First-trimester noninvasive prenatal diagnosis of seven facioscapulohumeral muscular dystrophy type 1 families using SNP-based amplicon sequencing: An earlier, rapid and safer way
Abstract
The study is to explore the feasibility and value of SNP-based noninvasive prenatal diagnosis (NIPD) for facioscapulohumeral muscular dystrophy type 1 (FSHD1) in early pregnancy weeks. We prospectively collected seven FSHD1 families, with an average gestational age of 8+6. Among these seven couples, there were three affected FSHD1 mothers and four affected fathers. A multiplex-PCR panel comprising 402 amplicons was designed to selective enrich for highly heterozygous SNPs upstream of the DUX4 gene. Risk haplotype was constructed based on familial linkage analysis. Fetal genotypes were accurately inferred through relative haplotype dosage analysis using Bayes Factor. All tests were successfully completed in a single attempt, and no recombination events were detected. NIPD results were provided within a week, which is 4 weeks earlier than karyomapping and 7 weeks earlier than Bionano single-molecule optical mapping (BOM). Ultimately, five FSHD1 fetuses and two normal fetuses were successfully identified, with a 100% concordance rate with karyomapping and BOM. Therefore, SNP-based NIPD for FSHD1 was demonstrated to be feasible and accurate in early weeks of gestation, although the risk of recombination events cannot be completely eliminated. In the future, testing of more cases is still necessary to fully determine the clinical utility.
1 INTRODUCTION
Facioscapulohumeral muscular dystrophy (FSHD, OMIM#158900) is an autosomal dominant neuromuscular disorder with an incidence of approximately 1/8000 to 1/20,000, second only to Duchenne muscular dystrophy and ankylosing muscular dystrophy (Deenen et al., 2014; He et al., 2018). Approximately 95% of FSHD cases are classified as FSHD1, which is resulted from reduced D4Z4 microsatellite repeat units in the subtelomeric chromosome region 4q35 (Sacconi et al., 2019). The D4Z4 repeats (DRs) decrease to 1–10 and show linkage with the downstream 4qA allele (Cortesi et al., 2019). The clinical manifestation of FSHD1 is mainly asymmetric, progressive muscle weakness (Kelly et al., 2022). Although the disease does not reduce life expectancy, loss of muscle strength can seriously reduce the quality of life and impose a substantial economic and psychological burden on families (DeSimone et al., 2017). Unfortunately, there is no curative treatment for FSHD1. Therefore, its detection and treatment deserve more attention and research (Bakker et al., 1996; Cohen et al., 2021).
In China, the majority of FSHD1 families express the desire for prenatal diagnosis of FSHD1, aiming to prepare in advance for the birth of affected children. Considering the complex structural variations in FSHD1 and the 99% homology of the 4q35 and 10q26 regions, molecular diagnosis of FSHD1 is a formidable challenge, and prenatal diagnosis even more so (Di Feo et al., 2022; Upadhyaya et al., 1999). Current methods for prenatal diagnosis of FSHD1 include Karyomapping (Zheng et al., 2020) and Bionano single-molecule optical mapping (BOM) (Dai et al., 2020; Stence et al., 2021). However, the above methods do not eliminate the risk of invasive procedures and can only be applied as early as the 11 week of pregnancy. Additionally, in most cases, chorionic villus sampling (CVS) does not directly meet the DNA input requirements for BOM, necessitating approximately 4 weeks of cell culture, which is not only extends the clinical turnaround time but also adds to the anxiety of pregnant women. Due to the autosomal dominant inheritance pattern of FSHD1 with a high 50% recurrence risk, and the current inability to perform preimplantation genetic testing for monogenic, there is a pressing desire among pregnant women for early noninvasive prenatal diagnosis (NIPD) of FSHD1.
