Expanding the SIAH1-Associated Phenotypic Spectrum: Insights From Loss-of-Function Variants
Funding: The authors received no specific funding for this work.
ABSTRACT
SIAH1 encodes for a RING-type E3 ubiquitin ligase involved in protein ubiquitination. More specifically, it positively regulates Wnt signaling through promoting the accumulation of β-catenin and mediates ubiquitination and degradation of Akt3 in neural development. Heterozygous de novo missense pathogenic variants in SIAH1 have been described in five unrelated individuals and are associated with developmental delay, hypotonia, and dysmorphic features. In this report, we present additional individuals from eight unrelated families and their clinical and genetic findings. We identified two missense and six predicted loss-of-function variants. Motor and speech delay and intellectual disabilities of varying severity were observed in all individuals. Neurodevelopmental issues, as well as infantile hypotonia and facial dysmorphism, were observed in the majority of individuals. Hearing loss, gastroesophageal reflux disease or other gastrointestinal issues, endocrinology abnormalities, and recurrent infections were observed in over 50% of individuals. This study expands the phenotypic spectrum of this syndrome and emphasizes the diverse impact of SIAH1 variation on multi-system clinical manifestations.
1 Introduction
Ubiquitination, a posttranslational modification of proteins, is an essential mechanism regulating a multitude of cellular processes, such as cell division and differentiation, response to environmental stressors, immune response, DNA repair, and apoptosis. This process involves three core components: ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). Among these, E3 ubiquitin ligases are pivotal for the substrate specificity of ubiquitin ligation and are classified into three main groups: RING E3s, homologous to the E6AP carboxyl terminus (HECT) E3s, and RING-between-RING (RBR) E3s; each exhibiting a distinct mechanism of action (Damgaard 2021). The seven in absentia homolog (Siah) protein family belong to the RING E3 class. Siah proteins, the mammalian homologs of the Drosophila sina proteins, are instrumental in modulating the ubiquitination of substrate proteins to promote functional changes or degradation of these proteins through the proteasomal pathway (Zhang et al. 2022). Three SIAH genes—SIAH1, SIAH2, and SIAH3—have been identified in humans, each performing unique yet overlapping roles (Siswanto et al. 2018). Imbalance of ubiquitination-mediated protein degradation underpins various human diseases. Several reports have suggested that SIAH1 functions as a tumor suppressor gene, as evidenced by its reduced expression in several types of cancer (Zhang et al. 2022).
A previous study described individuals from five unrelated families presenting with developmental delay, infantile hypotonia, and facial dysmorphism. Genetic testing revealed heterozygous de novo missense variants in SIAH1, leading to Buratti–Harel syndrome (BURHAS; MIM: 619314) (Buratti et al. 2021). Functional in vitro assays of the five missense variants demonstrated a loss of Wnt stimulatory activity, suggesting variant pathogenicity and expanding the spectrum of diseases linked to impaired ubiquitination.
To date, these cases represent the only published reports connecting SIAH1 to neurodevelopmental disorders (Buratti et al. 2021). In this study, we present findings from an additional eight unrelated individuals harboring distinct SIAH1 variants, including six predicted truncating variants. The individuals exhibited a broader multi-system phenotypic spectrum, underscoring the diverse clinical manifestations associated with likely pathogenic variants in SIAH1. Our results offer insights into SIAH1-related disease and the role of E3 ligases in complex, multi-systemic clinical presentations.
2 Methods
2.1 Ethics
All families provided written informed consent for clinical genetic testing. Consent for publication and participation in research was obtained according to the individual institution regulations, and, if necessary, following approval by institutional review boards.
2.2 Exome Sequencing
Proband-only or trio (parents and child) exome sequencing was pursued on whole blood. Details including capture kits, DNA sequencer specifications, read alignment and variant calling tools, and total reads and coverage are provided in Table S1. Annotation and filtering were done at the participating laboratory. All SIAH1 variants are provided in accordance with RefSeq transcript NM_003031.4; positional data are available in Table S1.
