Ocular manifestations in a cohort of 43 patients with KBG syndrome
Abstract
Ophthalmological conditions are underreported in patients with KBG syndrome, which is classically described as presenting with dental, developmental, intellectual, skeletal, and craniofacial abnormalities. This study analyzed the prevalence of four ophthalmological conditions (strabismus, astigmatism, myopia, hyperopia) in 43 patients with KBG syndrome carrying variants in ANKRD11 or deletions in 16q24.3 and compared it to the literature. Forty-three patients were recruited via self-referral or a private Facebook group hosted by the KBG Foundation, with 40 of them having pathogenic or likely pathogenic variants. Virtual interviews were conducted to collect a comprehensive medical history verified by medical records. From these records, data analysis was performed to calculate the prevalence of ophthalmological conditions. Out of the 40 participants with pathogenic or likely pathogenic variants, strabismus was reported in 9 (22.5%) participants, while astigmatism, myopia, and hyperopia were reported in 11 (27.5%), 6 (15.0%), and 8 (20.0%) participants, respectively. Other reported conditions include anisometropia, amblyopia, and nystagmus. When compared to the literature, the prevalence of strabismus and refractive errors is higher than other studies. However, more research is needed to determine if variants in ANKRD11 play a role in abnormal development of the visual system. In patients with established KBG syndrome, screening for misalignment or refractive errors should be done, as interventions in patients with these conditions can improve functioning and quality of life.
1 INTRODUCTION
KBG syndrome was first reported in 1975 by Herrman et al. (1975) and is named after the surnames of the first three families diagnosed (K, B, and G). It is associated with dental, developmental, skeletal, and craniofacial abnormalities (Brancati et al., 2006). Clinical findings in affected individuals include macrodontia, intellectual disability, short stature, brachydactyly, and costovertebral anomalies (Libianto et al., 2019; Sirmaci et al., 2011; Skjei et al., 2007). Other research has expanded the phenotype of KBG syndrome to include the occurrence of congenital heart defects, seizures, and gastrointestinal problems (Auconi et al., 2022; Digilio et al., 2022; Low et al., 2016). Currently, the prevalence of KBG syndrome is unknown but it is thought to be underdiagnosed due to the nonspecific and wide spectrum of symptoms (Sirmaci et al., 2011). Genetic or genomic testing are ways to confirm the diagnosis of suspected KBG syndrome (Swols et al., 2017).
The genetic etiology of KBG syndrome is from variants in Ankyrin Repeat Domain (ANKRD11) and deletions in 16q24.3 (Sirmaci et al., 2011). Variants in ANKRD11 have also been found in patients clinically diagnosed as possibly having Cornelia de Lange syndrome (CdLS), another developmental disorder that overlaps clinically with KBG syndrome (Cucco et al., 2020).
Among the clinical symptoms commonly attributed to KBG syndrome, ophthalmologic findings are sparsely mentioned in the literature. Refractive errors (myopia, hyperopia, astigmatism) and strabismus have recently been reported in patients (Nizette & Duchesne, 2021; Swols et al., 2017). Even though ophthalmologic evaluation is recommended in patients after initial diagnosis of KBG syndrome (Swols et al., 2017), there is a lack of research that investigates the link between KBG syndrome and ophthalmologic symptoms, if one is present. The prompt diagnosis and management of strabismus is essential in preventing permanent amblyopia (Helveston, 2010). Refractive errors should also be promptly addressed to improve visual deficits and life outcomes in pediatric patients (Braverman, 2007).
Since KBG syndrome is a rare condition, there have been efforts to sufficiently describe the phenotypical spectrum of diagnosed patients as well as describe novel clinical presentations (Alfieri et al., 2019; Ashraf et al., 2022; Ockeloen et al., 2015). The aim of this study is to describe the ophthalmological manifestations in a cohort of 43 patients with diagnosed KBG syndrome and compare descriptively the prevalence of refractive errors and strabismus in our cohort to the literature.
2 METHODS
Forty-three patients (19 females, 24 males) with a variant in ANKRD11 (n = 41) or deletions in 16q24.3 (n = 2) were interviewed via secure video conference platforms between January 2021 and May 2022. Video interviews were conducted to collect a thorough medical history of each patient, including problems with vision or other ophthalmological complaints. Past medical records, including genetic testing results confirming variants in ANKRD11, were collected as well. Interviews were approximately 1–2 h long and consisted of questions investigating the function of all organ systems as well as a characterization of their social, cognitive, and developmental history. All interviewed research participants were self-referred or recruited via a private Facebook group monitored and hosted by the KBG foundation. Written consent was obtained for the collection of medical records.