Since Lo et al. proposed the presence of cell-free fetal DNA in maternal peripheral blood, NIPD has become widely adopted and popular (Liang et al., 2019; Lo et al., 1997; Zhong & Chiu, 2022). Relative haplotype dosage (RHDO) is the mainstream for NIPD for monogenic diseases (Lo et al., 2010), which is not limited to mutation type. Indeed, RHDO-based NIPD has been successfully applied for various single-gene disorders such as spinal muscular atrophy, congenital adrenal cortical hyperplasia, and duchenne muscular dystrophy (Chen et al., 2017; Kazmi et al., 2017; Zhao et al., 2021). Our team has also explored NIPD for various recessive genetic disorders (Kong et al., 2021; Kong et al., 2023), with a high success rate. Qin et al. first proposed NIPD of FSHD1 based on target capturing and hidden Markov model in a paternal pedigree (Qin et al., 2022). However, this study did not demonstrate the diagnostic efficacy when the pregnant woman is an FSHD1 patient. Moreover, the pregnant woman was in second trimester, and the feasibility in early pregnancy was not validated.
In this study, we propose a single-nucleotide polymorphism (SNP)-based amplicon sequencing method in seven FSHD1 families, aiming to achieve rapid NIPD of FSHD1 in early pregnancy.
2 METHODS
2.1 Editorial policies and ethical considerations
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2019-KY-286), and all participants signed informed consent forms.
2.2 Workflow
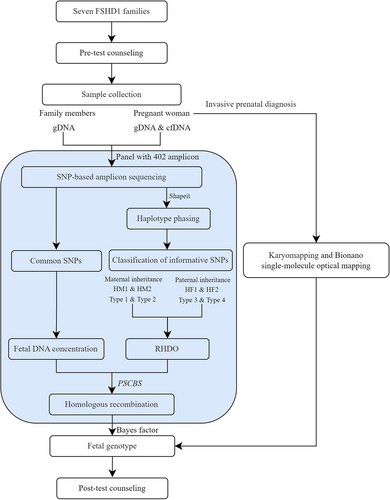
The workflow of this study is illustrated in Figure 1. Seven FSHD1 families were recruited. After genetic counseling, 10 mL blood sample from the pregnant women and 2 mL from family members were collected. Genomic DNA (gDNA) from the family members and cell-free DNA (cfDNA) from the pregnant women were extracted. DNA libraries were constructed by amplicon sequencing and were sequenced simultaneously on the Ion Proton platform after barcode ligation. Subsequently, the fetal fraction, haplotype phasing, recombination events, and RHDO were analyzed using sequencing data that met quality control. The fetal genotype was inferred by combining the Bayes Factor with RHDO. Invasive prenatal diagnosis was also performed. The NIPD results were provided by post-test counseling.
2.3 Sample collection
Seven families with a family history of FSHD1 were recruited from March 2022 to September 2023, with an average gestational age of 8+6 weeks (Table 1). Each family exhibited a dominant inheritance pattern and had at least two accessible FSHD1 patients (Supplementary Figure S1). All affected members had been diagnosed as FSHD1 patients through genetic testing. In the seven couples, three pregnant women were affected by FSHD1, representing maternal inheritance, and four husbands of the pregnant women were FSHD1, representing paternal inheritance. When constructing family risk haplotypes, if the core family structure was incomplete, samples from siblings could assist in haplotype phasing. As mentioned above, 10 mL of peripheral blood was collected from each pregnant woman, whereas 2 mL of peripheral blood was obtained from the other family members.