2.3 Sanger Validation
Sanger sequencing of the proband and available parents was pursued to confirm the de novo status of the SIAH1 variant. Amplicons containing the SIAH1 variants were amplified by conventional PCR of genomic data and analyzed by Sanger dideoxy nucleotide sequencing.
3 Results
3.1 Clinical Case Reports
Detailed clinical reports are provided as Data S1 and Table S1. The main neurological clinical manifestations across all reported cases included varying degrees of speech and motor delays, moderate to significant intellectual disability, and neurological and behavioral issues. Four probands were diagnosed with autism spectrum disorder and/or exhibited behavioral challenges such as impulsivity, aggression, or obsessive-compulsive behaviors. Four had infantile hypotonia. Other than a single individual with febrile seizures, no convulsions were reported. Brain magnetic resonance imaging (MRI) had nonspecific findings in three individuals and was reported as normal in two others (Tables 1 and S1).
| Family/proband | P-1 | P-2 | P-3 | P-4 | P-5 | P-6 | P-7 | P-8 | P-9 | P-10 | P-11 | P-12 | P-13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | This report | This report | This report | This report | This report | This report | This report | This report | Buratti et al. (2021) | Buratti et al. (2021) | Buratti et al. (2021) | Buratti et al. (2021) | Buratti et al. (2021) |
| Motor developmental delay (HP:0001270) | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Speech delay (HP:0000750) | + | + | + | + | + | NA | + | + | + | + | + | + | + |
| Intellectual disability (DQ or IQ) (HP:0001249) | + | + | + | + | + | NA | NA | + | NA | + | + | NA | + |
| Neurodevelopmental issues (HP:0012759) | + | + | + | − | − | NA | + | + | NA | NA | NA | NA | + |
| Hypotonia (HP:0008947) | − | − | NA | + | + | + | − | + | + | + | + | + | + |
| Laryngomalacia (HP:0001601) | − | − | + | + | − | + | + | + | + | + | + | − | + |
| Hearing loss (HP:0000365) | + | − | NA | + | − | − | + | + | NA | NA | NA | NA | NA |
| GERD (HP:0002020) | − | − | + | + | + | − | − | + | + | − | + | + | + |
| Gastrointestinal (GI) | − | − | + | + | + | + | NA | + | NA | NA | NA | NA | NA |
| Recurrent infections (HP:0002719) | − | − | NA | + | + | NA | − | + | − | − | − | + | + |
| Dysmorphism (HP:0001999) | + | + | + | + | + | + | − | + | + | + | + | + | + |
| Cleft lip/palate (HP:0000175, HP:0410030); Bifid uvula (HP:0000193) | − | + | NA | − | + | + | + | + | + | − | + | + | + |
| Cardiac malformation | − | − | NA | − | + | + | + | + | NA | − | − | − | + |
| Scoliosis (HP:0002650) | − | − | + | + | NA | + | NA | NA | NA | NA | − | NA | NA |
| Dental abnormalities (HP:0000164) | − | − | NA | + | − | + | NA | NA | NA | NA | NA | NA | NA |
| Endocrinology issues (HP:0031072) | − | + | NA | + | NA | NA | NA | + | NA | NA | NA | NA | NA |
| Nucleotide (NM_003031.4) | c.15_16insA | c.19dup | c.71_75del | c.104T>A | c.165T>A | c.337_338del | c.455A>G | c.529G>C | c.121T>G | c.149C>T | c.383G>T | c.502A>G | c.520G>C |
| Amino acid | p.(Ala6Serfs29*) | p.(Thr7Asnfs*28) | p.(Ala24Aspfs*9) | p.(p.Leu35Ter) | p.(Cys55Ter) | p.(Glu113Lysfs*5) | p.(His152Arg) | p.(Asp177His) | p.Cys41Gly | p.Pro50Leu | p.Cys128Phe | p.Thr168Ala | p.Gly174Arg |
- Abbreviations: DQ, developmental quotient; GERD, gastroesophageal reflux disease; IQ, intelligence quotient; NA, not available.
