Genotype and phenotype characterization of primary hypertrophic osteoarthropathy type 2 and chronic enteropathy associated with SLCO2A1: Report of two cases and literature review
Tamara N. Kimball and Pamela Rivero-García contributed equally to this work.
Abstract
Autosomal recessive type 2 primary hypertrophic osteoarthropathy (PHOAR2) and chronic enteropathy associated with SLCO2A1 (CEAS) are two entities caused by pathogenic variants (PVs) in the SLCO2A1 gene that can coexist or occur independently from one another. We report two cases of PHOAR2 in Mexico with concomitant CEAS and conducted a review of the literature of the reported cases of PHOAR2 and/or CEAS to analyze the relationship between their genotype and phenotype presentation. The patients from our Institution with classical PHOAR2 phenotype and CEAS, harbored SLCO2A1 c.547G > A and c.1768del variants. We reviewed 232 cases, of which 86.6% were of Asian origin, and identified 109 different variants in SLCO2A1. Intron 7, exon 13, and exon 4 were predominantly affected. The two most common PVs were c.940 + 1G > A and c.1807C > T. We found a statistically significant association between SLCO2A1 variants located in intron 7, exons 12, and 13 and the development of CEAS. Missense variants were more frequent in isolated PHOAR2, while a greater proportion of protein-truncating variants (PTVs) were found in CEAS. Further investigation is imperative to elucidate the underlying pathophysiological mechanisms associated with CEAS, thereby facilitating the identification of effective therapeutic interventions.
1 INTRODUCTION
Pachydermoperiostosis, also known as Familial Touraine-Solente-Golé Syndrome or primary hypertrophic osteoarthropathy (PHO), is a genetic disorder characterized by a triad of symptoms: pachydermia (which presents as the thickening of facial skin, cutis verticis gyrata, and coarse facial features), digital clubbing and periostosis of long bones (Lu et al., 2022). Other features of this disease include painful joint enlargement, acroosteolysis, ptosis, eczema, acne, hyperhidrosis, seborrhea, and gastrointestinal (GI) abnormalities such as diarrhea, duodenal or gastric ulcers, chronic gastritis, and hypertrophic gastropathy with reported incidence rates ranging 10%–17.2% (Castori et al., 2005; Wang et al., 2019). This disease can be inherited through both autosomal dominant and recessive modes (Xu et al., 2021).
Until now, two causal genes have been identified. The first, 15-hydroxyprostaglandin dehydrogenase (HPGD), codifies for a member of the short-chain nonmetalloenzyme alcohol dehydrogenase protein family results in autosomal recessive primary hypertrophic osteoarthropathy type 1 (PHOAR1, OMIM #259100) (Uppal et al., 2008). The second, solute carrier organic anion transporter family member 2A1 (SLCO2A1), which encompasses 12 transmembrane domains and encodes for a prostaglandin transporter responsible for the autosomal recessive primary hypertrophic osteoarthropathy type 2 (PHOAR2, OMIM #614441) (Zhang et al., 2012). In recent years, studies have linked the GI features with a new entity: chronic enteropathy associated with SLCO2A1 (CEAS) (Umeno et al., 2018). CEAS is characterized by intestinal ulcers which lead to hypoalbuminemia and chronic anemia.
PHOAR2 with CEAS has been predominantly described in the Asian population. Therefore, in this study we describe two non-related Mexican patients with PHOAR2 and CEAS confirmed through molecular testing. In addition, we reviewed 232 worldwide reported cases of PHOAR2 and/or CEAS and analyzed the relation between their genotype and phenotype presentation.
2 SUBJECTS AND METHODS
Peripheral blood sample was obtained prior to informed consent attained from two unrelated patients with clinical diagnosis of PHO. Next-Generation sequencing (NGS) Invitae™ was performed in search of pathogenic variants (PV) in the genes SLCO2A1 and HPGD. The medical records and clinical inquiry were used to obtain clinical data. Complete physical exam, imaging, biochemical, and endoscopic studies were performed. Variants were classified according to the American College of Medical Genetics and Genomics criteria (ACMG) (Richards et al., 2015). Serum prostaglandin E2 (PGE2) was measured using a competitive enzyme-linked immunosorbent assay.