| Family | Gestational weeks | Genetic diagnosis | NIPD | IPD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Southern blot | BOM | FFa | Type1/Type2 | Type3/Type4 | D-SNPb | Rc | BFd | Result | Karyomapping | BOM | Consistency | ||
| F1 | 11+2 | 15.0 kb | - | 3.5% | 14/19 | - | 75 kb | No | 6.27E+17 | Affected | Risk | 3DRs | Y |
| F2 | 8+6 | - | 4DRse | 4.2% | 50/93 | - | 216 kb | No | 5.30E+21 | Affected | Risk | 4DRs | Y |
| F3 | 9+0 | 20 kb | - | 18.6% | 17/6 | - | 75 kb | No | 1.80E+22 | Affected | Risk | 3DRs | Y |
| F4 | 7+0 | 13.5 kb | - | 5.7% | - | 24/36 | 257 kb | No | 2.96E+159 | Affected | Risk | 2DRs | Y |
| F5 | 8+4 | 23.5 kb | - | 4.9% | - | 34/16 | 75 kb | No | 9.10E-61 | Not-affected | Non-risk | 4DRs | Y |
| F6 | 8+6 | 25.0 kb | - | 3.2% | - | 19/20 | 140 kb | No | 1.43E+120 | Affected | Risk | 5DRs | Y |
| F7 | 8+1 | - | 4DRs | 9.1% | - | 7/21 | 128 kb | No | 4.40E-113 | Not-affected | Non-risk | 4DRs | Y |
- a Fetal fraction.
- b The distance of the nearest SNP from the D4Z4 locus.
- c Whether the recombination events exist.
- d Bayes factor.
- e D4Z4 repeats.
2.4 Multiplex PCR primer design
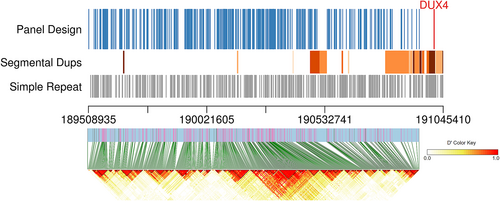
Allele frequency data were obtained from 1000G phase 3 and downloaded from the UCSC genome browser database (Karolchik et al., 2003) (http://genome.ucsc.edu/). After checking for minor allele frequency (MAF), linkage disequilibrium, and target specificity, 402 PCR amplicons were designed (see details in Supplementary Methods), which covered 402 highly heterozygous SNPs (MAF >0.2) around the DUX4 gene (Figure 2). Notably, the amplicons were designed to avoid simple repeats and segmental duplications upstream of the DUX4 gene and distributed within distinct linkage disequilibrium blocks whenever possible. In addition, 203 autosomal SNPs (MAF >0.45) were included in the panel to calculate the fetal fraction.
2.5 Library preparation and next-generation sequencing
Nucleic acid extraction and purification kits were used to extract gDNA from the family members and cfDNA from the pregnant women according to the manufacturer's protocol and instructions (Nahai Bio, Chengdu, China). The cfDNA should be purified before amplification. The library was constructed using two-step PCR. A total of 10 ng gDNA and at least 5 ng cfDNA were used for the first round of PCR. To form the complete PCR products, we implemented 15 rounds of amplification with high-fidelity DNA polymerase and multiplex PCR primers. The second round of amplification used universal primers with barcodes, aiming to achieve simultaneous sequencing of multiple samples using the Ion Proton platform (Thermo Fisher Scientific). Detailed information is provided in the Supplementary Methods. In total, 2 M, 2 M, and 1 M reads were obtained for the maternal cfDNA, maternal gDNA, and other family members gDNA, respectively. If the average sequencing depth was ≤30× for gDNA or ≤ 70× for cfDNA, the quality control of the sequencing data failed, and the sample should be resequenced.
2.6 NIPD of FSHD
- Measurement of the fetal fraction: To calculate the fetal fraction, we selected homozygous autosomal SNP loci in both parents but with different genotypes. The fetal fraction was calculated by the following equation: FF = ∑2a/(∑ 𝑎 + ∑ b) (Jiang et al., 2016), where FF represents the proportion of fetal DNA in maternal plasma, 𝑎 is the read depth of the allele that the fetus inherited from the father, and b is the read depth of the allele that the fetus shares with the mother.
