Epilepsy with faint capillary malformation or reticulated telangiectasia associated with mosaic AKT3 pathogenic variants
Martina De Bortoli, Marta Ivars as well as Laurence M. Boon, Eulalia Baselga, and Miikka Vikkula contributed equally to this study.
Abstract
Capillary malformations (CMs) are the most common type of vascular anomalies, affecting around 0.3% of newborns. They are usually caused by somatic pathogenic variants in GNAQ or GNA11. PIK3CA and PIK3R1, part of the phosphoinositide 3-kinase–protein kinase B–mammalian target of rapamycin pathway, are mutated in fainter CMs such as diffuse CM with overgrowth and megalencephaly CM. In this study, we present two young patients with a CM-like phenotype associated with cerebral anomalies and severe epilepsy. Pathogenic variants in PIK3CA and PIK3R1, as well as GNAQ and GNA11, were absent in affected cutaneous tissue biopsies. Instead, we identified two somatic pathogenic variants in the AKT3 gene. Subsequent analysis of the DNA obtained from surgically resected brain tissue of one of the two patients confirmed the presence of the AKT3 variant. Focal cortical dysplasia was also detected in this patient. Genetic analysis thus facilitated workup to reach a precise diagnosis for these patients, associating the vascular anomaly with the neurological symptoms. This study underscores the importance of searching for additional signs and symptoms to guide the diagnostic workup, especially in cases with atypical vascular malformations. In addition, it strongly emphasizes the significance of genotype–phenotype correlation studies in guiding clinicians' informed decision-making regarding patient care.
1 INTRODUCTION
Mutations in the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT)–mammalian target of rapamycin (mTOR) and RAS–RAF–mitogen-activated protein kinase (MAPK) pathways lead to the onset of vascular anomalies (Queisser et al., 2021). Among them, capillary malformations (CMs) are the most prevalent vascular malformations, affecting ∼0.3% of newborns (Uebelhoer et al., 2012). CMs typically appear as red or purple macules on the limbs and/or head and neck region, and they are primarily associated with mutations in the GNAQ and GNA11 genes upstream of the RAS–MAPK pathway (Uebelhoer et al., 2012). CMs can be associated with hypertrophy or overgrowth (Alcantara et al., 2017; Bolli et al., 2023; Davies et al., 2022). In rare cases, CM is rather accompanied by hypotrophy or undergrowth (Cubiró et al., 2020).
Focal cortical dysplasia (FCD) belongs to the broader spectrum of malformations of cortical development (MCD), and it has also been associated with pathogenic variants in genes belonging to the PI3K–AKT–mTOR pathway. However, CM has been rarely associated with FCD (Jansen et al., 2015; Luca et al., 2023; Pirozzi et al., 2022). In other MCDs, such as megalencephaly-capillary malformation (M-CM) and megalencephaly-pachygyria-polydactyly-hydrocephalus syndrome, CMs or vascular malformations of the skin are observed more often (D'Gama et al., 2017; Mirzaa et al., 2012; Pirozzi et al., 2022; Rivière et al., 2012). Somatic pathogenic PIK3CA variants have been described in these syndromes.
AKT, also known as protein kinase B, is a serine/threonine kinase and a key downstream effector of PI3K-mediated signaling. Dysregulation in its activation due to mutations in any of its three isoforms (AKT1, AKT2, and AKT3) can lead to the development of various types of cancers, including breast, ovarian, and prostate cancer (Mundi et al., 2016). The AKT3 isoform is enriched in the brain and pathogenic mosaic variants in AKT3 were first identified in children with hemimegaloencephaly (HMEG) (D'Gama et al., 2017; Jansen et al., 2015; Lee et al., 2012; Poduri et al., 2012). In this study, we describe two patients with cutaneous CM and severe epilepsy associated with two different mosaic AKT3 variants. This underscores the involvement of AKT3 in atypical CMs associated with cerebral phenotypes.
