Maternal CHD7 gonosomal mosaicism in a fetus with CHARGE syndrome
Abstract
Parental mosaicism is important in families with de novo mutations. Herein, we report a case of fetal CHARGE syndrome (CS) with a CHD7 variant inherited from maternal CHD7 gonosomal mosaicism. The variant was detected through trio-based whole-exome sequencing and Sanger sequencing. High-depth whole-exome sequencing was performed for the identification of parental mosaicism. A novel heterozygous CHD7 nonsense mutation (c.5794G>T/ p.E1932*) was detected in the tissue from the aborted fetus. The parents were wild-type, indicating that the mutation was a de novo variant. The mutation was suspected to be the cause of the fetal CS. However, high-depth whole-exome sequencing revealed maternal gonosomal mosaicism at a variant allele frequency of 3.2%–23.3%. The variant was identified in various tissues (peripheral blood, hair follicles, buccal epithelia, and pharyngeal epithelia) from the asymptomatic mother. We confirmed maternal CHD7 gonosomal mosaicism as a genetic cause of fetal CS. Our results emphasize the importance of clinical analysis in accurately determining the parents’ status in detecting the CHD7 de novo variant in fetal CS, as this analysis has vital implications for evaluating the recurrence risk for genetic counseling.
1 INTRODUCTION
CHARGE syndrome (CS, OMIM 214800) is a congenital malformation syndrome characterized by the variable occurrence of coloboma (C), heart defects (H), choanal atresia (A), growth retardation and/or anomalies of the central nervous system (R), genitourinary anomalies (G), and ear anomalies (E). This rare autosomal dominant disorder has a prevalence of 1 in 8500–12,000 live births (Issekutz et al., 2005; Källén et al., 1999) in approximately two-thirds of cases ascribed to a CHD7 mutation (Vissers et al., 2004). CHD7 encodes a member of the chromodomain helicase DNA-binding (CHD) protein family of evolutionarily conserved chromatin-remodeling proteins, whose members are involved in tissue-specific regulation of gene expression and play important roles during embryonic development (Woodage et al., 1997). The 2016 diagnostic criteria were revised by Hela, in which CHD7 was described as one of the four main diagnostic criteria, providing more possibilities for the prenatal diagnosis of CS (Hale et al., 2016).
Mosaicism refers to the presence of two or more genomes in one individual and is caused by postzygotic de novo genetic events, such as variants or abnormalities in chromosome replication, segregation, and/or methylation. (Thorpe et al., 2020). Specific types of mosaicism involve parts of the body that harbor variant cells and the potential for transmission to offspring. Mosaicisms can be classified as somatic mosaicism, germline mosaicism, and gonosomal mosaicism (Biesecker & Spinner, 2013). Gonosomal mosaicism arises at an early embryonic stage and can involve both somatic and germ cells, potentially leading to higher rates of intergenerational recurrences among the three mosaicism types (Veltman & Brunner, 2012; Campbell, Stewart et al., 2014; Wright et al., 2019; Thorpe et al., 2020). Few cases of parental CHD7 mosaicism regarding CS have been reported (Bartels et al., 2010; Delahaye et al., 2007; Jongmans et al., 2006; Jongmans et al., 2008; Lalani et al., 2006; Matías-Pérez et al., 2018; Pauli et al., 2009; Vuorela et al., 2007). Herein, we report a case of fetal CS inherited from maternal CHD7 gonosomal mosaicism. To the best of our knowledge, this is the first report of fetal CS resulting from maternal CHD7 gonosomal mosaicism.
2 CLINICAL DATA AND METHODS
2.1 Patients and ethics statement
We identified a pregnant woman with four previous adverse pregnancy outcomes (APOs) and an affected fetus showing multi-malformations according to a follow-up detailed ultrasound examination. Ethical approval for the study was obtained from the Medical Ethics Committee of the West China Second University Hospital, Sichuan University. Written informed consent was obtained from the parents.