We used Pubmed to search for articles related to PHOAR2 or CEAS with a confirmatory molecular test from 1991 to 2022. The keyword used was “SLCO2A1”. We excluded articles in languages other than English and those that did not specify the clinical or molecular data of the patients. We included all articles with variants of uncertain significance, likely pathogenic (LP), and PV, with a total of 62 articles. We documented whether they had complete (pachydermia and periostosis) or incomplete (lacking pachydermia) PHO phenotype, presence, or absence of CEAS, and the age of onset. Chronic diarrhea, intestinal ulcers, or gastrointestinal bleeding were considered suggestive of CEAS. Patients with unspecified GI manifestations were placed in the unknown category and excluded from the clinical features analysis.
We used chi-square (χ2) for categorical variables, and H-Kruskal–Wallis for numerical variables without normal distribution. Statistical analyses were carried out using IBM SPSS Statistics version 25.0. A p-value <0.05 was considered statistically significant. We performed a bivariate association analysis between the presence or absence of CEAS and the location of the variant within the gene, using χ2. Predictors with statistical significance associated with the CEAS phenotype were incorporated into a logistic regression model. All maintained statistical significance, while none of the other variables reached statistical significance to be incorporated into the model.
3 RESULTS
3.1 Case presentation
Patient 1 is a 39-year-old male born to non-consanguineous parents. At 12 years of age, the proband underwent Kimura's diamond-shaped-duodenoduodenostomy due to partial duodenum stenosis as a complication of recurrent ulceration. Seven years later a panendoscopy and biopsies were performed, which documented multiple duodenal ulcers limited to the mucosal and submucosal layer with lymphocyte infiltration, as well as erythematous gastropathy. It was later complicated with upper gastrointestinal bleeding, requiring gastrectomy with Roux-en-Y reconstruction. Of note, his sister died at the age of 15 years due to the same gastrointestinal complications. The proband perceived progressive enlargement of hands, digital clubbing, and palmoplantar hyperhidrosis beginning at the age of 16 years, which was later accompanied by Cutis verticis gyrata (Figure 1a). Clinical diagnosis of PHO was integrated. PGE2 was elevated at 329 pg/mL (normal range 30–170), NGS of the SLCO2A1 gene revealed homozygous missense PV c.547G > A(p.Gly183Arg) located in exon 4. This variant has been previously reported in the literature in patients with PHOAR2 (Umeno et al., 2018). The sequence change replaces glycine (neutral and non-polar), with arginine (basic and polar), at codon 183 of the SLCO2A1 protein.
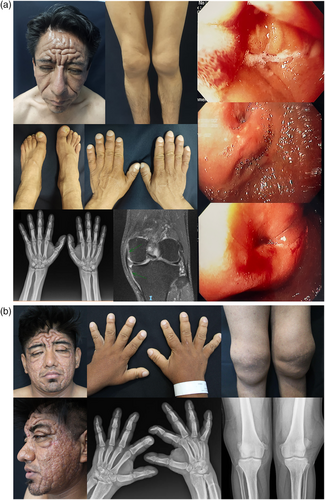
Patient 2 is a 24-year-old male born in Mexico from healthy parents denying consanguinity. At 15 years of age, he presented acne on his face and thorax. Three years later, he noticed enlargement of hands, digital clubbing, and progressive pachydermia predominantly in the forehead and skull. Joint augmentations of the knees became evident as well as generalized hyperhidrosis and mechanical blepharoptosis (Figure 1b). Upper gastrointestinal endoscopy (UGID) reported congestive gastropathy of gastric antrum as well as superior esophageal trachealization. He had normocytic normochromic regenerative anemia. A novel homozygous variant in SLCO2A1 c.1768del(p.Arg590Glyfs*39) was identified. This frameshift variant is located in exon 13 and according to the criteria of the ACMG it is classified as LP, meeting the following evidence: extremely low frequency in gnomAD population databases (PM2, allele frequency 0.132%) and null variant in a gene where loss of function is a known mechanism of disease, 19 pathogenic null variants have been reported in Clinvar for this gene, 2 of them in exon 13 (PVS1).
3.2 Literature review
In addition to our two patients, we reviewed 232 cases with molecularly confirmed PHOAR2 and/or CEAS, harboring 109 different variants in SLCO2A1 (Figure 2a; Table S1). The male-to-female ratio was 5.2:1. Most of the cases were either compound heterozygous (47.9%, 112/234) or homozygous (43.2%, 101/234), with only 9.0% (21/234) being heterozygous. Out of the 109 variants, 50.5% (55/109) were missense, 16.5% (18/109) splicing, 15.6% (17/109) nonsense, 15.6% (17/109) frameshift and one deletion and large rearrangement, each.