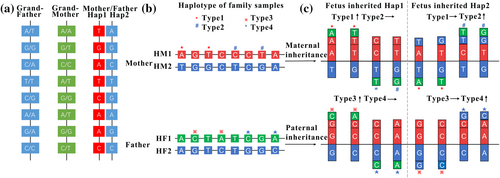
- Family haplotype phasing: Pathogenic haplotype H1 (HM1 from mother or HF1 from father) and normal haplotype H2 (HM2 or HF2) were constructed by family linkage analysis using the genotypes of family members via Shapeit software (Delaneau et al., 2011) (Figure 3a).
- Classification of informative SNPs: Informative SNPs were identified by various dosage changes when the fetus inherited different haplotypes (H1/H2). For maternal inheritance, informative SNPs were heterozygous in the pregnant woman and homozygous in the father. For paternal inheritance, informative SNPs were heterozygous in the father and homozygous in the mother (Figure 3b). To further distinguish whether fetal inherited H1 or H2, informative SNPs were classified into four categories. Maternal informative SNPs were classified as Type 1 and Type 2, and paternal informative SNPs were classified as Type 3 and Type 4. Type 1 and Type 3 had elevated dosages when the fetus inherited H1 (HM1/HF1), and Type 2 and Type 4 had elevated dosages when the fetus inherited H2 (HM2/HF2) (Figure 3c). Detailed information is provided in the Supplementary Methods.
- Determination of fetal genotype: The balance of alleles in maternal plasma was disrupted due to the existence of fetal DNA. The fetal allele frequencies (AF) were calculated by the equation , where i represents the informative SNP index, and AFcfDNA and AFgDNA reveal AF of maternal cfDNA and gDNA, respectively. The presence or absence of chromosomal recombination events was identified by the R package “paired parent-specific circular binary segmentation,” as based on the circular binary segmentation algorithm (Olshen et al., 2011). Type 1–4 SNPs were further divided into one or multiple segments. We selected the segment that was closest to D4Z4 to calculate the dosage change (DC) by using the formula: or . We applied a Bayesian model to predict further which haplotype the fetus inherited. The calculation formula of Bayes factors (BF) was as follows: , where H1 assumes that the fetus inherited a pathogenic haplotype and the expected DC is FF/2 and H2 presumes that the fetus inherited a normal haplotype and the expected DC is −FF/2 (see details in Supplementary Methods). When BF ≥10, the H1 hypothesis was accepted, and the fetus inherited the pathogenic haplotype. When BF ≤0.1, the H2 hypothesis was accepted, and the fetus inherited the normal haplotype. If BF fell within the interval (0.1, 10), the statistical significance was insufficient to guarantee the accuracy, and the result “no call” was given.
2.7 Invasive prenatal diagnosis
For all pregnancies, an invasive prenatal diagnosis at 11 weeks of gestation was performed. Fetal chorionic villus samples were obtained, and a portion was used for karyomapping analysis (Zheng et al., 2020). DNA was extracted using an Omega blood/tissue DNA kit (Georgia) according to the instructions. The DNA was scanned using an Illumina iScan after whole-genome amplification, DNA fragmentation, and hybridization. BlueFuse Multi v4.4 (Illumina) software was applied for data analysis to obtain informative SNP loci. Following the lineage linkage analysis, fetal and parental haplotypes were constructed to determine the fetal genotype.
Another portion of the villus sample was utilized for BOM analysis (Dai et al., 2020). After cell culture, extra-long DNA molecules were extracted using the SP Blood and Cell Culture DNA Isolation Kit (Bionano Genomics), and pulsed-field gel electrophoresis was performed to investigate the length of fragments. The average DNA length should be above 230 kb. DLS DNA Labelling Kit (Bionano Genomics) was used for genome-wide site-specific fluorescent labeling. The Saphyr chip helped with linearization of DNA molecules to achieve ultralong single-molecule high-resolution fluorescence imaging, generating more than 400 Gb of data per sample. Data were analyzed and visualized via Bionano Solve v3.5.1 software.