2 MATERIALS AND METHODS
2.1 Editorial policies and ethical considerations
Informed consent was obtained for the participant, as approved by the ethical committee of the Medical Faculty at the UCLouvain, Brussels, Belgium (B403201629786) and from the ethics Committee Fundació Sant Joan de Deu, Barcelona, Spain (PIC-181-21).
2.2 Sample collection and genetic testing
Samples of the patients were collected at the Saint Luc University Hospital, Brussels, Belgium and at Hospital Sant Joan de Deu, Barcelona, Spain. For Patient 1, tissue biopsies were collected at two different time points from a biopsy on the left leg and the affected cerebral tissue during programmed surgeries. DNA samples were screened via IonTorrent technology, using an IonAmpliseq in-house designed panel (Thermo Fisher Scientific) containing 53 genes belonging to pathways involved in vascular anomalies. Libraries were prepared using the Ion AmpliSeqTM Library Kit 2.0, according to the manufacturer's protocol (Pub. No. MAN006735; Life Technologies). The aimed theoretical panel coverage was 1000x, the average depth of coverage for the target was 1317x for the leg skin biopsy and 843x for the brain biopsy. For Patient 2, only a skin biopsy was available. Targeted sequences were designed to cover the entire coding sequences of genes included in the panel. Libraries were prepared using Agilent SureSelect XT HS DNA Reagent Kit for Illumina (Ref. G9706 with Index Primers 1–32), following the manufacturer's protocol. Theoretical vertical coverage was set to a minimum of 500x, the average depth of coverage achieved for the target was 980x. Reads were aligned to the human reference genome (hg19/GRCh37). The bioinformatics pipeline was optimized to detect somatic variant calling with a low variant allele frequency (VAF). The utilized NGS panel allows for the detection of variants down to 1% VAF. The NGS panel was validated using digital PCR as an alternative method for targeted detection of common hotspot variants. A complete list of the genes covered by each panel is reported in Table S1.
3 RESULTS
3.1 Patient 1
Patient 1, now aged 18, was conceived spontaneously and born at term, after a pregnancy marked by flu at 6 months of gestation. Growth parameters were normal: weight 3500 g (60th C), length 51 cm (80th C), and occipital frontal circumference 34 cm (35th C). The parents were healthy and non-consanguineous, and the family history was unremarkable. At birth, a red cutaneous stain on the left leg was observed. The patient also had skin hypotrophy on the lower limbs. The initial diagnosis was cutis marmorata telangiectatica congenita (CMTC).
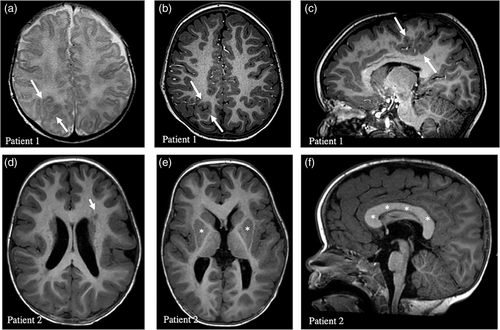
At the age of 2 weeks, he presented epileptic spasms; Othahara syndrome was suspected. EEG showed posterior right epileptic activity. Magnetic resonance imaging (MRIs) performed at the age of 1 month and 2 years showed parieto-occipital right FCD (Figure 1a–c). After the failure of pyridoxine treatment, he was treated with vigabatrin, and the seizures disappeared. A long-lasting EEG performed 1 month after starting vigabatrin showed no seizures and only a few slow waves in the right parieto-occipital region. The epilepsy was controlled with vigabatrin for 11 months, after which a recurrence of focal seizures was noted with the introduction of valproic acid, then levetiracetam, and the gradual reduction of vigabatrin. The epilepsy was difficult to stabilize, even with the addition of topiramate and clobazam. A temporal occipital parietal disconnection was performed at the age of 3. Despite this surgery and 4 antiepileptic drugs (valproic acid, levetiracetam, oxcarbazepine, and clobazam) tried over time, he continued to suffer from daily epileptic seizures. At the age of 5, he had severe epileptic decompensation, with more than 70 seizures a day. Vigabatrin was reintroduced with marked improvement. At the age of 5, a resection of the dysplastic brain lesion was performed.