In the present cohort, five individuals presented with laryngomalacia and/or tracheomalacia. Ophthalmological findings included iris coloboma, strabismus, and other vision-related abnormalities. Five individuals had conductive, sensorineural, or mixed hearing loss or required myringotomy and ventilation tube placement. Gastrointestinal issues, including poor feeding and gastroesophageal reflux disease, were encountered in five cases, with severe feeding issues requiring nasogastric tube or gastrostomy in three individuals.
Cardiac and renal structural malformations were prevalent, although not consistent within the cohort. Four individuals had structural heart defects, including tetralogy of Fallot in a single report, and patent foramen ovale, patent ductus arteriosus, or right aberrant subclavian artery in the others. Renal abnormalities included vesicoureteral reflux with a history of recurrent urinary tract infections in one individual and microalbuminuria at age 17 years in another.
Skeletal issues included scoliosis in three individuals, clubfoot in one, and broad or abnormal phalanges in two reports. Oligodontia was reported in two individuals. Endocrinology abnormalities included growth deficits, transient neonatal hypothyroidism, and type I diabetes mellitus. Three individuals had recurrent infections, including pneumonia, suggesting the possibility of immune dysregulation; however, no individual underwent a comprehensive immunological work-up.
Common dysmorphic characteristics included hypertelorism, epicanthal folds, broad or flat nasal bridges, low-set ears, micrognathia, high-arched or bifid uvula, and abnormal dentition. Figure 1A provides summary statistics for phenotypes encountered in previously reported cases and the present cohort.
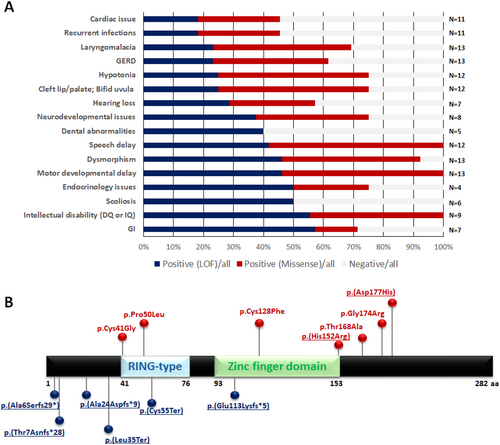
3.2 Next-Generation Sequencing Identifies SIAH1 Variants
Exome or genome sequencing identified frameshift or nonsense variants in SIAH1 in six individuals and two missense variants (Figure 1B). The reported variants were found to be de novo by either exome or Sanger sequencing, except for families B (p.(Thr7Asnfs*28)) and F (p.(Glu113Lysfs*5)), where either one or both of the parents' samples were not available, respectively (Table S1). This finding allows variant classification as “pathogenic” or “likely pathogenic” according to the American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al. 2015). Four variants led to presumed premature truncation variants at or before amino acid Leu35, among 282 total amino acids in the protein. No clustering of missense variants was noted.
4 Discussion
In the present study, we report distinct SIAH1 variants in eight unrelated individuals ranging from 15 months to 21 years of age and representing diverse ethnic backgrounds. These individuals exhibited more complex multi-systemic manifestations than previously reported in the Buratti et al. study (Buratti et al. 2021). Clinical features included motor and speech delays and varying degrees of intellectual disability, as well as autism spectrum disorder and behavioral issues. Dysmorphic facial features were prevalent, as were a bifid uvula or cleft lip/palate. Five individuals had laryngomalacia, whereas cardiac, renal, and skeletal abnormalities were varied. Endocrine abnormalities and recurrent infections were noted in several individuals.