3 RESULTS
3.1 Sequencing date
DNA from family members was sequenced using the panel described above (Supplementary Table S1). For gDNA, the average sequencing read was 2,017,026, the average sequencing depth was 2863×, and the average targeting ratio was 89.98%; the average sequencing data for cfDNA were 3,994,300, 5817×, and 79.88%, respectively. The sequencing results of all samples met the quality control criteria, and no resequencing was needed.
3.2 NIPD for FSHD1
3.2.1 Three FSHD1 families that pregnant women were affected
For F1–F3, pregnant women were FSHD1 patients, and RHDO was used to determine whether the fetus had inherited HM1 or HM2. The average fetal fraction was calculated to be 8.77% (3.5%–18.6%). According to the classification criteria of informative SNPs, average 66 informative SNPs were measured, including 27 Type 1 loci and 39 Type 2 loci (Table 1).
The average distance of the nearest SNP from the D4Z4 locus was 122 kb (75–216 kb). And no chromosomal recombination events were observed in the detected informative SNPs. The dosage changes of informative SNPs in maternal plasma were presented as scatter plots, and solid lines highlighted the mean values. For example, the result of F1 was illustrated in Figure 4a, the Type 1 allele dosage increased significantly and was roughly equal to FF/2, and Type 2 allele dosage remained unchanged. The violin diagram displayed the same result in Figure 4b, and the BF was calculated as 6.3 × 1017,which represented the positive result. Similar results were observed in two other families. Therefore, sufficient evidence supported that the three fetuses inherited HM1 and were more likely to be FSHD1 patients.
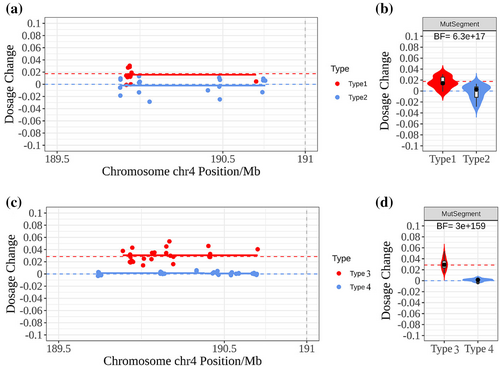
3.2.2 Four FSHD1 families that husbands of pregnant women were affected
In the remaining four families, the husbands of pregnant women were FSHD1 patients. The average fetal fraction was measured to be 5.73% (3.2%–9.1%). Although there was no mutation interference in maternal plasma, the complex structural variation of FSHD1 could not be directly detected; thus, we still used the haplotype-based method. The haplotypes of fathers were constructed through their parents. Average 44 informative SNPs were measured and classified as 21 Type 3 and 23 Type 4 (Table 1). The average distance of the nearest SNP from the D4Z4 locus was 150 kb (75–257 kb). Similarly, no chromosomal recombination events were observed. The result of F4 as shown in Figure 4c and 4d, we observed that the dosages of the Type 3 allele were significantly increased, and the BF was calculated as 3.0 × 10159. Ultimately, we identified two FSHD1 fetuses in F4 and F6 and two normal fetuses in F5 and F7.
3.3 Invasive prenatal diagnosis
Invasive prenatal diagnosis was performed to verify the accuracy of NIPD at 11 weeks of gestation. Karyomapping revealed that the fetus inherited the non-risk chromosome from the father in F5 and F7. However, in other five families, the fetus inherited the risk haplotype, which had a high probability of being an FSHD1 patient. At the single-molecule level, BOM indicated the presence of fetal abnormal chromosome 4 in the above five families. The result of F1 and F4 was illustrated in Supplementary Figure S2. In brief, the invasive prenatal diagnosis was all in agreement with the NIPD result (Table 1).