Microsoft Excel was used to organize and determine prevalence data of ophthalmological conditions reported by interviewed participants. Of most relevance was the prevalence of strabismus, astigmatism, myopia (near-sightedness), and hyperopia (far-sightedness). Other conditions were reported by our cohort, including anisometropia (n = 3), nystagmus (n = 1), amblyopia (n = 2), and cortical visual impairment (n = 1), but were not deemed relevant due to a low number of affected individuals in our cohort.
A PubMed search was conducted to find prevalence data of strabismus, astigmatism, myopia, and hyperopia of populations with similar demographics to ours (pediatric and non-Hispanic). Search criteria included the keywords “KBG syndrome” AND “eye OR refractive OR vision OR myopia OR strabismus OR hyperopia OR astigmatism OR ocular” to gather literature regarding the known reports of visual impairment in KBG syndrome.
A second search was conducted using the keywords “astigmatism OR hyperopia OR myopia OR strabismus” AND “prevalence” to collect and analyze studies describing the prevalence of these visual problems in various patient populations. Studies were prioritized if they analyzed populations that were Caucasian, non-Hispanic, and under 18 years, as this matches the demographics of most of our cohort (mean age 13.4, 95.3% Caucasian, 86.0% non-Hispanic). However, studies utilizing patient populations outside these parameters were also considered for comparison to their appropriate demographic in our cohort. Prevalence data from the literature for each condition was compared to our calculated prevalence, and a descriptive statistical analysis was performed.
3 RESULTS
3.1 Participant demographics
Patients come from 12 countries (55.8% from The United States) with a mean age of 13.4 years (range 1–59). Among our cohort, 41 patients (95.3%) were Caucasian, 37 patients (86.0%) were non-Hispanic, and 32 patients (74.4%) were under the age of 18 years. Table 1 outlines the demographic information of interviewed participants with KBG syndrome.
| Characteristics | Study participants (n = 43), n (%) |
|---|---|
| Sex | |
| Female | 19 (44.2%) |
| Male | 24 (55.8%) |
| Race | |
| Caucasian | 41 (95.3%) |
| Asian | 2 (4.7%) |
| Ethnicity | |
| Non-Hispanic | 37 (86.0%) |
| Hispanic | 6 (14.0%) |
| Mean age at the time of video conference (years) ± SD | 13.4 ± 13.4 |
| Median age at time of video conference (years) | 9 |
| Age range (years) | 1–59 |
| Country of residence | |
| United States | 24 (55.8%) |
| Spain | 5 (11.6%) |
| United Kingdom | 4 (9.3%) |
| Germany | 2 (4.7%) |
| Argentina | 1 (2.3%) |
| Australia | 1 (2.3%) |
| Canada | 1 (2.3%) |
| Ecuador | 1 (2.3%) |
| France | 1 (2.3%) |
| Lebanon | 1 (2.3%) |
| Mexico | 1 (2.3%) |
| Portugal | 1 (2.3%) |
3.2 Variants in ANKRD11
All patients were confirmed to have variants in ANKRD11 or deletions of 16q24.3, which is criteria for a diagnosis of KBG syndrome. Table 2 describes the genetic variants of each proband, as well as their age, sex, pathogenicity of variant, and inheritance pattern. As of October 2022, proband 24 was documented as having a de novo pathogenic variant in ANKRD11, but the exact change has not been provided by the clinicians involved. However, documentation from this proband's geneticist in April 2021 confirmed that “the analysis of a panel of 44 genes allowed the identification of a variation in the ANKRD11 gene… since this variation is by nature pathogenic and occurs de novo, all of these elements support its involvement in [proband 24]'s phenotype.” Variant analysis was done using ACMG standards, which describes each variant as PVS1 (very strong evidence for pathogenicity where null variant in a gene where loss of function is a known mechanism of disease), PS1-4 (strong support for pathogenicity), PM1-6 (moderate support for pathogenicity), and PP1-5 (supporting evidence for pathogenicity) (Richards et al., 2015). These descriptors are used to describe these variants as “pathogenic,” “likely pathogenic,” or a “variant of unknown significance.” More information regarding the variant analysis of our cohort according to ACMG guidelines can be found in Table S1.