2.2 Molecular analysis
Traditional G-banded karyotyping was performed using standard protocols (Steele & Breg Jr, 1966). Copy number variation sequencing (CNV-seq) was conducted by sequencing the DNA libraries on a NextSeq 500 platform (Illumina, San Diego, CA, USA), and the reads were uniquely and precisely mapped using the Burrows–Wheeler algorithm. Identified and mapped CNVs compared against publicly available databases according to the manufacturer's protocols and a previous study (Wang et al., 2018).
Trio-based whole-exome sequencing (trio-WES) was performed using tissues from the aborted fetus and parental peripheral blood mononuclear cells (PBMCs). Exome sequences were captured using a Nano WES Human Exome V1 (Berry Genomics, Beijing, China), and the exome libraries were sequenced on a NovaSeq6000 platform (Illumina) after enrichment and purification. The Burrows–Wheeler Aligner software tool was used to align the sequencing reads with GRCh38/hg18. The sequencing, variant filtration, and interpretation were conducted as previously described (Yang et al., 2021). Sanger sequencing was performed after primer design using Primer Premier software (Premier Biosoft, Rockville, MD, USA), and polymerase chain reaction amplification was performed using standard methods. The sequencing results were analyzed using Chromas 2.1 software. Mosaicism levels were analyzed using high-depth WES on a HiSeq 4000 PE150 system (Illumina) with a sequencing depth of 1000×. Exon trapping and library construction were performed using the Agilent SureSelect Human All Exon V6 Kit (Agilent Technologies, Santa Clara, CA, USA). Effective data were mapped to the reference genome (GRCh37/hg19) using BWA software. Insertion–deletion and single-nucleotide polymorphism sites were identified using SAM tools and annotated using ANNOVAR software. The variants were selected if their minor allele frequencies were less than 0.05 in gnomAD (http://gnomad.broadinstitute.org/), the 1000 Genomes Project Database (http://www.internationalgenome.org/), the Exome Aggregation Consortium (ExAC) browser (http://exac.broadinstitute.org/), the Novogene database, and the Chinamap database (http://www.mbiobank.com/info/), or they were removed. The detrimental effects of the variants were classified according to the American College of Medical Genetics and Genomics standards and guidelines (Richards et al., 2015). The detected variants were systemically evaluated based on scientific and medical literature, PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), ClinVar (http://www.ncbi.nlm.nih.gov/clinvar), Online Mendelian Inheritance in Man (OMIM, http://www.omim.org/), the Human Gene Mutation Database (http://www. hgmd.org), and the Human Genome Variation Society (http://www.hgvs.org/dblist/dblist.html). Data were analyzed using Integrative Genomics Viewer for putative mosaic indels.
3 RESULTS
3.1 Clinical data
A 28-year-old G5 woman (gravida 5) was referred at 18 weeks of gestation (WG) for amniocentesis because she had four previous APOs. Three of the four APOs were detected as abnormal ultrasound findings, including a male fetus affected by oligohydramnios, male fetus affected by the absence of the cerebellar vermis, and female fetus affected by multi-malformations, such as congenital heart disease (right aortic arch, right catheter arch, left subclavian artery vagus, and narrow diameter of the aortic valve ring), cleft lip and palate, and absence of the cerebellar vermis and the left subclavian artery vagus. The fourth fetus was miscarriaged at 8 WG.
Ultrasound examination for anomaly screening was normal during the current pregnancy, and her pregnancy was uneventful. The patient denied the consumption of alcohol, drugs, tobacco, or any other toxic substances. The father was 33 years old. The couple was non-consanguineous with no recognizable phenotype or significant underlying disease and had no family history of hereditary diseases. The karyotyping and CNV-seq results were normal. The prenatal invasive diagnosis procedure was performed at 18 + 6 WG without complications, and amniocentesis analysis via karyotyping and CNV-seq revealed a normal fetal karyotype 46, XX.
