Homozygous deletion of the DSG3 terminal exon associated with acantholytic blistering of the oral and laryngeal mucosa
Abstract
We report a novel homozygous 49.6 kb deletion of chromosome 18q12.1 involving the last exon of DSG3 in dizygotic twins with phenotype consistent with acantholytic blistering of the oral and laryngeal mucosa (ABOLM). The twin siblings presented predominantly with friability of the laryngeal and respiratory mucosa. This is only the second report in the literature of this unusual autosomal recessive blistering disorder. The diagnosis explains the mucosal phenotype of a pemphigus-like disorder without evidence of autoimmune dysfunction. The exclusion of an autoimmune basis has management implications. The deletion also involved the DSG2 gene, which is associated with arrhythmogenic right ventricular dysplasia (ARVD). The affected siblings and heterozygous parents do not show any cardiac phenotype at this time. Functional studies would further clarify how deletions resulting in loss of function of DSG3 may cause the reported phenotypes of DSG3-related ABOLM.
1 INTRODUCTION
Desmosomes are intercellular adhesive junctions that tether intermediate filament cytoskeleton to plasma membrane and provide strong adhesion between cells to maintain tissue integrity. They are therefore prolific in tissues that experience high mechanical stress such as epithelium and cardiac muscle. The desmosomal cadherins, which are grouped into desmocollin (DSC1-DSC3) and desmoglein (DSG1-DSG4), form heterodimers or homodimers with partner molecules from adjacent cells (Delva et al., 2009). Different desmosomal cadherin isoforms are expressed in a tissue-dependent manner (Kowalczyk & Green, 2013). DSC2 and DSG2, ubiquitously expressed in all desmosome-containing tissues, are the only demosomal cadherin isoforms expressed in heart and simple epithelia (Hegazy et al., 2022). In skin, DSC1 and DSG1 express throughout the epidermis (though are more prominent in superficial layers), while DSC3 and DSG3 are more predominantly expressed in the lower layers (Hegazy et al., 2022; Mahoney et al., 1999). In mucosal epithelium, DSG1 is less predominant, whereas DSG3 is expressed in all layers (Hegazy et al., 2022; Mahoney et al., 1999). Disruptions of desmosomal cadherins lead to diseases in different tissue types, such as cardiomyopathies and blistering diseases of the skin (Lee & McGrath, 2021).
In this study, we report a family of El Salvadorian descent with 9-month-old dizygotic twins who presented with blistering of the oral and laryngeal mucosa, normal skin, and non-dysmorphic features. By single nucleotide polymorphism (SNP) oligonucleotide microarray, a novel homozygous 49.6 kb interstitial deletion including the last exon of DSG3 and the first eight exons of DSG2 on chromosome 18q12.1 region was identified in both twins.
2 CLINICAL REPORT
The twin siblings were born at full term after an uncomplicated pregnancy by planned caesarian section to a then-26-year-old G2P1 mother. The children were noted at a few days of age to have increased oropharyngeal secretions and mucus production, but they were otherwise well and were discharged home from the newborn nursery.
The twin sister subsequently experienced multiple episodes of acute respiratory failure requiring pediatric intensive care unit admission on four occasions. These episodes were presumed to have been caused by recurrent viral respiratory infections. At 7 months of life, she again presented to the Emergency Department in acute respiratory failure, this time complicated by cardiac arrest for approximately 2 min. Intubation during the resuscitation was difficult, ultimately achieved on the third attempt. Given her history of recurrent acute respiratory failure, tracheostomy was performed 12 days later. Due to persistently bloody tracheostomy secretions, laryngoscopy was performed, demonstrating friable respiratory mucosa with pale, plaque-like lesions of the epiglottis, arytenoids, and aryepiglottic folds that were concerning for a blistering dermatological disorder (Figure 1a). She had no skin findings.
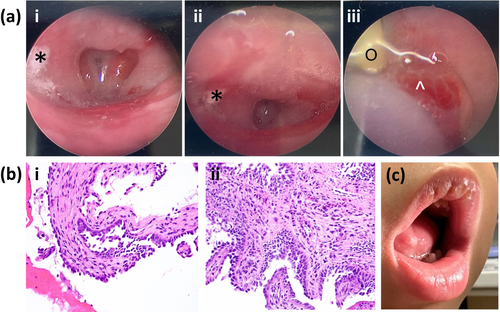
She continued to have bloody tracheostomy secretions despite intermittent courses of oxymetazoline and ciprofloxacin + dexamethasone drops via her tracheostomy. Repeat direct visualization via laryngoscopy, bronchoscopy, and esophagoscopy revealed edematous and friable mucosa of the pharynx, larynx, and esophagus, as well as edematous and inflamed tracheal tissue. Post-cricoid, endolaryngeal, and esophageal biopsies contained epithelial cells that showed possible viropathic effect, though immunostains were negative for herpes simplex virus (HSV), varicella zoster virus, adenovirus, and cytomegalovirus; and respiratory syncytial virus (RSV) stains were nondiagnostic. GMS stains were negative for fungi. Additionally, the endolaryngeal biopsy demonstrated evidence of acantholysis admixed with neutrophils, fibrin, and blood. Overall, the pathologist noted that the findings could be compatible with pemphigus vulgaris in the proper clinical setting, though the samples were nondiagnostic (Figure 1b).
