Expanding the phenotypic and genotypic spectrum of GGPS1 related congenital muscular dystrophy
Ruqaiah Altassan and Hanan AlQudairy contributed equally to this work.
Abstract
Congenital muscular dystrophies are a group of progressive disorders with wide range of symptoms associated with diverse cellular mechanisms. Recently, biallelic variants in GGPS1 were linked to a distinct autosomal recessive form of muscular dystrophy associated with hearing loss and ovarian insufficiency. In this report, we present a case of a young patient with a homozygous variant in GGPS1. The patient presented with only proximal muscle weakness, and elevated liver transaminases with spared hearing function. The hepatic involvement in this patient caused by a novel deleterious variant in the gene extends the phenotypic and genotypic spectrum of GGPS1 related muscular dystrophy.
1 INTRODUCTION
Congenital muscular dystrophies (CMDs) are a group of progressive disorders with a wide range of symptoms and ages of onset (Mercuri et al., 2019). CMDs are associated with various mechanisms and pathways, including sarcomeric, extracellular, membrane-centered, nuclear, mitochondrial, and metabolic mechanisms (Foley et al., 2020). Recently, congenital hearing loss and primary ovarian insufficiency have been linked to muscular dystrophy caused by mutations in GGPS1 (Foley et al., 2020). GGPS1 protein and the farnesyl pyrophosphate synthase (FPPS) (Roca-Ayats et al., 2018) are involved in protein prenylation, serve as precursors for the squalene cholesterol pathway (Kainou et al., 1999; Kuzuguchi et al., 1999), and play essential roles in various important cellular functions such as osteoclast formation and function, cytoskeletal actin dynamics, and signal transduction (Foley et al., 2020; Roca-Ayats et al., 2018; Vicent et al., 2000) and regulate negative feedback of the mevalonate pathway by stimulating ubiquitination and degradation of HMG-reductase (Foley et al., 2020). GGPS1 is expressed in skeletal muscle, female reproductive organs at different developmental stages, and the inner ear of rodents (Kaiyrzhanov et al., 2022). Accordingly, GGPS1 mutations have been found to specifically affect muscle, inner ear, and ovary cell mechanisms. In this report, we present a patient with a homozygous GGPS1 variant who exhibited muscular dystrophy with liver involvement but did not have congenital sensorineural hearing loss.
2 SUBJECTS AND METHODS
2.1 Patients and ethics
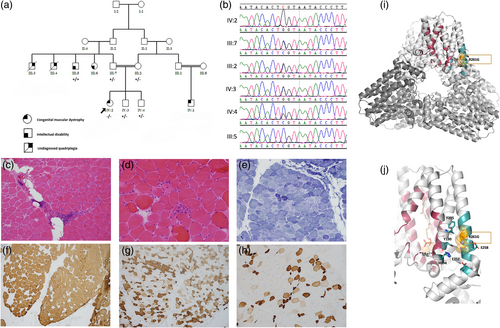
The patient and her family members (Figure 1a) ascertained/consented under an approved project at King Faisal Specialist Hospital and Research Centre, KFSHRC-RAC# 2120022.
2.2 DNA isolation, polymerase chain reaction
DNA was extracted from the blood using Puregene Blood Kit (Qiagen, Venlo, Netherlands), and measured using NanoDrop ND-1000 Spectrophotometer (ThermoFisher, Waltham, MA, USA). Primers were designed to target GPPS1 using Primer-3 Web-Tool.
2.3 Whole exome sequencing, whole genome sequencing, and mtDNA sequencing
Genomic DNA was fragmented, pooled, paired-ended, and sequenced on an Illumina platform with an average on-target coverage of approximately 30×, following the manufacturer's instructions. The mtDNA was amplified using long-range PCR and sequenced using the Illumina HiSeq2500 platform. The calls were generated by the DRAGON pipeline from Illumina.
2.4 Confirmatory sanger sequencing
Sanger sequencing was performed according to standard protocols on PCR fragments. The results were analyzed using SeqMan software (DNASTAR Inc., Madison, WI, USA).
2.5 Pathogenicity prediction of the novel variant
Pathogenicity was predicted using various in-silico classifiers (Supplementary file 1).
2.6 Cell passaging and harvesting
Epstein Barr Virus (EBV) immortalized lymphocyte cell line (LCL) was generated from the patient using whole blood samples collected in sodium heparin tubes according to standard protocols.
2.7 Histological analyses
A muscle biopsy was taken from the patient's right quadriceps at the age of 7 years. Frozen sections were stained with hematoxyline and eosin (H&E), routine histochemical and enzyme histochemical stains, and immunostains for the myosin heavy chain isoforms and dystrophy-associated proteins.
2.8 Computational structural analysis of mutant
Crystal structures of human GGPS1 (PDB ID 2Q80) was used for modeling and structural analysis. Models were manually inspected, and mutations were evaluated using the PyMOL program (pymol.org).
