A novel variant in CYFIP2 in a girl with severe disabilities and bilateral perisylvian polymicrogyria
Abstract
Bilateral perisylvian polymicrogyria (BPP) is a structural malformation of the cerebral cortex that can be caused by several genetic abnormalities. The most common clinical manifestations of BPP include intellectual disability and epilepsy. Cytoplasmic FMRP-interacting protein 2 (CYFIP2) is a protein that interacts with the fragile X mental retardation protein (FMRP). CYFIP2 variants can cause various brain structural abnormalities with the most common clinical manifestations of intellectual disability, epileptic encephalopathy and dysmorphic features. We present a girl with multiple disabilities and BPP caused by a heterozygous, novel, likely pathogenic variant (c.1651G>C: p.(Val551Leu) in the CYFIP2 gene. Our case report broadens the spectrum of genetic diversity associated with BPP by incorporating CYFIP2.
1 CLINICAL REPORT
The patient is the third of four children of a nonconsanguineous couple. She was born by urgent cesarean section at 30 + 3 weeks of gestational age because of sinus bradycardia. Her birth weight was 1310 g (−1.2 standard deviation (SD)), height 38 cm (−1.1 SD) and head circumference 27.5 cm (−0.6 SD). Apgar scores were 8/5/8. Soon after birth bilateral hip dislocation, left clubfoot, multiple joint contractures, muscular hypotonia and atrial septal defect were detected. Sick sinus syndrome was identified at 4 months of age. Since the age of 4 months, the patient has suffered from treatment-resistant epilepsy and has been sensitive for upper and lower respiratory tract infections associated with asthma-like wheezing. Osteoporosis was diagnosed at 5 years and central sleep apnea at 7 years of age. Osteoporosis has been treated with intermittent bisphosphonate infusions and central sleep apnea with continuous positive airway pressure. The patient has undergone several surgical procedures. A tracheostomy tube was placed at 3 months, gastrostomy tube at 5 months and pacemaker at 7 months of age. Gingival overgrowth has been operated twice, at 4 and 6 years of age.
At present, at the age of 8 years, she is of normal size but has microcephaly. Her weight is 28 kg (−0.1 SD), length 131 cm (−0.1 SD) and head circumference 48.8 cm (−2.5 SD). Her dysmorphic features include micro- and retrognathia, smooth philtrum, puffiness of the legs and tapered fingers (Figure 1). She communicates with expressions, gestures and making different sounds. She can choose from two or three alternatives by focusing her eye into the chosen object. She can hold her head straight, turn her head side to side, grasp the objects and shake the toys. She can sit and stand with appropriate support for a short period. She is socially interactive. Based on the Bayley Scales III test, she is profoundly intellectually disabled.
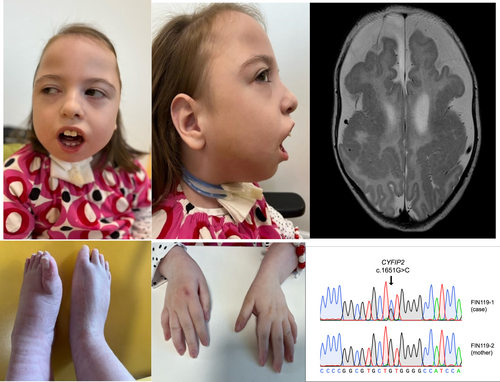
Brain magnetic resonance imaging (MRI) showed (Figure 1) extensive polymicrogyria with thickening involving bilateral perisylvian cortex of frontal and temporal lobes extending to parietal lobes. Antero-posterior diameter of the skull was large, with relative narrowing of the transverse diameter suggesting the scaphocephaly. Central, temporal, frontal, and parietal cerebro-spinal fluid spaces were wide and corpus callosum was hypoplastic.
Via molecular karyotyping and optical genome mapping, a 400 kb tandem duplication [ogm[GRCh38] 7q36.2(154342577_154742890)x3] was identified in the patient's (FIN119-1) and her mother's sample (FIN119-2) and thus held as a variant of unknown significance. Clinical exome sequencing in 2015 did not report any likely pathogenic nor pathogenic variants. In 2022, novel exome sequencing identified a heterozygous variant NM_001291722.2: c.1651G>C: p.(Val551Leu) in the cytoplasmic FMRP-interacting protein 2 gene (CYFIP2, 606323.0001) (OMIM # 618008). The variant was confirmed using Sanger sequencing (Figure 1) and was not present in the maternal sample. A paternal sample was not obtained.
CYFIP2 is an evolutionary highly conserved cytoplasmic protein that interacts with the fragile X mental retardation protein (FMRP). It is also a member of the canonical WAVE regulatory complex (WRC), which controls actin cytoskeletal dynamics (Zweier et al., 2019). The gene is highly intolerant to missense variants (gnomAD Z = 6.01) and contains subdomains (DUF1394 and FragX_IP) that are tightly interlocked at the CYFIP2-WAVE1 interface. The p.(Val551Leu) variant is present in the conserved FragX_IP subdomain of CYFIP2, and further confirmed by the bioinformatics tool Domain Logo Protein for Human Information (DOLPHIN) to be located in a functional domain affecting a key residue (WT: 2.265; ∆: −6.45) (Corcuff et al., 2023). It is absent from all databases (gnomAD, TopMed, All-of-Us), highly conserved across species (GERP_RS: 5.63; phyloP100: 9.947) and predicted deleterious (CADD: 27; MutationTaster: D). According to the American College of Medical Genetics, the variant would classify as likely pathogenic (PM1, PM2, PP2, and PP3) (Richards et al., 2015).