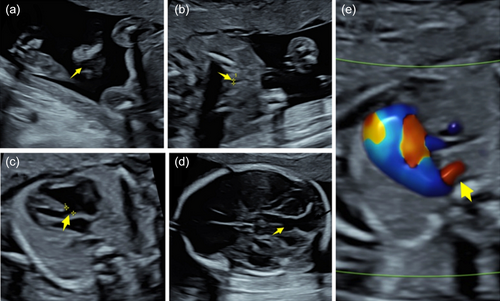
However, detailed ultrasound examination for anomaly screening on follow-up scans at 22 + 1 WG showed multi-malformations, including congenital heart disease, cleft lip and palate, absence of the cerebellar vermis, and enlargement of the posterior fossa cisterns and right subclavian artery vagus (Figure 1). The pregnancy was terminated at 32 WG with careful consideration of the parents after genetic counseling, in accordance with Chinese law.
4 IDENTIFICATION OF A NOVEL CHD7 MUTATION
Tissues from the aborted fetus and parental PBMCs were examined using trio-WES and Sanger sequencing. One heterozygous variant in a known disease-causing gene was identified in the fetus, which is a novel single-nucleotide substitution (c.5794G>T/p.E1932*) detected in exon 29 of CHD7. Sanger sequencing validated that both parental PBMCs were homozygous for the wild-type allele (Figure S1), suggesting that the mutation was de novo”. This sequence change creates a premature translational stop signal in CHD7, which is expected to result in an absent or disrupted protein product. This nonsense mutation has not been reported in the 1000 Genomes Project or ExAC databases. According to the American College of Medical Genetics and Genomics variant interpretation guidelines (Richards et al., 2015), the variant was classified as “pathogenic.” To the best of our knowledge, this mutation in CHD7 has not been previously reported. Our data support the clinical diagnosis of CS based on Hale's diagnostic criteria (Hale et al., 2016).
4.1 Identification of maternal gonosomal mosaicism
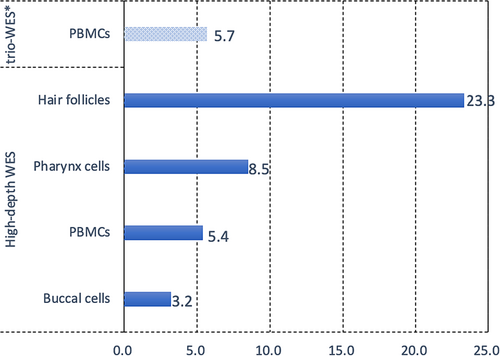
Although no CHD7 variants were detected in the parental PBMCs using trio-WES and Sanger sequencing, after carefully reviewing the BAM files of trio-WES using the Integrative Genomics Viewer, a variant allele fraction (VAF) of 5.7% (6/106) for this variant was observed in the maternal PBMCs. However, it was not detected because it was below the detection threshold. To determine whether the mosaicism of this mutation was present in the mother, high-depth WES was performed on DNA extracted from her PBMCs, hair follicles, buccal cells, and pharyngeal cells. Notably, the same variant was detected in all four tissue types (Figure S2), and the distribution of VAFs was estimated as 3.2%–23.3% (Figure 2). The highest level of mosaicism was observed in the hair follicles, with an abnormal allele count of 127 of 544 reads (23.3%). The other three tissues tested positive for low-level somatic mosaicism (<10%) as follows: approximately 8.5% (54/632) in pharyngeal cells, 5.4% (29/542) in PBMCs, and 3.2% (28/877) in buccal epithelial cells. Clinical examination of the mother revealed no recognizable phenotype associated with CS, suggesting maternal gonosomal mosaicism for the CHD7 mutation. The findings for CHD7 mosaicism in CS in the asymptomatic parents following definitive detections are summarized in Table 1. These findings may be helpful, as our literature review provided few reports on this mosaicism (Bartels et al., 2010; Jongmans et al., 2006; Jongmans et al., 2008; Matías-Pérez et al., 2018; Pauli et al., 2009).