| Proband | Age (years) | Sex | cDNA change | Protein change | Classification | Inheritance |
|---|---|---|---|---|---|---|
| 1 | 0–4 | M | c.1903_1907del | p.(Lys635fs) | Class 1: pathogenic | De novo |
| 2 | 5–9 | M | c.3770_3771delAA | p.(Lys1257RArgfs*25) | Class 1: pathogenic | Unknown |
| 3 | 0–4 | F | c.1181delA | p.(Asn394llefs*33) | Class 1: pathogenic | De novo |
| 4 | 5–9 | F | c.2329_2332del | p.(Glu777Argfs*5) | Class 1: pathogenic | Unknown |
| 5 | 15–19 | M | c.6472G > T | p.(E2158*) | Class 1: pathogenic | De novo |
| 6 | 10–14 | M | c.7354C > G | p.(Arg2452Gly) | Class 2: likely pathogenic | De novo |
| 7 | 30–34 | F | c.5233_5234del | p.(Ser1745Hisfs*51) | Class 1: pathogenic | Maternal |
| 8 | 60–64 | F | c.5233_5234del | p.(Ser1745Hisfs*51) | Class 1: pathogenic | Unknown |
| 9 | 5–9 | F | c.2404_2407del | p.(Leu802Lysfs*60) | Class 1: pathogenic | De novo |
| 10 | 21–24 | M | c.4384dupA | p.(Arg1462Lysfs*92) | Class 1: pathogenic | Unknown |
| 11 | 5–9 | M | 158 kB loss in 16q24.3 | - | Class 1: pathogenic | Unknown |
| 12 | 5–9 | M | c.6628G > T | p.(Glu2210*) | Class 1: pathogenic | Unknown |
| 13 | 0–4 | F | c.3991del | p.(Asp1331Thrfs*14) | Class 1: pathogenic | De novo |
| 14 | 20–24 | F | c.2177_2178del | p.(Lys0726Argfs*15) | Class 1: pathogenic | De novo |
| 15 | 20–24 | M | c.1018dup | p.(Thr340Asnfs*9) | Class 2: likely pathogenic | Unknown |
| 16 | 5–9 | F | c.1903_1907del | p.(Lys0635Glnfs*26) | Class 1: pathogenic | De novo |
| 17 | 10–14 | M | c.7825C > T | p.(Gln2609*) | Class 1: pathogenic | De novo |
| 18 | 5–9 | M | c.4489_4490del | p.(Arg1497Glyfs*56) | Class 1: pathogenic | De novo |
| 19 | 10–14 | M | c.6015dupA | p.(Gly2006Argfs*26) | Class 1: pathogenic | de novo |
| 20 | 0–4 | F | c.1318C > T | p.(Arg440*) | Class 1: pathogenic | De novo |
| 21 | 5–9 | F | c.2409_2412 del | p.(Glu0805Argfs*57) | Class 2: likely pathogenic | Unknown |
| 22 | 5–9 | M | c.3770_3771delAA | p.(Lys1257Argfs*25) | Class 1: pathogenic | De novo |
| 23 | 5–9 | M | c.7607G > A | p.(Arg2536Gln) | Class 2: likely pathogenic | De novo |
| 24 | 5–9 | F | Not reported | Not reported | - | De novo |
| 25 | 35–39 | F | c.1756G > A | p.(Val0586Met) | Class 3: VUS | Unknown |
| 26 | 0–4 | M | c.1756G > A | p.(Val0586Met) | Class 3: VUS | Maternal |
| 27 | 0–4 | M | c.1756G > A | p.(Val0586Met) | Class 3: VUS | Maternal |
| 28 | 5–9 | M | c.1318C > T | p.(Arg0440*) | Class 1: pathogenic | De novo |
| 29 | 0–4 | M | c.2175_2178delCAAA | p.(Asn0725Lysfs*23) | Class 1: pathogenic | De novo |
| 30 | 5–9 | F | c.2398_2401delGAAA | p.(Glu800Asnfs*62) | Class 1: pathogenic | De novo |
| 31 | 15–19 | M | c.7789A > T | p.(Lys2597*) | Class 2: likely pathogenic | Paternal |
| 32 | 55–59 | M | c.7789A > T | p.(Lys2597*) | Class 2: likely pathogenic | Unknown |
| 33 | 10–14 | F | c.2273dup | p.(Arg759Glu*23 fs) | Class 1: pathogenic | Unknown |
| 34 | 20–24 | F | c.6596_6597insA | p.(Ala2201Cysfs*6) | Class 1: pathogenic | De novo |
| 35 | 10–14 | F | c.5659C > T | p.(Gln1887*) | Class 1: pathogenic | Unknown |
| 36 | 5–9 | M | c.5227C > T | p.(Gln1743*) | Class 1: pathogenic | Maternal |
| 37 | 15–19 | M | c.3224_3227delAAAG | p.(Glu1075Glyfs*242) | Class 1: pathogenic | Unknown |