Her physical examination at 8 months of age (while still hospitalized) showed height at <1st percentile, weight at 4th percentile, and head circumference at 18th percentile. Other than a narrow palate, the child was non-dysmorphic with no oral lesions. She had normal digits and nails, and normal-appearing skin without blistering, scarring, or pigmentary abnormalities. Her teeth were slow to erupt. Echocardiography did not show any structural heart defects or myocardial abnormalities. After a hospital stay of approximately 2 months, she was discharged with the tracheostomy in place.
Her follow-up examination at 27 months of age showed height at <1st percentile, weight at 1st percentile and head circumference at 8th percentile. Her tracheostomy remained in place, and she was otherwise medically stable.
The twin brother was also noted at several days of life to have thick mucosal secretions. He was subsequently admitted to the hospital with an RSV infection that required intubation for approximately 2 months. The intubation was noted to have caused severe irritation, and the patient subsequently demonstrated laryngeal edema and friability of the respiratory mucosa that was concerning for a blistering disorder. After approximately 3 months in the hospital, he was discharged without a tracheostomy or further intubation. A cleft soft palate and left multicystic kidney were noted during his hospital stay. Echocardiography did not show any significant abnormalities. He had failure to thrive on follow up, necessitating nasogastric tube feeds that resulted in subsequent weight gain. He had developmental delays and received approximately once weekly physical therapy, speech therapy, and occupational therapy. His teeth were slow to erupt. At 21 months of age, he was hospitalized with HSV and blistering of the tongue, exacerbated by the use of mouthwash (Figure 1c). Physical examination at 27 months of age was notable for right exotropia, mild hypertelorism, and hypotonia. He measured at <1st percentile for height, weight, and head circumference. He had no other mucosal involvement, and his skin was normal.
The twins' parents and older brother were reportedly healthy without medical issues. The parents were both of El Salvador descent and reportedly nonconsanguineous.
3 GENETICS TESTING AND RESULTS
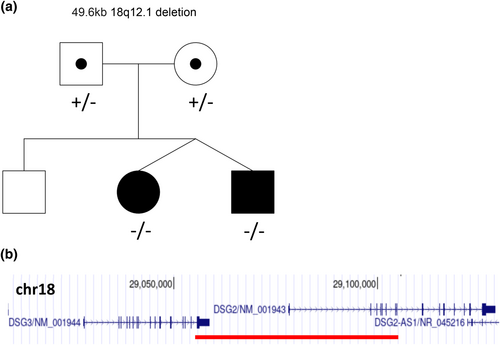
SNP oligonucleotide microarray analysis using Affymetrix GeneChip Human Mapping HD SNP array and Affymetrix Chromosome Analysis Suite 3.3 (Affymetrix, Santa Clara, CA) identified a homozygous 49.6 kb interstitial deletion on the long arm of chromosome 18 (arr[GRCH37] 18q12.1(29,055,092_29,104,698)x0) in both twin siblings (Figure 2a). The deleted region contains the last exon (exon 16) of the DSG3 gene (NM_001944.3) and the first eight exons (including the 5′ UTR), of the DSG2 gene (NM_001943.5) (Figure 2b). Long contiguous stretches of homozygosity (>3 megabases) were seen in multiple chromosomes, including chromosome 18, in both siblings, indicative of common ancestry. Parental testing demonstrated both parents were heterozygous for the chromosome 18q12.1 deletion (Figure 2a).
Trio exome sequencing was performed on both siblings independently by two different clinical laboratories. The twin sister was negative for any clinically significant variants, while the twin brother was found to have a homozygous missense variant of uncertain significance, c.4705A>G, p.M1569V, in the EPG5 gene (NM_020964.2), which is associated with Vici syndrome (OMIM#: 242840); the twin brother's phenotype was not suggestive of this condition. With an in-house-developed copy number variant detection tool, the second laboratory also confirmed the homozygous 18q12.1 deletion in the twin brother and the heterozygous deletion in both parents.
4 DISCUSSION
In this study, we identified a novel homozygous 49.6 kb deletion on chromosome 18q12.1 in dizygotic twins who presented with friability of the laryngeal and respiratory mucosa but intact skin. The deletion contains the last exon of DSG3 and the first 8 exons of DSG2, which leads to predicted loss of function of DSG3 and complete loss of function of DSG2. Homozygous deletion of this 18q12.1 region has not previously been reported. Both DSG3 and DSG2 are members of desmosomal cadherins, which are cell adhesion molecules that are essential to maintain tissue integrity and normal function.