Histology of the resected cerebral specimen showed non-specific changes, with the presence of reactive gliosis and some neuronal grouping (Figure S1A), well highlighted by immunohistochemistry with Neu N (Figure S1B). Subcortically, there were some widened spaces of Virchow–Robin with several capillaries, some lymphocytes, and macrophages (Figure S1C).
The patient's progress was characterized by a reduction in the number of seizures, but without complete remission. At present, the patient is being treated with multiple antiepileptic medications (valproate, vigabatrin, and oxcarbazepine). It is impossible to reduce vigabatrin because the seizures recur significantly. The patient also receives intermittent clobazam in the event of new seizures. The last EEG performed at the age of 15 showed an abnormal sleep–wake recording due to the presence of interictal epileptiform abnormalities on the right temporo-parieto-occipital regions, rare during wakefulness and increased during sleep, as well as sequences of rapid right frontotemporal rhythms. Other features included hyperlaxity, moderate axial hypotonia, global developmental delay, and moderate intellectual deficit. He acquired a sitting position at 9 months, began walking at 2 years, and spoke his first words at around 17 months. He attends a special school for intellectually disabled children and receives multidisciplinary care, including speech therapy, physiotherapy, and TEACCH teaching. It was not possible to carry out a standardized intellectual assessment. He was diagnosed with autism spectrum disorder (ASD) at the age of 7. In addition, he is affected by spastic cerebral palsy with left hemiplegia. Although his hearing is not impaired, he has vision problems, including astigmatism and hyperopia. He also presents with hypotrophy (length and girth) of the left leg, with a difference in leg length estimated at 2 cm at the age of 8.5 years and 3 cm at the age of 11 years. The patient did not have any pain in the leg, and further imaging was not performed. On physical examination at the age of 18, the growth parameters were as follows: height 190 cm (95th C), and weight 59 kg (15th C). The frontal occipital circumference was 55 cm (35th C) (at age 15). The clinical course was marked by thoracolumbar scoliosis and dorsal kyphosis diagnosed at the age of 13 years (Figure 2a). Due to deterioration over time, corrective surgery is planned. The patient also had severe planovalgus of the feet, which was managed with orthotics.
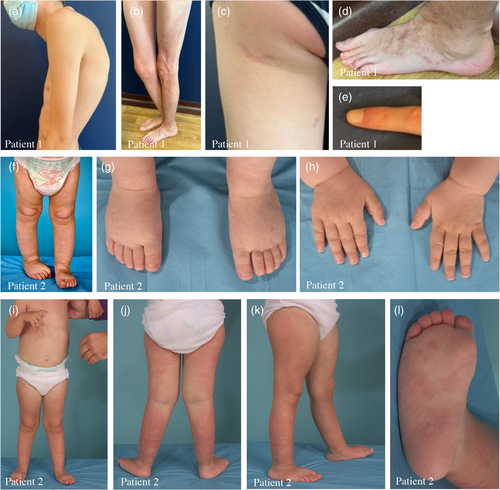
At the age of 11, a skin examination unraveled a large, well-geographically demarcated CM on the left leg with prominent dilated veins (Figure 2b) and three additional small CMs on the left thigh (Baek et al., 2015), left foot, and left index finger (Alcantara et al., 2017) (Figure 2c–e). Following genetic counseling, it was hypothesized that the PIK3CA gene could be involved. Targeted NGS analysis of a sample of the patient's blood using a panel comprising a total of 26 cancer genes (with a detection limit of 4% mosaicism) did not reveal any pathogenic variants. Subsequently, a skin biopsy was taken specifically from the site of the CM for tissular sequencing to identify somatic variants. A somatic pathogenic variant in the AKT3 gene (ENST00000366539) (c.49G>A; E17K) at 3% of variant allele fraction (29/1117) was identified. The cerebral tissue previously obtained during cortectomy surgery was subsequently studied, and the same variant was identified with a 1% variant allele fraction (13/1466). In both resected tissues, no variant in the PIK3CA gene was detected.
