Biallelic variants of the first Kunitz domain of SPINT2 cause a non-syndromic form of congenital diarrhea and tufting enteropathy
Abstract
Biallelic SPINT2 pathogenic variants cause a syndromic form of congenital diarrhea and enteropathy (OMIM 270420). To date, 35 patients have been reported and all presented with additional extra-intestinal features, apart from one case. We report on a 5-year-old girl who presented early in life with diarrhea and was found to have a novel homozygous variant in SPINT2. Pathological studies confirmed tufting enteropathy, and during her 5 years of life, she has not developed any extra-intestinal features. Molecular analysis detected a homozygous variant (NM_021102.4: c.203A>G (p. [Tyr68Cys]) in SPINT2. This is the first missense variant reported in the first Kunitz domain (KD1) of SPINT2 in humans. In vitro functional studies of this variant confirmed the deleterious effect leading to the loss of inhibitory activity of the intestinal serine proteases. This is the first description of SPINT2-related diarrhea in a patient who lived without long-term total parenteral nutrition. This study expands the clinical and molecular characteristics of SPINT2-related conditions.
1 INTRODUCTION
Infantile diarrhea and malnutrition are common clinical presentations and can be attributed to several acquired or maternally derived factors, including congenital infections and dietary protein intolerance. Congenital diarrheas and enteropathies (CDEs), a rare cause of severe, life-threatening diarrhea, are monogenic intestinal epithelial disorders. They usually present within weeks of life and are associated with feeding intolerance and malabsorption (Thiagarajah et al., 2018). Infants with congenital diarrheas usually require parenteral nutrition to maintain proper growth, electrolyte, and nutrient requirements. Although many of these disorders are linked to altered immune system function, disruption of intestinal epithelial structure and function is a characteristic that unites all these diseases (Babcock et al., 2023). The etiology of CDEs includes the following five categories: intestinal epithelial electrolytes transport defect, dysfunction of epithelial enzymes and metabolisms, disorders of epithelial trafficking and polarity, defects of enteroendocrine cells, and anomalies in the regulation of the intestinal immune response (Cai et al., 2020; Thiagarajah et al., 2018).
The SPINT2 gene, also known as HAI2 (hepatocyte growth factor activator inhibitor-2), encodes a protein that contains two Kunitz domains (Kunitz Domain 1 [KD1] and Kunitz Domain 2 [KD2]) and is expressed in most epithelial cells. It acts as an inhibitor of several proteases, including matriptase-1, a serine protease. Biallelic variants of SPINT2 as a cause of a syndromic form of congenital sodium diarrhea (SCSD) (OMIM: 270420) were first described in 10 families (Heinz-Erian et al., 2008). Non-intestinal features include choanal or anal atresia, hypertelorism, corneal erosions, renal double collecting system, cleft palate, and digital anomalies. Recently Holt-Danborg and colleagues (Holt-Danborg et al., 2019) reviewed all 34 patients reported at the time, and compiled the 13 different SPINT2 variants reported; since, at least one additional variant was reported (Zhang et al., 2022). All cases that were reviewed by Holt-Danborg and colleagues had syndromic form, and they all needed total parental nutrition (TPN), but one patient managed to be weaned off at the age of 12 years. Of the 14 different SPINT2 variants reported, five are missense variants and are localized to the second Kunitz domain (KD2) of SPINT2. To date, no patient with biallelic stop variants was identified, suggesting that at least one SPINT2 allele encoding a protein with residual function is necessary for survival.
We report on a 5-year-old girl with novel biallelic variants of SPINT2 (NM_021102.4: c.203A>G (p. [Tyr68Cys]) which was previously confirmed to cause a loss-of-function effect (Faller et al., 2014). This is the first missense variant in the 1st Kunitz domain (KD1) of SPINT2 to be identified. Our patient presented with non-syndromic SPINT2-related congenital diarrhea and enteropathy and did not receive long-term TPN.