| References | Patient sex | Age at clinical evaluation (year) | Exon | Nucleotide change | Amino acid change | Segregation | Mosaicism cells | Mosaicism level |
|---|---|---|---|---|---|---|---|---|
| Jongmans et al., 2008 | F | 14 | 8 | c.2520G>A | p.Trp840X | Paternal somatic mosaicism | PBMCsa | ND |
| M | 11 | |||||||
| Jongmans et al., 2006 | M | 7 | 30 | c.5982G>A | p.W1994* | Maternal somatic mosaicism | PBMCsa | ND |
| M | 3 | |||||||
| Bartels et al., 2010 | 35 | c.7636G>T | p.E2546X | Paternal somatic mosaicism | PBMCsa | ND | ||
| Matías-Pérez et al., 2018 | F | 24 | 31 | c.6184C>T | p.Arg2062Trp | Maternal somatic mosaicism | PBMCsa | ND |
| M | 9 | |||||||
| Pauli et al., 2009 | F | 6 | 34 | c.7302dupA | p. (Glu2435Argfs*35) | Paternal germline mosaicism | Spermatozoab | 27.10% |
| M | 3 | |||||||
| This article | F | 32 WGc | 29 | c.5794G>T | p.E1932X* | Maternal gonosomal mosaicism | PBMCs, hair follicles, buccal cells and pharynx cells | 3.2%–23.3% |
- Note: *, Nonsense mutation; F, female; M, male; ND, not done; NK, not known; PBMCs, peripheral blood mononuclear cells; WG, weeks of gestation.
- a The study only identified PBMCs but did not identify other tissues.
- b The mutation was not detected in the father's PBMCs, hair follicles, and buccal cells.
- c Termination of the pregnancy at 32 WG.
5 DISCUSSION
We report a case of fetal CHARGE syndrome (CS) with a CHD7 variant inherited from maternal CHD7 gonosomal mosaicism. This is the first report arising from a prenatal study. CS is a rare genetic disorder with a highly variable phenotype and possibly severe prognosis because of combined congenital anomalies, sensory deficits, variable intellectual prognosis, the need for recurrent hospitalizations and surgeries, and high morbidity and mortality (Hefner & Fassi, 2017). However, CS has a wide spectrum of abnormalities, most of which cannot be visualized by prenatal ultrasound. Therefore, the prenatal diagnosis rate should be improved. As shown in our study and others, prenatal diagnosis of CS by identifying mutation in CHD7 is critical but rarely reported (Biard et al., 2021; Busa et al., 2016; Colin et al., 2012; Legendre et al., 2012).
We detected a nonsense mutation (c.5794G>T/p.E1932*) in CHD7 in the fetus affected by CS in our study; however, this mutation was not initially observed in the parents. When a child has a phenotype related to CS and the parents do not, a de novo variant (DNV) or parental mosaicism is clinically suspected (Zlotogora, 1998). The transmission of parental mosaicism may explain up to 10% of apparent DNVs in some pathologies, and mosaicism rates may be underestimated (Acuna-Hidalgo et al., 2016; Stosser et al., 2018). Based on the suspicion of maternal CHD7 mosaicism because of her pregnancy history of APOs and the ignored low-level VAF below the threshold of detection in the maternal PBMCs detected using trio-WES, the same mutant allele was detected using high-depth WES in the peripheral blood, hair follicles, buccal epithelia, and pharyngeal epithelia, which originated from all three maternal germ layers (ectoderm, endoderm, and mesoderm), suggesting maternal gonosomal mosaicism in the unaffected mother. Ultimately, we observed maternal CHD7 gonosomal mosaicism at 3.2%–23.3% VAF. Additionally, we summarized previous studies in which CHD7 mosaicism was detected in asymptomatic parents with CS-affected offspring (Table 1) (Bartels et al., 2010; Jongmans et al., 2006; Jongmans et al., 2008; Matías-Pérez et al., 2018; Pauli et al., 2009), most of which had more than one affected child. In previous studies with a limited number, the cases were diagnosed after birth. Our study adds to the understanding of this rare condition in prenatal diagnosis and expands the molecular and clinical spectrum of CS.