4 DISCUSSION
This study proposes an SNP-based amplicon sequencing method for NIPD of FSHD1 in early pregnancy. Currently, there is only one report on NIPD for FSHD1 that included a family the husband of pregnant woman was affected (Qin et al., 2022). In this study, we recruited seven families, including three cases where the pregnant women were FSHD1 patients representing maternal inheritance, and four cases where the husbands of pregnant women were FSHD1 patients representing paternal inheritance. All NIPD were successfully achieved in early pregnancy weeks. To the best of our knowledge, this is currently the largest cohort for NIPD of FSHD1, and the first successful maternal inheritance detection of FSHD1 in cases where pregnant women are the FSHD1 patients. This method significantly bringing forward the timing of prenatal diagnosis for FSHD1 and reduces clinical turnaround times, holding profound clinical significance.
The complex structural variation in the 4q35 region hinders the development and application of FSHD1 prenatal testing technology (Cortesi et al., 2019). Additionally, the earlier the gestational age, the lower the fetal DNA content in maternal peripheral blood, posing greater challenges for NIPD. However, early and rapid testing is essential for FSHD1 families, which can not only reduce maternal anxiety, but also prepare for postnatal treatment for the FSHD1 fetus. Haplotype analysis is undoubtedly an applicable method for complex variation, which is not limited to mutation type (Hanson et al., 2022). Our study is an innovative attempt to apply SNP-based amplicon sequencing combined with RHDO for NIPD of FSHD1. In the study, the significant advantages are the early detection time and shortened testing process. For example, NIPD for the F4 family was implemented at 7+0 weeks of gestation, and the result was obtained within 1 week. In comparison, CVS was carried out at 11+0 weeks of gestation, and the results of karyomapping and BOM were reported at 12+2 and 15+1 weeks, respectively. Thus, the noninvasive result was obtained 4 weeks earlier than that of karyomapping and 7 weeks earlier than that of BOM. Our previous studies have also shown that NIPD in early pregnancy has 100% accuracy (Kong et al., 2021). Therefore, NIPD is the earliest known method to provide accurate prenatal detection of FSHD1.
Regarding haplotype analysis, a primary concern is the false prediction of fetal genotype due to recombination events (Barat-Houari et al., 2010; Lathrop et al., 1985). Haplotype dosage analysis cannot identify when homologous recombination occurs between the closest upstream SNP locus and the DUX4 gene. Based on genetic distance, the calculated recombination events within the panel established in this study were 3.29%. However, previous research found a 14% recombination rate within 550 kb upstream of the D4Z4 region (Pini et al., 2023), which is higher than expected. In our study, 87 SNPs were found to be located within this high recombination region, with the nearest SNP being only 64 kb from D4Z4, minimizing the inference of recombination events. In clinical tests of seven FSHD1 families, the distance of the nearest SNP from the D4Z4 locus ranged from 75 kb to 257 kb. Although the risk of recombination was not exactly the same in different families, considering that our panel range was 2 M, the nearest informative SNP location (75–257 kb) had a small impact on recombination evaluation, all of which were less than 14%. The CBS algorithm was used to predict recombination events, which was shown to be effective in our previous study (Kong et al., 2021). No recombination events were discovered in the seven FSHD1 families. Unfortunately, due to the complexity and repeatability of the 4q35 region, no effective informative SNPs were found downstream of the DUX4 gene in our panel. The risk of recombination still cannot be completely eliminated.
To adequately guarantee the accuracy of NIPD, strict quality control criteria were established, including sequencing depth, fetal fraction, and the number of informative SNPs. For sequencing depth, 30× of gDNA and 70× of cfDNA were set as the cut-off, with substandard samples needing to be resequenced. In our team's previous NIPD study, 1% was established as the lower bound of the fetal fraction (Kong et al., 2021). Given that the DUX4 gene is located at the subtelomeric region and there are many repetitive fragments upstream, causing fewer informative SNPs to be available, the threshold of the fetal fraction increased to 2% for FSHD1. A lower fetal fraction does not guarantee the accuracy of NIPD, and resampling after 2 weeks to obtain a higher fetal fraction should be considered for these pregnancies. The expected number of informative SNPs was calculated as 96 ± 32 by randomly selecting individuals from the East Asian population to form mates in 1000G phase 3. Actually, the number limit is 10 for Type 1/2 SNPs and 5 for Type 3/4 SNPs. As there is no maternal background interference in paternally inherited families, the requirement is relaxed. If the number is below the threshold, NIPD might be considered inappropriate for that family, and an invasive prenatal diagnosis should be recommended. The FSHD1 families included in this study met the above quality control requirements, and the experimental results are credible.