2 CASE REPORT
The patient was a term female neonate born to healthy first-cousin parents. Her birth weight was 3.6 kg (50th centile), length was 54 cm (75th centile) and head circumference was 37 cm (75th centile). There was no antenatal history of polyhydramnios. The patient has two healthy older siblings, and there was no family history of chronic diarrhea or malabsorption syndromes. At the age of 3 months, she started having frequent episodes of non-bloody, non-mucoid diarrhea up to seven times per day. It was initially associated with vomiting but no fever. She was managed at the local hospital was diagnosed with acute gastroenteritis and was treated with intravenous fluids. However, as the symptoms persisted, she started to lose weight, and her weight centile dropped to −2 SD. At the age of 8 months, she was referred to a tertiary hospital for further investigations. Initial examination revealed a small-for-age child with no facial dysmorphism. features. She had parched buccal mucosa, depressed anterior fontanel, and sunken eyes. Her weight was 5.8 kg (−3 SD), and her height was 66 cm (normal for her age). Abdominal examination revealed a distended but soft abdomen with no organomegaly. Eye examination was normal.
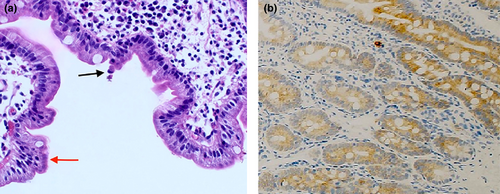
Investigation revealed complete blood counts, hyponatremia (128 mmol/L [135–145]), normal potassium at 4 mmol/L (3.5–5), chloride (100 mmol/L [98–107]), urea, and creatinine. She had hypoalbuminemia (26 g/L [35–50]), elevated alkaline phosphatase at 571 U/L (90–270), and normal bilirubin and transaminase levels. She was investigated for all possible causes of chronic diarrhea in infancy, and her work revealed a normal sweat test, normal celiac serology, normal serum immunoglobulin levels, and normal lymphocyte subset test. Her stool tests were negative for any pathogens. The trial of elemental formula for possible cow's milk protein allergy was unsuccessful. She underwent a gastroscopy and colonoscopy. Histological examination of the duodenal biopsy reveals villous blunting with associated disorganization of the surface epithelium without an increase in the intraepithelial lymphocytes. The surface reveals pseudo-stratification and multifocal tufted enterocytes (Figure 1a). Immunohistochemistry with Ber-Ep4 shows positive staining (Figure 1b). The histological features are consistent with diagnosing Tufting enteropathy with positive Ber-Ep4. Hence, the patient was put on parenteral nutrition, which resulted in weight gain. However, her parents declined the home TPN. Therefore, she was sent home on oral feed (extensively hydrolyzed formula), multivitamins supplements, and oral bicarbonate. Exome sequencing revealed a homozygous SPINT2 variants (NM_021102.4): c.203A>G (p.[Tyr68Cys]). Both parents were heterozygous for the same variant. This variant is absent in the public genomic database and in-house exome database. In-silico pathogenicity score predicated damaging effect. Previously, Faller and colleagues functionally confirmed the deleterious effect of the Y68C variant in the 1st Kunitz domain (KD1) of SPINT2 and showed to cause a loss of its function on inhibiting prostasin (Faller et al., 2014).
Currently, the patient is 5 years old, and she still has semi-formed stools up to four times per day with intermittent diarrhea that requires hospitalization. She continued to have a bloated abdomen. Her weight centile remains at the 10th centile, but her height is below the 3rd centile with normal developmental milestones. She underwent a complete medical checkup, and no other syndromic features have been identified so far.