| 38 | 5–9 | F | c.1977C > G | p.(Tyr0659*) | Class 2: likely pathogenic | Unknown |
| 39 | 5–9 | M | c.2409_2412del | p.(Glu805Argfs*57) | Class 1: pathogenic | De novo |
| 40 | 20–24 | M | c.3770_3771del | p.(Lys1257Argfs*25) | Class 1: pathogenic | De novo |
| 41 | 0–4 | M | c.4396_4397 | p.(Arg1466Glyfs*87) | Class 1: pathogenic | De novo |
| 42 | 5–9 | F | c.1893delA | p.(Lys0631fs) | Class 1: pathogenic | De novo |
| 43 | 30–34 | F | c.5227C > T | p.(Gln1743*) | Class 1: pathogenic | Unknown |
- Abbreviation: VUS = variants of unknown significance.
3.3 Ocular findings in past KBG syndrome cohorts
A thorough literature search was conducted via PubMed in order to determine what has been established in regard to ophthalmological conditions in patients with KBG syndrome. Search criteria included (KBG syndrome) AND (eye OR refractive OR vision OR myopia OR strabismus OR hyperopia OR astigmatism OR ocular). From this search, seven articles were screened, with six of them describing instances of ophthalmological manifestations in patients with KBG syndrome. Brancati et al. (2006) reported an 18% prevalence of strabismus as well as describing rare instances of congenital bilateral cataracts and megalocornea. Gnazzo et al. (2020) reported a 13% prevalence of strabismus, whereas Choi et al. (2023) reported a single case of strabismus among their cohort. Chen et al. (2021) and Nizette and Duchesne, (2021) both reported isolated cases of patients with KBG syndrome having binocular refractive errors, with the latter also reporting concurrent bilateral corneal clouding. Finally, Novara et al. (2017) reported four cases of astigmatism and two cases of myopia among their cohort.
Due to the small amount of literature reporting ophthalmological conditions in patients with KBG syndrome, this work further describes an ophthalmological profile in patients with KBG syndrome.
3.4 Strabismus, astigmatism, myopia, and hyperopia
Due to the uncertainty of pathogenicity, all variants with Class 3 variants (variants of unknown significance) were not included in prevalence data. Therefore, the cohort of interest (referred to as “our entire cohort”) are the 40 patients with Class 1 or Class 2 variants.
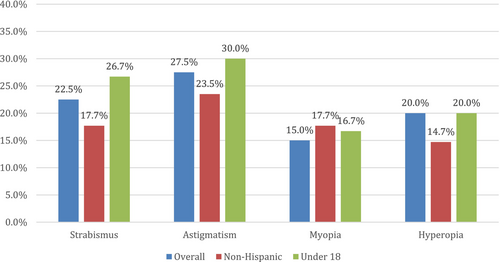
The prevalence of strabismus in our entire cohort was calculated to be 22.5% (n = 9). This result is slightly above that reported in Brancati et al. (2006) and Gnazzo et al. (2020), who reported prevalences of strabismus of 18% and 13%, respectively. In non-Hispanic participants (n = 34), the prevalence of strabismus was 17.7% (n = 6). For our participants who were under 18 years (n = 30), the prevalence of strabismus was 26.7% (n = 8).
Among our entire cohort, the prevalence of astigmatism was 27.5% (n = 11). The prevalence of astigmatism in our non-Hispanic patients (n = 34) was 23.5% (n = 8). For our participants who were under 18 years (n = 30), the prevalence of astigmatism was 30.0% (n = 9).
For myopia (nearsightedness), the prevalence among our entire cohort was 15.0% (n = 6), while the prevalence among our non-Hispanic (n = 34) participants was 17.7% (n = 6). The prevalence of myopia among patients under 18 (n = 30) was 16.7% (n = 5).