This case report is a part of a large multicenter research study and was approved by ethics committee at the Helsinki University Central Hospital and the Institutional Review Board of Columbia University (IRB-AAAS3433). Consent was obtained allowing the publication of the medical history and photographs of the case.
Three research groups have lately reported de novoCYFIP2-related impairments. Table 1 includes the brain MRI findings of our patient with those reported by Nakashima et al. (2018), by Zweier et al. (2019), and by Begemann et al. (2021).
| MRI-findings | CYFIP2 variants | |
|---|---|---|
| Our patient | Bilateral polymicrogyria around the sylvian fissures | p.(Val551Leu) |
| Nakashima et al. (2018) (n = 3) | Diffuse cerebral and mild cerebellar atrophy, dysmorphic corpus callosum, mild enlargement of the subarachnoid spaces | p.(Arg87Cys), p.(Arg87Leu), p.(Arg87Pro) |
| Zweier et al. (2019) (n = 7) | Moderate diffuse atrophy, frontal lobe hypoplasia with simplified gyration, mild diffuse cerebellar parenchymal volume loss, marked subarachnoid spaces, hypoplasia of the chiasma and retrochiasmatic region | p.(Ala455Pro), p.(Arg87Cys) (3), p.(Glu1174Aspfs*3), p.(Gln725Arg), p.(Tyr108His) |
| Begemann et al. (2021) (n = 7) | Diffuse cerebral atrophy, marked atrophy of the frontal and anterior temporal lobes, corpus callosum hypogenesis, hypomyelination of the right hemisphere | p.(Arg87Cys), p.(Arg87Leu), p.(Asp724Tyr), p.(Glu468Asp), p.(Met456Val), p.(Phe888Ser), p.(Thr490Met) |
- Abbreviation: MRI, magnetic resonance imaging.
2 DISCUSSION
This is the first report to describe CYFIP2 causing bilateral perisylvian polymicrogyria (BPP). CYFIP2 encodes the cytoplasmic FMR1-interacting protein 2 (Schenck et al., 2001). CYFIP2 is thought to have an influence on neurodevelopment via WAVE regulatory complex, which plays a role in synapse morphology and in the function of axons (Schenck et al., 2004). Previously, CYFIP2 variants have reported to cause intellectual disability, early-onset epileptic encephalopathy and dysmorphic features. CYFIP2 has been found to affect gyration and brain structural anomalies (Begemann et al., 2021; Nakashima et al., 2018; Zweier et al., 2019).
The BPP is a congenital anomaly of cerebral cortex (Kuzniecky et al., 1993). Extensive genetic heterogeneity has been identified to cause BPP (Dobyns et al., 2008; Guerrini & Dobyns, 2014). Congenital cytomegalovirus infection and intrauterine ischemia have reported to be non-genetic causes of BPP (Barkovich & Lindan, 1994; Van Bogaert et al., 1996) and twins have described to have an increased risk for polymicrogyria (Park et al., 2021). The major manifestations of BPP are intellectual disability, epilepsy, pyramidal signs, and orofacial dyspraxia (Guerrini & Dobyns, 2014). The radiological and clinical features of BPP are variable depending on the distribution of BPP and underlying causative factors (Guerreiro et al., 2002; Guerrini & Dobyns, 2014; Leventer et al., 2010). Children with microcephaly, wide polymicrogyria, severe dysarthria and abnormal neurological examination tend to have the most severe outcomes (Barkovich & Kjos, 1992; Jansen & Andermann, 2005).
When comparing the clinical features except the brain MRI findings of our patient with the other individuals with CYFIP2 variant, no significant differences were observed in single clinical symptoms. Most of individuals with CYFIP2 variant have severe of profound intellectual disability, epileptic encephalopathy, dysmorphic features, microcephaly, muscle hypotonia, visual impairment and eating difficulties. On the other hand, our patient has in its entirety more severe clinical symptoms than individuals with a CYFIP2 variant on average. This may be explained by widely distributed polymicrogyria, which has proved to cause severe clinical outcomes (Guerrini & Barba, 2010).
CYFIP2 variants (Begemann et al., 2021; Nakashima et al., 2018; Zweier et al., 2019) seem to underlie several structural findings distributed widely in the brain (Table 1). The main cortical malformations in these studies were cerebral and cerebellar atrophy especially in the frontal lobe. One patient was reported to have frontal lobe hypoplasia with simplified gyration, but none of 17 patients has polymicrogyria. It is noteworthy, that from totally reported 32 patients, 15 patients have a normal brain MRI.
3 CONCLUSION
Bilateral perisylvian microgyria is a highly heterogeneous disorder with most of the underlying genes remaining uncharacterized. Our case report extends the genetic heterogeneity of perisylvian polymicrogyria to include CYFIP2. As it is likely that genetic panel testing will miss some of the genetic abnormalities causing bilateral perisylvian microgyria, we suggest genome or exome sequencing when diagnosing brain malformations.
AUTHOR CONTRIBUTIONS
Tommi Salokivi, Irma Järvelä, Maria Arvio and Isabelle Schrauwen designed the study. Tommi Salokivi collected the data and wrote the first draft of the manuscript. Riitta Parkkola analyzed the MRI scans and Yasmin Rajendran, Thashi Bharadwaj, Anushree Acharya, Suzanne M. Leal and Isabelle Schrauwen analyzed the genetic data. All authors were contributors in revising the manuscript and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This study was funded by the National Institute of Neurological Disorders and Stroke (NINDS) grant R21 NS123325 to I.S. and National Institute of Child Health and Human Development (NICHD) grant R01 HD109342 to S.M.L and I.S. We thank the patient and her mother for their participation.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The variant has been deposited into ClinVar under #accession number: SCV003920947. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.