3 RESULTS
3.1 Clinical descriptions
3.1.1 Patient
The patient was an 8-year-old female firstborn of consanguineous parents. She was delivered vaginally at full term after an uneventful pregnancy. Her family history included childhood and adulthood deaths in two paternal uncles who had undiagnosed quadriplegia. The patient was asymptomatic until the age of three when she developed symptoms following a 2-week febrile illness. She experienced lower limb weakness, frequent falls, and difficulty standing, walking, and climbing stairs. Her creatine kinase (CK) level was elevated during her illness. She did not have weakness in her upper limbs, ptosis, swallowing difficulties, abnormal limb movements, or seizures.
Her developmental history was appropriate for her age. She achieved her initial motor milestones on time and her cognition, speech, and vision were intact. Later, her myopathy affected her lung function and airway clearance during respiratory illnesses. Her latest physical examination showed that she was a non-dysmorphic child with normal growth parameters. Her occipitofrontal circumference was 53 cm (95th percentile), weight was 35 kg (90th percentile), and height was 131 cm (90th percentile). The neuromuscular exam revealed proximal muscle weakness in her upper and lower limbs, a positive Gowers sign, waddling gait, and mild lordosis. Her deep tendon reflexes were +1. Her ocular motility test was normal and her cranial nerves were intact. Nerve conduction studies, including median motor and repetitive stimulation, were unremarkable. Her audiology assessment was normal bilaterally. Ovarian function assessment was deferred due to her pre-pubertal age. An abdominal ultrasound showed mild liver enlargement with moderate diffuse increase in parenchymal echogenicity and her echocardiography was normal.
Biochemical analyses identified high levels of creatine kinase, ranging from 2000 to 7426 U/L (normal range 24–195 U/L), and persistent elevation of liver transaminases in multiple readings (AST 198 U/L and ALT 298 U/L; normal range 10–45 U/L). Her complete blood count, coagulation profiles, and lipid profiles were unremarkable. WES/mtDNA-screening was inconclusive. Therefore, WGS was employed and revealed a homozygous variant in the GGPS1 (NM_001037277.1: c.781C>G; p.Arg261Gly) was confirmed by Sanger sequencing (Figure 1b). The variant was absent in local databases, present in GnomAD (0.000003981) and in 1000 Genomes (0.0000041), and has been described in compound heterozygous status in three individuals but has never been reported in homozygosity (Foley et al., 2020).
3.1.2 Muscle histopathology
The H&E stain (Figure 1c) showed moderate variation in myofiber size with many atrophic roundly contoured fibers and occasional hypertrophic fibers. Rare atrophic fibers were elongated or angulated and a rare pyknotic nuclear clump was seen. There was moderate perivascular mononuclear infiltration of T lymphocytes (CD3+) in the perimysium, which also contained several adipocytes. Occasional necrotic fibers and rare regenerative fibers were noted (Figure 1d), some with myophagocytosis. The number of internalized nuclei was within the normal range. Rare subsarcolemmal vacuoles showed glycogen and mitochondria on periodic acid Schiff (PAS) and oxidative enzyme stains, respectively. The modified Gomori trichrome showed mild and focal increase in endomysial connective tissue; no rimmed vacuoles, ragged red fibers or other inclusions were seen. The nicotine amide dehydrogenase-tetrazolium reductase (NADH-TR) and succinic dehydrogenase (SDH) stains showed a disrupted intermyofibrillar network (so-called moth-eaten fibers), occasional fibers with increased sarcoplasmic enzyme activity and many others with increased subsarcolemmal activity (Figure 1e). Rare atrophic angular fibers were darkly stained. There were no COX-negative fibers. The myosin heavy chain (MHC) immunostains showed a predominance of type 2 (fMHC) fibers (Figure 1f), with apparent poor fiber type differentiation as many of these fibers also expressed sMHC (Figure 1g). The developmental (dMHC) and neonatal (nMHC) isoforms were dually expressed in many normal-sized and atrophic fibers. Severely atrophic and angular fibers were apparent with these stains (Figure 1h). Dystrophin, sarcoglycans, and dysferlin were normally expressed.
3.1.3 Structural analysis and predicted effect
Human GGPS1, a 300-amino acid protein, adopts an all-helical trans-prenyltransferase fold consisting of 12 α-helix microdomains. GGPS1 monomers associate into a trimer of dimers, forming a hexamer (Figure 1i). In certain circumstances, an octamer can also form (Kavanagh et al., 2006; Muehlebach & Holstein, 2023). GGPP binds to a mostly hydrophobic GGPS1 pocked formed by residues R28, L31, F35, H57, R73, L122, L155, F156, Al159, V160, and F184 (Consortium PD-K., 2020; Kavanagh et al., 2006) (Figure 1i). Magnesium ions bind to aspartate-motifs on helices 4 and 8 in each of the six monomers (Consortium PD-K., 2020; Kavanagh et al., 2006).