3.2 Patient 2
Patient 2, now aged 3, was conceived by in vitro fertilization using the parent's gametes. Although macrocephaly was diagnosed prenatally, no alteration was evident at birth. However, she had axial hypotonia, “doughy skin,” and hydrocephalus, which had not been operated on. In addition, mild laryngomalacia was diagnosed during the neonatal period. At the age of 7 months, the patient was admitted to the hospital for an assessment of neurological development, which revealed a delay. Brain MRI at 17 months of age revealed extensive areas of biparietal, posterior bitemporal, and left posterior insular pachygyria with probable associated dysplastic changes (loss of gray–white matter differentiation) (Figure 1d–f). Furthermore, opercular pachygyria was detected in the left frontal area, along with a few foci of periventricular cortical pachygyria and a diffuse increase in corpus callosum volume. Axial hypotonia was also assessed, revealing hyperlaxity of the joints. Further physical examination at 18 months of age showed subtle reticulated telangiectatic CM without atrophy in both legs (Figure 2g,h). No other anomalies were observed, such as sandal gap, syndactyly/polydactyly, macrodactyly, or apparent asymmetric growth. Although no obvious CM is currently detectable in the forehead or philtrum, photographs of the patient as a newborn, provided by the patient's family, revealed that a faint CM was visible in the philtrum previously. Despite a prolonged period without a definitive diagnosis, she was ultimately diagnosed with macrocephaly-capillary malformation syndrome (MCAP). In the recent evaluation of the patient at 3 years of age, the reticulated CM was found to be more marked, especially in the posterior region of the legs (Figure 2i–l).
The patient suffers from multiple episodes of epilepsy, the first of which occurred at just 17 days of age, leading to hospitalizations due to the difficulties in managing the epileptic seizures, which were also associated with cardiorespiratory arrest. The parents are now equipped with a manual resuscitator and trained in cardiopulmonary resuscitation procedures. The patient is currently being treated with zonisamide, clobazam, and lacosamide and is under medical care in a specialized epilepsy unit. The introduction of a ketogenic diet has recently contributed to improved seizure management. Hypothyroidism, diagnosed at the age of 6 months, is now treated with levothyroxine (25 mcg).
A genetic study of a DNA sample from the CM-affected skin was performed and revealed the presence of a pathogenic variant in the AKT3 gene (NM_005465.7): c.863C>T; T288I with a VAF of 14.31% (101/766). Variants in the PIK3CA gene were not detected.
4 DISCUSSION
Classical CMs are generally associated with pathogenic variants in GNAQ and GNA11, which are upstream of the RAS–RAF–MAPK signaling pathway. However, mutations in the PI3K–AKT–mTOR signaling pathway, involving both somatic and germline mutations, are also associated with a spectrum of CM-related conditions. Specifically, mutations in PIK3CA and PIK3R1 genes, encoding the catalytic and regulatory subunits of the PI3K kinase, respectively, have been observed in CM patients with hypertrophy or overgrowth, such as in diffuse CM with overgrowth and M-CM (Cottrell et al., 2021; Cubiró et al., 2020; De Bortoli et al., in revision; Lee et al., 2013).
MCD encompasses a heterogeneous group of disorders resulting from defects in various processes such as neuronal proliferation, migration, and maturation. These disorders are characterized by developmental delay, severe epilepsy, and seizures (Barkovich et al., 2012). Interestingly, some of these malformations are associated with germline or mosaic mutations in genes associated with the PI3K–AKT–mTOR pathway (Baldassari et al., 2019; Jansen et al., 2015).
