cerebrovascular diseases. These findings indicate the potential value of screening for MA in SERPINB7 cohorts and individuals with NPPK, as there may be an opportunity for stroke prevention if cerebrovascular pathology is identified early. Cohort-level screening may thus be a multifaceted mechanism for preventing neurological insult and linking the hypothesized association between SERPINB7 and MA, serving to bridge the current gaps in knowledge about the etiology of MA in these populations.
Author Contributions
Lilia Kazerooni: data curation and chart review, literature review, obtaining subject consent, writing – original manuscript draft, writing – review and editing. Benjamin N. Vogel: data curation and validation, literature review, writing – review and editing. Abhik K. Banerjee: formal analysis, providing expert opinion, writing – review and editing. Saba Jafarpour: data interpretation, providing expert opinion, writing – review and editing. Jonathan D. Santoro: study conceptualization, data acquisition, providing expert opinion, writing – review and editing. All authors revised and approved the manuscript.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The following case report was performed after obtaining written informed consent from the subject. The signed Patient Consent Form was received from the subject indicating consent to participate in the following case report and its inclusion of de-identified photographic images.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