R261 is located in the C-terminal of the 11th helix, close to the catalytic site of the hexamer, where R261 engages in several interactions that stabilize the surrounding helices (Figure 1j). It forms ionic interactions with E252 and E258, hydrogen bonds with Y190 and H194, and its aliphatic moiety helps anchor F295. The loss of these interactions due to the R261G mutation could destabilize the region and lead to distortions and instabilities in the adjacent catalytic site. The R261G variant is predicted to be deleterious by MutationTaster (Schwarz et al., 2014), probably damaging (score: 0.977; sensitivity: 0.76; and specificity: 0.96) by Polyphen2 (Adzhubei et al., 2010), not tolerated by SIFT (Sim et al., 2012) and others (Supplementary file 1).
4 DISCUSSION
CMD resulting from biallelic mutations in the GGPS1 is associated with extra-muscular manifestations, mainly hearing loss and primary ovarian failure in affected females. Detailed phenotyping and genotyping of 26 individuals with GGPS1 mutations have been recently reported (Foley et al., 2020; Kaiyrzhanov et al., 2022). The onset of presentation and severity of symptoms were heterogeneous among affected patients, ranging from antenatal presentation with decreased fetal movements to slowly progressive muscle weakness. Elevated CK levels were a constant finding in all affected patients. Sensorineural hearing loss is considered a variable phenotype of GGPS1 and has been reported in 70% of affected patients. Interfamilial variability has been observed and a variant-specific effect has been hypothesized (Kaiyrzhanov et al., 2022). The mechanism of ear involvement in GGPS1 defects is still being explored, but the expression of Ggps1 in the embryonic and postnatal mice cochlea has been studied (Foley et al., 2020; Kaiyrzhanov et al., 2022).
Importantly, only compound heterozygous R261G mutations in GGPS1 have previously been reported. Affected patients presented with variable symptoms such as sensorineural hearing loss, wheelchair dependence, respiratory insufficiency, scoliosis, and failure to thrive. Our patient is homozygous for the R261G mutation.
All post-pubertal females affected by GGPS1 defects had primary ovarian failure. GGPS1 was strongly expressed in the embryonic mice female gonads. Furthermore, GGPS1 is essential for folliculogenesis and oocyte maturation. As a result, GGPS1 deficiency in oocytes could impact female fertility (Jiang et al., 2017). Decreased GGPS1 expression in the testis has been associated with infertility in men, suggesting a role for this enzyme in both male and female reproductive organs (Diao et al., 2016). None of the post-pubertal affected males with GGPS1 were tested for fertility function, but none had offspring.
Previous histopathological muscle examinations in patients showed myopathic and dystrophic changes, including necrosis, regeneration, and mitochondrial accumulation of myofibers. In contrast, our patient's muscle examination showed the presence of inflammation, type 2 fiber predominance (not type 1), coexpression of sMHC and fMHC in a significant subset of fibers, and a possible neurogenic component.
Unlike previous reports (Table S1), our patient's motor function was initially normal. Her myopathy became evident after a viral infection of the upper respiratory tract. At the age of 3 years, she presented with slowly progressive proximal muscle weakness that gradually began to interfere with her ability to walk. Her hearing was preserved, but future hearing loss cannot be ruled out. Surprisingly, screening of her liver function showed elevated liver transaminase levels, which had not been previously reported in individuals with GGPS1 defects. The mechanism of liver involvement in GGPS1 defects is not well understood. Deficiency of GGPP can result in the accumulation of FPP, leading to the farnesylation of liver kinase B1 (LKB1). This may cause mitochondrial dysfunction and hepatic inflammation via activation of the AMP-activated protein kinase (AMPK) pathway, release of pro-inflammatory cytokines, and macrophage infiltration (Muehlebach & Holstein, 2023).
In summary, our findings reveal an additional phenotype of this ultra-rare congenital muscular dystrophy, expanding the pathophysiology of GGPS1-related human diseases.
AUTHOR CONTRIBUTIONS
Namik Kaya, Ruqaiah Altassan, and Stefan T. Arold: Conceptualized, designed, and supervised the project, and drafted and revised the manuscript. Ruqaiah Altassan: Recruited the patients to the project. Ruqaiah Altassan and Mohammed AlMuhaizea: Performed clinical examinations, and reviewed the patient's charts and revised the clinical descriptions. Hazem Ghebeh and Amer Almzroua: Performed flow cytometry experiments and related data analysis. Dilek Colak: Performed in silico prediction analyses of the variant, involved in drafting. Hanan AlQudairy and Sarah AlJebreen: Performed the experiments, reviewed the charts, involved in the initial drafts of the clinical description and manuscript. Karla A. Peña-Guerra and Stefan T. Arold: Performed the structural modeling analysis. All authors involved in drafting/revising the manuscript, and read the final draft.
ACKNOWLEDGMENTS
We are grateful to the patients and their family.
FUNDING INFORMATION
King Salman Center for Disability Research (RAC, 2180004) support for Dr. Namik Kaya. The research by Stefan T. Arold and Karla A. Pena-Guerra was supported by the King Abdullah University of Science and Technology (KAUST) through the baseline fund and the Award No. FCC/1/1976-33 and REI/1/4446-01 from the Office of Sponsored Research (OSR).
CONFLICT OF INTEREST STATEMENT
We have no conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