AKT plays a central role as one of the main effectors of the PI3K–AKT–mTOR pathway and consists of three homologous isoforms: AKT1, AKT2, and AKT3. The E17K activating somatic mutation in AKT1 causes overgrowth disorders, such as the Proteus syndrome (Lindhurst et al., 2011), while the E17K heterozygous constitutional mutation in AKT2 is associated with hypoinsulinemic hypoglycemia with hemihypertrophy (Hussain et al., 2011). Mutations in AKT3, the isoform enriched in brain tissue, are less common and have been identified in children affected by HMEG (Alcantara et al., 2017; Davies et al., 2022; D'Gama et al., 2017; Lee et al., 2013).
Patient 1 described here was diagnosed at birth with undergrowth of the left leg associated with CM with dilated veins (De Bortoli et al., in revision), FCD, and severe epilepsy. A mosaic pathogenic variant in the AKT3 gene (E17K) was found in leg and brain tissues. Involvement of PIK3CA was initially suspected, but tests were negative. The second patient reported in this study had a reticulated CM associated with macrocephaly and pachygyria and also suffered from refractory epilepsy. The clinical features of the CM were those typical of MCAP. However, refractory epilepsy is not a common feature of MCAP due to PIK3CA pathogenic variants. A mosaic pathogenic variant of the AKT3 gene (T288I) was found in affected skin tissue.
Somatic pathogenic variants in the AKT3 gene have been reported in four individuals with CM and undergrowth/hypotrophy (Alcantara et al., 2017; Bolli et al., 2023; Davies et al., 2022). These individuals all shared the same AKT3 E17K somatic change. However, the clinical features of the capillary stains reported in these patients were different from those presented here. In addition, only two out of four individuals had a cerebral phenotype (HMEG or megalencephaly) and impaired neurodevelopment (Davies et al., 2022). One individual showed signs of ASD (Davies et al., 2022).
Only one patient has been reported to carry the T288I AKT3 pathogenic variant identified in our second case (Lai et al., 2022). The reported patient had HMEG and overgrowth syndrome, with infantile hemangioma (capillary hemangioma), toe syndactyly, and intractable focal epilepsy, which required cortical tissue resection. No CM was mentioned.
The clinical characteristics of the CMs associated with AKT3 variants remain poorly defined. The considerable variability in clinical presentation observed among patients within the spectrum of AKT3-related diseases may arise from variations in the pathogenic variant type, degree of mosaicism, as well as differences in the specific cell types harboring the pathogenic variant and their presence in different tissues of the body. The few reported CMs have been described as vascular anomalies marked by a deep reddish-purple hue, coarse reticulation, and linear patterns on the skin, accompanied by skin atrophy resembling CMTC (Davies et al., 2022). These patients also had soft tissue hypotrophy, similar to our Patient 1. Another patient was reported to have a faint reticulated stain with visible veins (Bolli et al., 2023). We noted a distinct, well-defined geographic stain with dilated veins in one patient and a faint reticulated stain in the other. It thus seems that the AKT3-associated vascular lesions are mostly atypical CMs, which are reticulated, linear, or faint geographic stains with deep reddish-purple hue. Notably, both patients discussed in this study also experienced frequent and severe episodes of epileptic seizures. This is consistent with findings showing that seizures are frequent in mice harboring activating Akt3 mutations (Baek et al., 2015; Tokuda et al., 2011).
In vitro studies have provided evidence for the pathogenic effect of the E17K pathogenic variant by demonstrating constitutive activation of AKT3 and its elevated kinase activity as a direct consequence (Alcantara et al., 2017). Mutated AKT3–E17K exhibits intensified pathological localization to the plasma membrane, resulting in increased binding to phosphatidylinositol-(3–4)-biphosphate (PIP2), which serves as a substrate for PI3K and triggers activation of the downstream pathway (Carpten et al., 2007; Parikh et al., 2012). However, further studies are needed to fully understand the molecular mechanisms underlying the development of these clinical manifestations and how the same variant can cause overgrowth or undergrowth. The second AKT3 variant reported in this study, T288I, has not been functionally characterized.