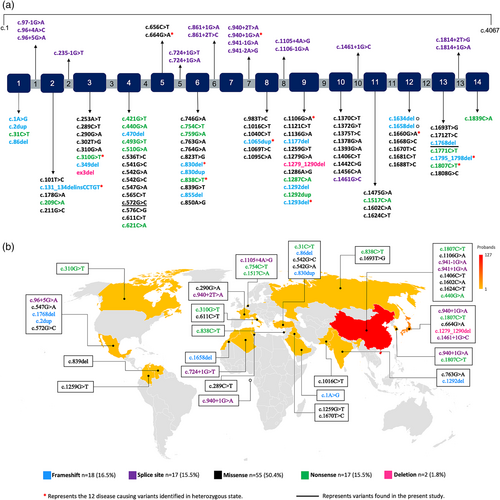
Intron 7, exon 13, and exon 4 were predominantly affected, accounting for 46.1% of the variants found in the sample. Among the patients whose place of origin was specified, 86.6% were of Asian origin (China 61.1%, Japan 17.3%, South Korea 7.2%, and India 1.0%) (Figure 2b).
We categorized 159 cases into three phenotypic groups: isolated PHOAR2 (60, 37.7%), isolated CEAS (16, 10.1%), and combined PHOAR2 and CEAS (83, 52.2%) (Table S2). Isolated CEAS was associated with an older age of onset (median 18.5), in comparison with isolated PHOAR2 and combined phenotype (12.5 and 15 years respectively), with a significant statistical difference (H-Kruskall–Wallis p 0.012).
We found isolated PHOAR2 exclusively in male patients (60/60, 100%), and while isolated CEAS was predominantly in female patients (14/16, 87.5%), it also showed statistical significance (χ2 p = 2.19E−17). When comparing compound heterozygous or homozygous with heterozygous patients, there was no difference in phenotypic presentation (χ2 p = 0.204). In the combined phenotype group, there was a greater association of complete PHO (69/81, 85.2%) in comparison with the isolated PHOAR2 group (43/60, 71.7%) (χ2 p 0.049).
We identified that having a PV in intron 7, exon 12, or exon 13 increases the likelihood of developing CEAS, with an OR of 2.2 (Wald test p 0.005, CI 1.27–3.70), 5.2 (Wald test p 0.036, CI 1.11–23.85) and 2.1 (Wald test p 0.024, CI 1.10–3.84), respectively (Table S2). The proportion of missense variants in isolated PHOAR2 (54/108, 50.0%) was greater than in both groups with CEAS (combined and isolated) (70/190, 36.8%), with statistical significance (χ2 p = 0.027). In contrast, nonsense (23.7% vs. 19.4%) and splicing (31.6% vs. 19.4%) variants were more prevalent in the CEAS group.
4 DISCUSSION
PHO is divided into type 1 (PHOAR1) and 2 (PHOAR2) according to the affected gene. The common pathologic mechanism in both is an alteration in the metabolism of prostaglandins, leading to an increase in the levels of PGE2. Consequently, at the skin level, there is epidermal and sebaceous gland hyperplasia accompanied by dermis hypoplasia, which manifests as pachydermia and hyperhidrosis. Bone alterations are the result of an unbalance between osteogenesis and bone resorption since PGE2 mimics the activity of osteoblasts and osteoclasts. In addition, prolonged local vasodilatation is the cause of digital clubbing (Souza et al., 2018). Therefore, non-steroidal anti-inflammatory drugs (NSAIDs), particularly COX-2 inhibitors such as etoricoxib, have proven to be effective in treating skin and joint features (Li et al., 2017, 2018).
To date, there have been no reports of clinical improvement in GI manifestations using NSAIDs. This could be explained by the fact that high levels of PGE2 serve as a protective barrier for the gastrointestinal mucosa. A previous study documented dilated microvessels in the histological GI reports of PHOAR2 patients, which could be exacerbated by the increased production of vasodilating molecules when NSAIDs are administrated (Harirforoosh et al., 2014; Wang et al., 2019).
Certain cardinal features may suggest the presence of CEAS, including patients with small intestine circular, oblique or longitudinal refractory ulcers (especially but not limited to ileum) with multiple stenoses in the distal jejunum and ileum and terminal ileal sparing (Hosoe et al., 2017; McAlindon, 2022). Histopathologic studies in CEAS patients have demonstrated spared muscular layer and nonspecific inflammatory infiltrates, such as those observed in patient 1 (Hosoe et al., 2017; Umeno et al., 2018). Patient 2 had congestive gastropathy visualized in endoscopy, which has been a feature previously documented in patients with PHOAR2 (Li et al., 2018).