The prevalence of hyperopia (farsightedness) in our entire cohort was 20.0% (n = 8). Among those of non-Hispanic origin (n = 34), the prevalence was 14.7% (n = 5). The prevalence of hyperopia among patients under 18 (n = 30) was 20.0% (n = 6).
Figure 1 outlines the results of each of these four ophthalmological conditions. Several of our patients also reported having more than one of the described eye conditions (strabismus, astigmatism, myopia, or hyperopia), with 25.6% (n = 11) reporting the presence of at least two conditions and 7.0% (n = 3) reporting at least three conditions.
Other eye conditions were reported among our cohort, but to a lesser extent. These conditions included anisometropia (n = 3), nystagmus (n = 1), unspecified blurred vision (n = 2), amblyopia (n = 2), ptosis (n = 1), Salzmann's nodular degeneration (n = 1), intermediate uveitis (n = 1), cortical visual impairment (n = 1), ocular immaturity (n = 1), and difficulty adjusting from light to dark (n = 1); 62.8% (n = 27) of all participants reported having at least one ophthalmological condition.
4 DISCUSSION
- The main limitation of our study is that we were unable to provide an age-matched control group from the same interview group without KBG syndrome, leaving us limited to a descriptive statistical analysis between our prevalence data and previously published studies. Furthermore, the subpopulation of our cohort belonging to Hispanic ethnicity had a small sample size of only six representatives, making the results difficult to extrapolate to larger Hispanic populations.
- Another limitation is that documentation of ophthalmological conditions was done mostly by self-reporting by the participants themselves. Medical records were collected and most provided documentation of visual impairment, but many did not have records from an optometrist or ophthalmologist showing exactly to what extent their visual impairment deviated (in diopters, for example) from what is considered healthy vision. Furthermore, proband 24 provided medical documents showing that there was a pathogenic variant in ANKRD11, but the exact cDNA and protein change is unknown at this time.
- Another limitation lies in the literature search done in order to find descriptions of ophthalmological conditions in patients with KBG syndrome. While our literature search found six works outlining ophthalmological findings in KBG syndrome, the number could be higher as some works may not have been found though a PubMed keyword search alone.
- A final limitation lies in the method by which we defined and compared refractive errors and astigmatism. Since inclusion of these conditions were given after a self-report by the interviewed patient, and since most of them had no documentation to provide the extent of refractive error (in diopters) or astigmatism (in spherical equivalents), it was hard for us to have the same inclusion criteria as the studies we compared our prevalence data to. The studies used had different thresholds for which a patient was determined to have a refractive error worth reporting. For example, one study defined astigmatism as a cylindrical refractive error equal to or greater than 1.50 diopters (D) in the worse eye, producing a prevalence of 16.8% among Hispanic children (Fozailoff et al., 2011). However, the prevalence of astigmatism with a worse eye cylindrical refractive error equal to or greater than 3.00 D in Hispanic children was determined to be 2.9% (Fozailoff et al., 2011). Without our own refractive measurements of our cohort to define the threshold of significant refractive error, and the fact that studies used different thresholds to determine significance, we were left limited in our comparisons of refractive error.
Although ophthalmological findings have been reported in some cases of KBG syndrome (Brancati et al., 2006; Chen et al., 2021; Nizette & Duchesne, 2021; Novara et al., 2017), there is currently a lack of research that estimates the incidence of visual problems among those with diagnosed KBG syndrome, or determines if there is a link between KBG syndrome and visual impairments. Our analysis of visual impairments in patients with KBG syndrome hints that a significant relationship may exist between the two.
Among our entire cohort, the prevalence of strabismus (23.3%) is much higher than studied pediatric populations, which have shown to have a prevalence between 2.1% and 3.6% depending on race and ethnicity (Friedman et al., 2009; McKean-Cowdin et al., 2013; Multi-ethnic Pediatric Eye Disease Study Group, 2008). This prevalence is also much higher than reported by an analysis from the Intelligent Research in Sight (IRIS) Registry, which determined that 2.75% of over 30 million patients in the United States had a diagnosis of strabismus (Repka et al., 2018).