Notably, for an individual family with a child having a genetic disorder caused by an apparent DNV, estimating the prevalence and levels of parental mosaicism for the recurrence risk is important, particularly for severe and highly penetrant genetic disorders for which medical therapy remains limited. First, identifying the parent in whom a mutation originates may be useful, as maternally transmitted mutations comprise a minority of alleles, and the risk of recurrence for apparent DNVs is 10-fold higher than when inherited from the fathers (Campbell, Yuan, et al., 2014; Jónsson et al., 2018). Second, it is important to distinguish between gonosomal and germline mosaicism because higher intrafamilial recurrence rates have been reported in gonosomal mosaicism cases than in germline mosaicism cases, which have a low to intermediate recurrence risk (Campbell, Stewart, et al., 2014; Rahbari et al., 2016; Ye et al., 2018). Finally, variants detected in the parental blood positively correlate with a substantially higher recurrence risk compared with those confined to the germline (Campbell, Stewart, et al., 2014). Rahbari et al. showed that when mosaicism for the variant is present in more than 1% of parental blood cells, the recurrence risk increases to 24%; this risk increases to 50% when mosaicism is found in more than 6% of parental blood cells (Rahbari et al., 2016). In our study, maternal gonosomal mosaicism showed a 5.4% VAF in PBMCs, indicating that the risk of recurrence in this family is high, which is important for genetic counseling.
Parental mosaicism was not initially detected in our study maybe because of two limitations to the clinical detection of mosaicism. First, low-level somatic mosaicism with a VAF of less than 10% is typically undetectable using current routine diagnostic methods (Gambin et al., 2020; Qin et al., 2016; Rodríguez-Martín et al., 2020). The ability of WES to detect mosaicism is proportional to the read depth of coverage or the number of reads that are available covering a given base position. In our study, the parents did not present with CHD7 mosaicism according to routine trio-WES and Sanger sequencing. The causative mutation was later found in the mother using high-depth WES, which not only detected 23.3% of DNA copies extracted from hair follicles but also revealed that 3.2%–8.5% of DNA copies extracted from the other three tissues carried the c.5794G>T variant, suggesting low-level mosaicism for this defect. Consistent with the findings of a previous study, we observed that the VAFs in non-blood tissues were similar to those in blood samples (Gambin et al., 2020). Second, multiple accessible tissues should be analyzed. Genetic screening of the parents of an affected child is typically performed using DNA extracted from lymphocytes. However, lymphocytes are an unstable source of genetic material, given that they can undergo multiple rounds of self-renewal during hematopoiesis, exhibit rapid turnover, and can undergo clonal expansion. The diversity of the clonal lineages that give rise to circulating blood cells appears to decrease with age (Busque et al., 1996; Jaiswal et al., 2014), and clonal expansion of peripheral blood lymphocytes may lead to an erroneous conclusion of an increased level of mosaicism over time. Moreover, some mutations are enriched in other tissues or are detectable only in sperm (Smith et al., 2016), such as in patients with Pallister–Killian syndrome, and patch-like patterns occurring in the skin may need to be sampled for tetrasomy of isochromosome 12p because mosaicism is limited at that site (Choo et al., 2002).
To our knowledge, this is the first report of a CS-affected fetal inherited from a clinically healthy, CHD7 gonosomal mosaicism mother, it adds to the understanding of this rare condition. More robust screenings for parental mosaicism, the determination of the parent of origin, and an analysis of multiple accessible tissues to detect parental mosaicism with affected fetuses should be conducted in larger trials to estimate the recurrence risk, which is important for genetic counseling.
AUTHOR CONTRIBUTIONS
Ting Bai: Investigation; writing—original draft; writing—review and editing. Ying Shen: Conceptualization; supervision. Yanting Yang and Siyu Dai: Investigation. Hongqian Liu: Conceptualization; funding acquisition; writing—review and editing; supervision.
ACKNOWLEDGMENTS
The authors would like to thank the family for their generous participation in this study.
FUNDING INFORMATION
This study was jointly funded by grants from the National Key Research and Development Program of China (No. 2022YFC2703400).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