The function of DSG3 has mostly been studied in pemphigus vulgaris, an autoimmune disease where DSG3 is targeted by autoantibodies, leading to mucosal blistering (Amagai et al., 1999). Dsg3-targeted knockout mice demonstrated loss of keratinocyte adhesion, mucocutaneous blistering, erosions, and randomly occurring skin lesions accompanied by patchy hair loss, recapitulating the phenotype of pemphigus vulgaris (Koch et al., 1997; Rötzer et al., 2016). Although the genetic function of DSG3 has been well studied in mice, only one human patient has previously been reported to harbor a biallelic loss-of-function variant of DSG3 (Kim et al., 2019). This patient carried a homozygous nonsense variant (p.Arg287*) in DSG3 and presented with acantholytic blistering of the oral and laryngeal mucosa but intact skin, very similar to our patients. Kim et al.'s case report suggested the association of DSG3 with acantholytic blistering of the oral and laryngeal mucosa (ABOLM) and an autosomal recessive mode of inheritance of the disease (OMIM#: 619226). Deletion of the last exon (exon 16) of DSG3 has not previously been reported and is not predicted to cause nonsense-mediated decay of the DSG3-encoded transcript (Hug et al., 2016). However, it truncates more than 10% of the DSG3 protein and approximately 50% of the intracellular domain of DSG3, which includes the C-terminus half of the intracellular cadherin-like segment (ICS) subdomain. In vitro functional studies that sequentially truncate the intracellular domain of DSG3 revealed the plakoglobin binding role of the C-terminus part of the DSG3 ICS subdomain (Andl & Stanley, 2001; Roh & Stanley, 1995). Deletion of the C-terminus of the DSG3 ICS subdomain disrupted the localization of DSG3 to desmosomes on the cell membrane, while the protein expression level was not affected (Andl & Stanley, 2001). Homozygous truncation of mouse Dsg3 ICS domain led to hair loss and a pemphigus vulgaris-like phenotype, including blisters on the tongue, foot pads, and eyelids (Koch et al., 1997; Montagutelli et al., 1997; Pulkkinen et al., 2002). Unlike what has been shown in cultured human epithelial cells (Andl & Stanley, 2001), the protein expression of Dsg3 was lost in the oral mucosa of mice with homozygous C-terminus Dsg3 truncation variants (Koch et al., 1997; Pulkkinen et al., 2002). However, the mRNA expression of Dsg3 was not affected in those mutated mice, suggesting that the truncated Dsg3 polypeptide, if synthesized, might have been rapidly degraded (Koch et al., 1997; Pulkkinen et al., 2002). Therefore, we predict that exon 16 deletion of DSG3 leads to its loss-of-function, highlighting the essential role of the ICS domain in exerting normal DSG3 functions.
Deletion of the first 8 exons of DSG2 (including the 5′UTR) is predicted to cause complete loss of function of the DSG2 protein. Unlike DSG3, which is expressed in skin and mucosa, DSG2 is predominantly expressed in myocardium. DSG2 has been reported to be associated with arrhythmogenic right ventricular dysplasia (ARVD) (OMIM#: 610193), arrhythmogenic right ventricular cardiomyopathy (ARVC), and dilated cardiomyopathy (OMIM#: 612877) with incomplete penetrance and variable expressivity. However, the disease mechanism linking DSG2 with these conditions has not been clearly determined. According to the ClinGen dosage sensitivity curation in 2022, DSG2 has emerging evidence for haploinsufficiency with a score of 2. On the other hand, DSG2 has a pLI score of 0 by gnomAD prediction, suggesting it is not dosage sensitive. The majority of the reported pathogenic DSG2 variants are missense variants that follow an autosomal dominant inheritance pattern (Awad et al., 2008), while heterozygous loss-of-function variants in DSG2 have also been reported in ARVC patients (Fernandes et al., 2015; Gehmlich et al., 2012). More recently, three individuals with juvenile/early adulthood onset arrhythmogenic/dilated cardiomyopathy have been reported to carry homozygous or hemizygous loss of DSG2 (Brodehl et al., 2021; Shiba et al., 2021), and their heterozygous carrier parents exhibit no significant cardiac phenotype. In our case, neither the siblings nor the parents present with any cardiac findings at this time, which may result from a different disease mechanism, or age-related penetrance/variable expressivity. We plan to continue monitoring the cardiac phenotype of the probands in case cardiac issues arise in the future.
In conclusion, we describe a novel homozygous 49.6 kb deletion on chromosome 18q12.1 that leads to the truncation of the last exon of DSG3 in dizygotic twins with features consistent with ABOLM. To the best of our knowledge, this is the second report of a biallelic loss-of-function variant in DSG3 identified in ABOLM patients. Our results further support the role of DSG3 in causing ABOLM.
AUTHOR CONTRIBUTIONS
Nan Jiang analyzed, curated the data and took the lead in writing the article. All authors assisted with clinical assessment, data collection, review and editing of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the patients' family for their time and willingness to expand our knowledge about DSG3-associated ABOLM.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to declare.
INFORMED CONSENT
Written informed consents were obtained from the patient's family for publication of clinical information and photos.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