3 DISCUSSION
SPINT2 variants can lead to CSD-predominant features or congenital tufting enteropathy (CTE) features. The genotype–phenotype correlation in SPINT2-associated disease is not well understood, and further studies are necessary to determine the molecular etiology of CTE, CSD, or mixed CTE–CSD phenotype (Babcock et al., 2023). CTE is characterized by chronic watery diarrhea that occurs within weeks to months after birth. It can be due to variants in the epithelial cell adhesion molecule (EPCAM) or SPINT2 genes (Das & Sivagnanam, 2021). It has a heterogeneous clinical manifestation. Generally, it is characterized by watery diarrhea (>50 mL/kg/day), eventually leading to malabsorption of macro and micronutrients. Eventually, it will lead to failure to thrive and impaired growth. Some patients might develop vomiting and abdominal distention, as what has happened to our patient. In syndromic CTE, the patients have extra-intestinal symptoms, including ophthalmologic signs (photophobia, cataracts, corneal erosions, and superficial punctuated keratitis), choanal atresia or anal atresia, dermatological anomalies, bone malformations, cholestatic liver disease, chronic arthritis, and skeletal dysplasia (Cai et al., 2020). Our patient has no extra-intestinal manifestations.
CTE is generally diagnosed by esophagogastroduodenoscopy, noting duodenal histological abnormalities. The most pathognomonic feature is the presence of disorganized surface epithelial cells with crowding and formations of epithelial tufts without an increase in intraepithelial lymphocytes. The latter feature excludes another enteropathy, which sometimes can show focal surface epithelial changes like celiac disease, infection, and cow milk allergy. Immunohistochemistry plays an essential role in the diagnosis as loss of anti-EPCAM (epithelial cell adhesion molecule) immunohistochemistry (MOC-31, Ber-Ep4) along with the morphological features show a sensitivity and a specificity of 100% (Cai et al., 2020). However, these stains are usually normal in patients with SPINT2 variants. The diagnosis is generally confirmed by identifying variants in EPCAM or SPINT2 genes. To date there are five reported missense variants that are localized to the second Kunitz domain (Holt-Danborg et al., 2019; Zhang et al., 2022). The Y68C variant reported in our patient localizes to the first Kunitz domain. It affects the same position in the first Kunitz domain as the most prevalent SPINT2 pathogenic variant Y163C, in the second Kunitz domain. SPINT2 is a potent inhibitor of different gastro-intestinal serine proteases. The Y163C variant in the second Kunitz domain resulted in a complete loss of inhibitory activity on two intestinal proteases, prostasin, and tmprss13. The result of the analogous Y68C variant in the first Kunitz domain is consistent with the idea that the two Kunitz domains of SPINT2 contribute differently to the inhibition of the protease prostasin (Faller et al., 2014). It is also suggested that HAI-2 regulates matriptase zymogen activity and thus may act as regulator of matriptase trans(auto)-activation (Skovbjerg et al., 2020).
The physiological targets of SPINT2 are proteases prostasin and matriptase, both in their zymogen states and in their activated states. However, The Y68C and Y163C variants in the 1st and 2nd Kunitz domains have differential effects depending on the serine proteases investigated. Importantly, Faller et al. (2014) showed a loss of function of the Y68C variant on inhibiting prostasin but not matriptase. Zymogens were not examined in this study.
Management of SPINT2 deficiency is dependent on the predominant phenotype. The main management is via parenteral nutrition and, in severe cases, intestinal transplant. In reviewing the literature, total autonomy was never achieved in CTE or CSD before the age of 11 (Holt-Danborg et al., 2019; Salomon et al., 2014). Current guidelines recommend avoiding early intestinal transplantation and preserving as much enteral nutrition as possible. Although previous publications in CTE suggest the possibility of parenteral nutrition, weaning occurs only after several years, our patient survived without TPN support, which points to the heterogeneity of the disease.
In conclusion, our study expands the knowledge regarding the clinical manifestations and molecular findings associated with SPINT2-related conditions with our patient having no extra-intestinal findings and achieving autonomy without the need for TPN at this point.
AUTHOR CONTRIBUTIONS
Yusriya Al Rawahi and Almundher Al-Maawali designed the study and wrote the manuscript and supervised the project. Mohammed Al-Masqari analysis of the pathology studies. Omar Al Sunaidi, Adawiya Al Jamei, and Dafalla Rahamtalla, clinical care, reviewed the manuscript and critically reviewed the data. All authors reviewed the manuscript and approve it.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




