Genetics of 21-hydroxylase deficiency: Clinical presentation should guide the investigation
Abstract
A young couple applied for preconception counseling because of a case of congenital adrenal hyperplasia in the family. They were concerned about their risk of giving birth to a child with classic congenital adrenal hyperplasia. The case presented here demonstrates the complexity of the genetics of 21-hydroxylase deficiency and the way the clinical presentation should guide the genetic inquiry.
1 INTRODUCTION
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is the most common form of CAH and it can present as a wide range of clinical syndromes. As it is an autosomal recessive disease, the heterozygous carriers are usually asymptomatic, mostly with normal biochemical testing, that is, normal basal level of 17-hydroxyprogesterone (17OHP) in serum, which is the biochemical marker of the disease. Those who have two abnormal genes may have a disease ranging from mild (non-classic 21OH-def (NCCAH)) through simple virilization (SVCAH) to a severe life-threatening form (salt wasting 21OH-def [SWCAH]). SWCAH can cause neonatal mortality and is a lifelong debilitating disease necessitating permanent medical treatment and follow up. Therefore, preventing the disease is of great importance (Speiser et al., 2018).
There is a good correlation between the genotype and phenotype in 21OH-def (Speiser et al., 2018). Different changes cause varying degrees of 21-hydroxylase functionality reduction. The rule is that only severely changed genes on both alleles cause classic CAH. If at least one allele is not severely changed, the phenotype should not be severe.
The genetics of 21OH-def is very complicated for several reasons: the presence of the 21-hydroxylase pseudogene (CYP21A1P) in a duplicated locus; the existence of numerous genetic changes of different kinds, including deletions and intronic changes; and wide copy number variation of both the coding gene and the pseudogenes. Double, triple, and even higher-order changes in cis position are also well known in the 21-hydroxylase gene (Pignatelli et al., 2019). Because of the complexity, full gene sequencing is not performed and the routine test includes a survey for common known changes and deletions.
The following report illustrates how remembering the above rule can help giving a correct genetic consultation.
2 REPORT
A 30y/o young couple (II-2 and II-3 in the pedigree, Figure 1) that applied for preconception counseling. They were concerned about their risk of giving birth to a child with classic CAH. The reason for their concern was II-1, the sister of II-2, who suffers from classic CAH due to 21-hydroxylase deficiency (21OH-def). She is 48y/o and treated with dexamethasone and fludrocortisone. II-2 and II-3 consulted a geneticist and he referred them, together with II-1, to perform a genetic study.
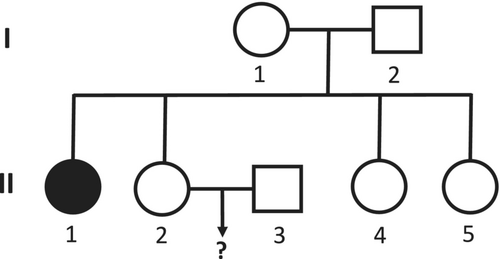
The three were genetically tested by a national center for 21OH-def. II-3, the husband, was found to be heterozygous for the p.Val282Leu change. His wife, II-2, was found to be a heterozygous carrier of the p.Gln319X change. Her sister, II-1 was found to be heterozygous for two changes, p.Val282Leu and p.Gln319X. Based on these results, the genetic consult advised the couple that their risk of conceiving a child with CAH is 25%.
As the genetic report of II-1 was not consistent with her clinical diagnosis (p.Gln319X/p.Val282Leu is expected to cause NCCAH and not classic CAH), two additional steps were undertaken. The first was to reevaluate her clinical diagnosis as it was made many years ago. Her old file revealed that her medical history was positive for at least three findings consistent with the diagnosis of classic CAH due to 21OH-def. The first is a measurement of very high levels of 17OHP (above the upper limit of the assay) on several occasions. Second, she underwent a clitoridectomy. Third, she was admitted to hospital for “salt-losing dehydration” on several occasions. The second step was to ask the genetic lab to carry out full sequencing of the 21-hydroxylase gene. This test is not a true full sequencing, but rather covers all the exons and parts of the introns of the gene. In this test, II-1 was found to be a heterozygous carrier of another change in the 21-hydroxylase gene, c.293-7C > G. This change lies in the acceptor splice site in intron 2. The change was reported by the genetic laboratory as a “variant of uncertain significance” according to the American College of Medical Genetic (ACMG) guidelines.
To further elucidate the distribution of the changes, we have asked the lab to check for the existence of c.293-7C > G in II-2, the sister of II-1. The result was negative. She does not carry this change.