When comparing our cohorts' prevalence of astigmatism (27.9%), it proved to also be higher than several studies of other populations. The prevalence range, like strabismus, varied with race and ethnicity between 6.3% in non-Hispanic white (NHW) children and 16.8% in Hispanic children in studies done by the Multi-Ethnic Pediatric Eye Disease Study Group (Fozailoff et al., 2011; Wen et al., 2023). In comparison, 15.6% of our non-Hispanic patients and 31.3% of our Hispanic patients reported a diagnosis of astigmatism. Another study determined the prevalence of astigmatism of nearly 19,000 school-aged children in Philadelphia to be 7.8%, which is much lower than our cohort (Mayro et al., 2018).
The previous studies also determined that the prevalence of myopia (nearsightedness) of various populations to be 1.20% in NHW children, 3.70% in Hispanic children, and 9.4% in Philadelphia school-aged children (Mayro et al., 2018; Multi-Ethnic Pediatric Eye Disease Study Group, 2010; Wen et al., 2023). Our overall cohort and our non-Hispanic participants clearly had much higher rates of 16.28% and 21.88%, respectively. Interestingly, none of our Hispanic patients reported myopia.
For studies done on the prevalence of hyperopia among populations, the data were quite variable. Studies have reported a range of 2.4–25.65% among children of various races and ethnicities (Mayro et al., 2018; Wen et al., 2023). Another study showed that the prevalence of hyperopia among a cohort of school-aged children ranged from 12.7% to 19.3% among Hispanic and white children, respectively (Kleinstein et al., 1960). Our cohort also showed wide fluctuations in data, with the rate of hyperopia being 20.93% in our entire cohort, 60.0% in our Hispanic population, and 9.4% among our non-Hispanic population. Depending on which race or ethnicity was being considered, hyperopia prevalence in our patients can be interpreted as higher or lower than what is reported in the literature.
The function of ANKRD11 when it comes to development of the central nervous system is not clear. Studies have shown that ANKRD11 is expressed in the brain and is present in nuclear inclusions in nonneuronal cells (Sirmaci et al., 2011; Zhang et al., 2004). This gene has also shown evidence of playing a role in craniofacial development, as ANKRD11 increases acetylation of p53, which is involved in the pathogenesis of craniofacial developmental syndromes (Bowen & Attardi, 2019; Neilsen et al., 2008). Both disruptions in the central nervous system and craniofacial development may explain a mechanism by which those with KBG syndrome develop ophthalmological conditions. Other related syndromes, including those with 16q24.3 microdeletion syndrome, have shown to overlap clinically with KBG syndrome while also showing ophthalmological abnormalities, including strabismus and refractive errors (Chopra et al., 2023; Novara et al., 2017; Sveden et al., 2023).
Regardless, a pattern of visual abnormalities exists within our cohort of 43 patients with KBG syndrome, and further research is indicated to investigate these associations.
5 CONCLUSIONS
While the presentation of KBG syndrome is classically associated with dental, developmental, skeletal, and craniofacial abnormalities, this analysis demonstrates that in a cohort of 43 individuals with diagnosed KBG syndrome, the occurrence of ophthalmological conditions occurs at a rate that is higher than other studied populations. Specifically, the prevalence of strabismus, astigmatism, and myopia among our patient population was higher than what was reported in other studies. The prevalence of hyperopia can be interpreted as higher or lower than what is reported in the literature depending on ethnicity. Nonetheless, more research is needed to determine what role ANKRD11 pathogenic variants have in the clinical manifestation of KBG syndrome, and if altered, ANKRD11 function can lead to impaired development of the visual system. If such a connection exists between KBG syndrome and ophthalmological conditions, patients with diagnosed KBG syndrome should receive prompt referral to ophthalmology for evaluation of misalignment or refractive error. Prompt intervention in pediatric patients with misalignment can prevent permanent issues, and intervention in refractive errors in patients of all ages can improve their quality of life and function.
AUTHOR CONTRIBUTIONS
Gholson J. Lyon conducted all virtual interviews with the participants and was responsible for primary data collection. Elaine Marchi was responsible for summarizing primary data. Drake C. Carter was responsible for data analysis and project conception along with Gholson J. Lyon. The first draft of the manuscript was written by Drake C. Carter, with critical revision performed by Ola Kierzkowska, Kathleen Sarino, and Gholson J. Lyon.
ACKNOWLEDGMENTS
We would like to thank all families who participated in this study, as well as the KBG foundation for referrals.
FUNDING INFORMATION
This research was supported by funds provided to G. J. L. from the New York State Office for People with Developmental Disabilities. Furthermore, additional funding was provided by several families with KBG syndrome and seed funding by the KBG Syndrome Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