Compared to the study by Qin et al. (2022), our research included a larger number of FSHD1 families, conducted testing at an earlier gestational age, and expanded to maternal genetic testing for pregnant women who are patients. It is worth emphasizing that our study achieved NIPD of FSHD1 in pregnancies with affected mothers for the first time. The research indicates that compared to paternal inheritance, NIPD in families where pregnant women were affected requires higher testing accuracy due to maternal background interference (Hanson et al., 2022). In order to minimize the risk of incorrect results, we established a stricter quality control criteria of informative SNPs for maternal inheritance. The threshold for the number of Type 1/2 SNP was set at 10, which was more stringent than that for Type 3/4 SNPs. Good testing performance was achieved in the three families (F1–F3) included in this study. Furthermore, we adopted amplicon sequencing to construct the DNA library, which reduces the cost to less than half of that of the hybridization capture method. Wu et al. demonstrated the feasibility of amplicon sequencing for NIPD by designing 20–30 amplicons flanking related pathogenic variants in seven different diseases (Wu et al., 2022). In consideration of the high recombination rate upstream of the DUX4 gene (Pini et al., 2023), we increased the number of SNPs to 402, which enabled more accurate detection of recombination events. The knowledge and experience gained from this study can be extended to the design of other panels.
Due to the rarity of FSHD1, we have only collected seven eligible pedigrees as of now. However, these families represented both maternal and paternal inheritance, providing a theoretical basis for further clinical verification. We will continue to expand the research cohort in the future. In this study, all SNPs were located upstream of the DUX4 gene, and therefore, the risk of recombination events could not be completely ruled out. Another limitation of our study was that family samples were still required and that the technique was not applicable for families with only one affected individual or fetal de novo mutations. Direct haplotyping approaches such as nanopore and linked-read sequencing are rapidly advancing, and future research should explore the possibility of implementing NIPD that does not rely on a proband.
AUTHOR CONTRIBUTIONS
Xiangdong Kong: Conceptualization, Funding Acquisition, Methodology, Project Administration, Supervision, Writing—Review & Editing. Yanan Wang: Conceptualization, Writing—Review & Editing. Di Wu: Conceptualization, Writing—Review & Editing. Xinyu Fu: Formal Analysis, Investigation, Validation, Visualization, Writing—Original Draft Preparation. Zhenhua Zhao: Data Curation, Project Administration. Lingrong Kong: Formal Analysis, Resources, Validation. Shaojun Li: Formal Analysis, Visualization, Software. Feifei Li: Resources, Validation. Xiujuan Han: Resources, Validation. Luming Sun: Funding Acquisition, Validation.
ACKNOWLEDGMENTS
The authors would like to thank all the participants who supported this study, and all the team members for their contributions to data collection and integrity.
FUNDING INFORMATION
Funding support was given to XK by the Henan Province Medical Science and Technique Foundation (SBGJ202102097), the Henan Province Fertility Protection and Eugenics Key Laboratory Open Project (SYLBHHYS2022-02), the funding was given to LS by the National Key Research and Development Program of China (2022YFC2704700), and the Henan Province Medical Science and technology project (joint construction) LHGJ20190113.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. Author Di Wu, Shaojun Li, Feifei Li, and Xiujuan Han are employed by Celula (China) Medical Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PATIENT CONSENT STATEMENT
All patients and their family members signed informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.