We identified 232 cases reported in the literature with molecularly confirmed PHOAR2 and/or CEAS. The two most common PVs were c.940 + 1G > A and c.1807C > T, with a mutant allele frequency of 19.0% (85/447) and 10.7% (48/447) respectively. Interestingly, these variants were only found in the Asian population and in a patient from Africa, suggesting a founder effect. In comparison with previous studies where the frequency of GI manifestations ranges from 10% to 17% (Castori et al., 2005), our study found a higher frequency of GI manifestations in patients with SLCO2A1 variants, 73.9% (173/234) had information regarding the presence or absence of GI manifestations of which 65.3% (113/173) had at least one clinical GI finding suggestive of CEAS.
Phenotypic presentation was analyzed in 159 cases, identifying 60 (37.7%) cases of isolated PHOAR2, 16 (10.1%) of isolated CEAS, and 83 (52.2%) of combined PHOAR2 and CEAS. The isolated CEAS group was characterized by an older age of onset and predominantly affecting the female population, while isolated PHOAR2 was exclusively found in men. As a possible theory, authors have suggested hormones and sex-related modifier genes, even though this association remains unclear (Umeno et al., 2021).
PVs in intron 7, exon 12 or exon 13 increased the likelihood of developing CEAS, with statistical significance, remarkably exon 12 showed the highest association, more than doubling the OR in comparison to exon 13 and intron 7.
Regarding the type of PV, missense variants were more prevalent in isolated PHOAR2, while the proportion of other PTVs was greater in the groups that presented CEAS (combined or isolated). This observation aligns with existing literature as CEAS has consistently shown clinical associations with PTVs in roughly two-thirds of cases (Umeno et al., 2015; Yanai et al., 2019). We posit that the susceptibility to CEAS in individuals carrying variants within intron 7 may be attributed to the predominance of a specific variant in this intron, namely c.940 + 1G > A, which was documented with an allele frequency of 39.4% in the CEAS group. This variant is recognized as a frameshift and splice site mutation, resulting in premature translation termination. Previous studies have provided compelling evidence suggesting an association between CEAS and variants conditioning a non-functional protein (Chan et al., 1999; Umeno et al., 2015; Van Poucke et al., 2009).
Evidence also exists regarding the missense variant c.1681C > T (p.Arg561Cys) located in exon 12, which has been linked to diminished protein activity in rodent models since amino acid 561 plays a pivotal role in substrate transportation and electrostatic binding of anionic substrates (Chan et al., 2002; Itoh et al., 1996).
Furthermore, our investigation revealed that the most frequently identified variants within exons 12 and 13, were distributed across the Transmembrane Domain 11 (TMD11) (c.1681C > T, c.1693 T > G and c.1660G > A) and between the two contiguous extracellular domains (c.1634del, c.1768del, c.1807C > T, c.1771C > T) (Omasits et al., 2014). TMD11 is known to be associated with ligand binding, and given the identified susceptibility to CEAS, we propose it may play a critical role in the inflammatory processes of the gastrointestinal tract. Nevertheless, further studies are necessary to elucidate this possibility.
Our study is one of the first to examine possible genotype–phenotype associations in the SLCO2A1 clinical spectrum. We found a statistically significant association between SLCO2A1 variants located in intron 7, exons 12, and 13 and the development of CEAS. In female patients with GI ulcers and no other identified cause (Crohn's disease, Chronic ulcerative colitis, H. pylori, and due to NSAIDs), CEAS should be ruled out.
While NSAIDs demonstrate efficacy in addressing the skin and skeletal manifestations of PHOAR2, it is imperative to underscore the need for additional research aimed at elucidating the underlying mechanisms linked to CEAS and ascertaining the appropriate treatment approaches.
AUTHOR CONTRIBUTIONS
Tamara N. Kimball: Data curation, Conceptualization, Formal analysis Investigation, Methodology and Writing-original draft. Pamela Rivero-García: Data curation, Conceptualization, Investigation Methodology and Writing-original draft. Alejandro Barrera-Godínez: Resources, Writing-review & editing, Validation. Judith Domínguez-Cherit: Resources, Writing-review & editing, Validation.
ACKNOWLEDGMENTS
The authors thank the study participants and families for their contribution.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