Three SIAH genes have been identified in humans: SIAH1, SIAH2, and SIAH3. These genes play distinct but overlapping functions (Siswanto et al. 2018). SIAH1 and SIAH2 share high protein sequence similarity and structural homology, with the primary distinction being additional amino acid residues at the N-terminus of SIAH2 (Dickins et al. 2002). Although they share high homology, SIAH1 proteins apparently operate in diverse signaling pathways, depending on their interactions with different proteins and molecular signals. Phenotypic studies in rodents underscore this divergence. Siah1-null mice exhibit severe growth delay, defective bone formation, early mortality, and male infertility that results from meiotic arrest at metaphase I with impaired spermatogenesis (Dickins et al. 2002). Siah2-null mice, on the other hand, are fertile and exhibit relatively normal development.
Emerging research highlights an important role for Siah1 in the innate immune signaling response to various infectious agents. The innate immune system, as the body's first line of defense, has evolved complex multilevel mechanisms, which include post-translational modifications such as ubiquitination, to control immune responses at the molecular level. Upon pathogen detection, a cascade of signaling molecules and cytokine production is triggered to prevent pathogen dissemination. Ubiquitination is central to modulating host type I interferon (IFN) signaling. SIAH1 interacts with ubiquitin-specific protein 19 (USP19), a member of the ubiquitin-specific protease family known to be involved in a variety of cellular processes. The SIAH1–USP19 interaction following viral infections enhances host innate immune defenses by positively regulating the type I IFN signaling pathway (Weerawardhana et al. 2024). Additionally, SIAH1 can activate pro-inflammatory transcription factors, including IL-1α and IL-6, and modulate key signal transducers or transcription factors (Schmitz et al. 2022). The functionality of SIAH1 in innate immunity may explain the recurrent infections observed in almost half of the patients.
The pathogenic mechanism of variants in SIAH1 has been proposed to be haploinsufficiency. The probability of loss-of-function intolerance (pLI) score for this gene is 1 (o/e = 0.09), with a Z-score of 4.84 for tolerance to missense variants. In 2021, Buratti et al. supported this hypothesis by showing that all of the missense variants identified in the affected individuals led to a loss of Wnt stimulatory activity (Buratti et al. 2021). To date, no germline deletions or truncating variants have been reported in this gene. The SIAH1 gene consists of two exons, with the coding sequence found entirely in the second exon in the canonical transcript. The reported nonsense and frameshift variants are found in the second exon, rendering nonsense-mediated decay unlikely. However, in this study, we report six truncating variants, five of which are located upstream or within the RING-type domain, while one is located at the initial sequence of the zinc finger domain. These variants are expected to lead to a loss of key functional sequences, and therefore are predicted to result in a partial or complete loss of protein activity. Cells were not available from the affected individuals, such that RNA and protein levels could not be assessed.
In summary, these findings broaden the phenotypic spectrum associated with SIAH1 variants, highlighting complex multi-systemic manifestations including neurodevelopmental delays, hearing loss, laryngomalacia, poor feeding, dental, skeletal, and endocrine abnormalities, and recurrent infections. Additional studies of individuals with SIAH1 variants will clarify whether cardiac and renal malformations constitute part of the core phenotype. This study underscores the importance of SIAH1 in critical cellular processes, emphasizing its role in pathophysiology and the potential implications of haploinsufficiency in affected individuals.
Author Contributions
L.D. and T.H. conceived and designed the study. P.F.A., M.F., L.H., T.L.H., M.B.I., J.K., A.K., A.L.A., D.L.D., H.L., O.M., D.S., and B.K.W. contributed clinical and molecular data. L.D. and T.H. wrote the draft, and all authors revised, edited, and approved the final submitted version.
Acknowledgments
The authors wish to thank all the patients, family members, and collaborators that participated in this study.
Conflicts of Interest
A.K. is an employee and stockholder of MyOme Inc. Other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in ClinVar at https://www.ncbi.nlm.nih.gov/clinvar/, reference number SCV005423748-SCV005423755.