In summary, we report here the natural history of the oldest patient with CM-undergrowth-FCD, associated with a mosaic pathogenic E17K AKT3 variant. In addition, we present the second documented case of a patient carrying the T288I AKT3 variant associated with MCAP. It is important to note that these two patients share severe epileptic episodes. With this study, we want to highlight the importance of performing genetic analyses to help delineate the spectrum of AKT3-related diseases. Although there are a few examples of patients with AKT3 pathogenic variants associated with vascular and brain anomalies, these cases remain rare, and many go undiagnosed due to the difficulty of obtaining lesional brain tissue for genetic analysis. The presence of CM-like features in these patients facilitates diagnosis, as genetic testing can be performed on the CM. Reporting cases such as this is essential to help clinicians make a definitive diagnosis and choose appropriate management. Currently, treatment options mainly target symptoms, such as reducing seizure episodes, without addressing the underlying cause. However, identification of the specific gene and mutation involved will pave the way for personalized treatments, such as the AKT-inhibitors already available, and thus make it possible to target the cause.
AUTHOR CONTRIBUTIONS
Martina De Bortoli: Conceptualization, formal analysis, data analysis, writing (original draft), writing (review and editing). Marta Ivars: Conceptualization, formal analysis, writing (original draft), writing (review and editing). Nicole Revencu: Conceptualization and design, clinical data analysis, writing. Marie-Cécile Nassogne: Clinical data analysis, writing. Cinzia Lavarino: Data analysis. Anne Renders: Clinical data analysis, writing. Dana Dumitriu: Clinical data analysis. Raphael Helaers: Software. Laurence M. Boon: Clinical data analysis, conceptualization, writing. Eulalia Baselga: Conceptualization. Miikka Vikkula: Conceptualization, writing (review and editing), funding acquisition. All authors reviewed the results and approved the final version of the manuscript. All authors: data curation.
ACKNOWLEDGMENTS
This work has been supported by the European Reference Network on Rare Multisystemic Vascular Diseases (VASCERN)—Project ID: 769036, partly cofounded by the European Union within the Third Health Program “ERN-2016—Framework Partnership Agreement 2017–2021”. We are grateful to patients for their invaluable participation. These studies were financially supported by Fonds de la Recherche Scientifique—FNRS Grants T.0240.23 and P.C005.22 (to Miikka Vikkula), T.00.19.22 and P.C013.20 (to Laurence M. Boon), and by Fund Generet by King Baudouin Foundation (Grant 2018-J1810250-211305), the Walloon Region through FRFS-WELBIO Strategic Research Program (WELBIO-CR-2019C-06), the MSCA-ITN network V.A. Cure No. 814316, the 21CVD03 Leducq Foundation Networks of Excellence Program grant “ReVAMP”, and the European Union's Horizon 2020 Research and Innovation Program under grant agreement No 874708 (Theralymph) (all to Miikka Vikkula). The project was also funded by the Swiss National Science Foundation under the Sinergia project nro CRSII5_193694 (LB/MV). The project was also supported by Pierre M. fellowship. The authors thank the Genomics Platform of UCLouvain for IonTorrent PGM Next Generation Sequencing. We also thank the National Lottery, Belgium and the Foundation against Cancer (2010-101), Belgium, for support to the Genomics Platform of UCLouvain and de Duve Institute, as well as Fonds de la Recherche Scientifique—FNRS Equipment Grant U.N035.17 for «Big data analysis cluster for NGS at UCLouvain».
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The datasets supporting the current study have not been deposited in a public repository because data are not public due to privacy laws on patients. Some of the data may be requested by contacting to corresponding author.