3 DISCUSSION
The p.Gln319X is a severe genetic change related to classic CAH (Pignatelli et al., 2019). However, p.Val282Leu is a mild change and is actually one of the most common 21-hydroxylase changes found in NCCAH (Weintrob et al., 2000). Thus, the combination p.Gln319X/p.Val282Leu is expected to cause NCCAH, a mild form of the disease. Based on the above, was the genetic consult accurate? It is true that II-1 was diagnosed with classic CAH and was shown to have the p.Gln319X/p.Val282Leu genotype by the genetic test. It is also true that a child of II-3 and II-2 has a 25% risk of carrying the p.Gln319X/p.Val282Leu combination. However, II-3 has no consanguinity with II-2 and II-1. Thus, even their offspring who would carry p.Gln319X/p.Val282Leu are expected to have a mild phenotype, which is usually seen with this genotype. Therefore, II-2 and II-3 should be advised that their risk of conceiving an offspring with classic CAH is very low. Their child has a 25% chance of having NCCAH with the p.Gln319X/p.Val282Leu genotype.
The c.293-7C > G change that was found in II-1 is a rare change and was described by Rubtsov et al. (2011). Their patient had SWCAH and was homozygote for the change. They performed a functional analysis assay in order to assess the effect of this change and concluded: “The mutation impairs the use of intron 2 acceptor splice site resulting in intron retention in mRNA.” Furthermore, even if it does not severely impair the enzymatic function by itself, it may do so with additional changes on the same gene (likep.Val282Leu). We have recently published a case in which two “mild” changes in cis position caused a severely defected gene and classic CAH in the homozygote (Ilany et al., 2021).
However, at this point we cannot conclude that all the three changes that were found in II-1 explain her phenotype, as we do not know the allelic distribution of these changes. For example, c.293-7C > G and p.Gln319X can lay in cis position on the same allele, leaving only p.Val282Leu on the second allele and the phenotype should be NCCAH.
The fact that II-2 does not carry the c.293-7C > G and p.Val282Leu changes proves that the genotype of II-1 is c.293-7C > G+ p.Val282Leu/p.Gln319X. This genotype might explain her phenotype and reassures us regarding the low risk of II-2 and II-3 giving birth to a baby with classic CAH. The fact that II-3 carries p.Val282Leu change should not affect this conjecture, as the c.293-7C > G change is very rare. The existence of rare genetic changes that are not included in the routine genetic test and of new “de novo” genetic changes prevent us from absolutely ruling out the possibility of an offspring with CAH. However, we can safely reassure the couple that their risk of having such a child is very low.
We found that the p.Val282Leu change is linked to the c.293-7C > G change in this family. The routine genetic test does not check for the c.293-7C > G change. Finding the p.Val282Leu change usually would cause the genetic consult to predict that the risk of a child with CAH is very low. However, in this family it denotes a carrier of a severely changed allele with a 50% chance of inheriting the change. Therefore, if any of this family's members is found to be positive for p.Val282Leu, a complementary test for c.293-7C > G is necessary. For the parent of II-1 and II-2 (I-1 and I-2 in the pedigree) who is found to carry the change, a detailed family tree should be drawn and all the relatives at risk should be offered genetic testing and counseling.
II-1 and II-2 have two more healthy sisters (II-4 and II-5 in the pedigree) who have been told in the past that they do not carry a severe genetic change. Based on our findings, we advised the sisters to complete repeat genetic testing and consider testing the parents as well.
The conclusion from the case presented here is that the rule that a patient with classic CAH must have two severely changed alleles is very solid. Recently, my colleague O. Cohen and I have shown that the clinical presentation is a strong predictor of the genotype in non-classic 21OH-def as well (Ilany & Cohen, 2021). Further genetic investigation should be undertaken in every case where the genetic test result is not consistent with the clinical diagnosis. In the case presented, the acceptance of inconsistent genetic results might have led to inappropriate genetic counseling. Digging deeper into the genetics provided a better understanding of the classic CAH of II-1. Furthermore, it led to the conclusion that an unusual, severely changed gene exists in her family. This gene would appear as mildly changed on routine genetic tests, thus leading to erroneous genetic counseling. This problem is true for any rare severe change not included in the routine test panel. However, here we can track the family members at risk and test them as the p.Val282Leu change is linked to the rare unexamined severe change. Finding it would necessitate further testing for the c.293-7C > G change followed by a prompt genetic consult.
4 LESSONS FOR THE CLINICIAN
- Classic CAH is a serious condition and accurate genetic counseling is important.
- The genetics of 21-hydroxylase deficiency is very complicated, making genetic consult complex.
- The rule is that only severely changed genes on both alleles cause classic CAH.
- In case of unsuitability between the clinical presentation and the genetic report, further genetic investigation should be performed.
ACKNOWLEDGMENTS
The author thanks Mr. David Dvash for his help in preparing the manuscript. The author declares that no funds, grants, or other support were received during the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




