EED related overgrowth: First report of multiple members in a single family
Abstract
EED is a core component of polycomb repressive complex 2 (PRC2) with EZH2 and SUZ12. PRC2 has H3K27 methyltransferase activity (HMTase) that catalyzes the addition of up to three methyl groups on histone 3 at lysine residue 27 (H3K27). Germline heterozygous variants in EED, SUZ12, and EZH2 have been identified in patients with overgrowth and multiple dysmorphic features. The clinical manifestations of these syndromes significantly overlap: generalized overgrowth, intellectual disability, and scoliosis. To date, 11 unrelated patients have been published with missense variants in EED at highly conserved amino acids. We report three affected members in a family with a previously reported missense variant. All three affected members manifested very similarly, and this represents a homogenous clinical phenotype associated with EED related intellectual disability and overgrowth. This disorder is appropriately called Cohen-Gibson syndrome.
Overgrowth syndromes manifest as an increased mass of multiple tissues and often include advanced bone age, facial dysmorphism, and intellectual disability. Pathogenic variants in epigenetic regulators frequently affect many aspects of growth and metabolism, resulting in either short or tall stature. Sotos (MIM #117550) and Weaver syndromes (MIM #277590) are caused by germline pathogenic variants in NSD1 and EZH2, respectively. The EZH2, EED, and SUZ12 genes encode proteins that are core components of the polycomb repressive complex 2 (PRC2), with H3K27 methyltransferase activity (HMTase). This catalyzes the addition of up to three methyl groups on histone 3 at lysine residue 27 (H3K27). The HMTase activity of PRC2 is conferred by the SET domain contained at the C terminus of EZH2 in mammals. EZH2 activity is dependent on the formation of a proper complex with EED and thus, pathogenic variants in EED are expected to lead to a similar phenotypic outcome as seen in patients with EZH2 pathogenic variants. De novo pathogenic variants in EED were reported in two patients with Weaver-like syndromes (Cohen & Gibson, 2016; Cohen et al., 2015). Furthermore, three new patients with Weaver-like overgrowth syndrome with EED pathogenic variants were described (Cooney et al., 2017; Imagawa et al., 2017; Smigiel et al., 2018). Subsequently, three patients were reported with novel de novo variants in EED and compared with patients with pathogenic variants in other genes involved in the protein complex (Griffiths et al., 2019). Three more unrelated individuals were reported with de novo missense variants in EED (Spellicy et al., 2019). Appropriately, this novel disorder is now called Cohen-Gibson syndrome (COGIS).
Here we report on an Australian family with three adults with overgrowth, facial dysmorphism, and intellectual disability, caused by a previously reported variant in EED (Griffiths et al., 2019). This is the first report of a familial COGIS showing a largely homogenous phenotype in the affected members of the family.
1 CASE REPORT, OUR FAMILY
Our proband (1.1), her deceased son (1.2), and her daughter (1.3) had significant learning disabilities, language disorder, tall stature, and similar facial features that were different from those of other members of the family (Figure 1).
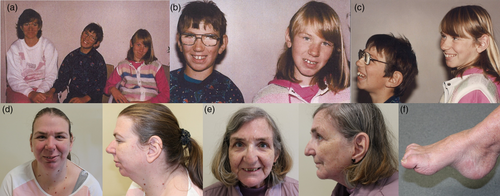
1.1 Proband (1.1)
Our proband first presented to clinical genetics clinic in 1995 at 36 years of age with her son, aged 8 years and daughter aged 10 years regarding their intellectual impairment. Her height was 175.5 cm (Z-score = 1.8, 97th percentile), upper segment:lower segment ratio was 0.85, arm span was 174 cm, weight was 119 kg (Z-score = 3.7, >99th percentile), and her head circumference was 55.5 cm (Z-score = 1.08, 86th percentile). Her body mass index was 38.7 kg/m2 (Z-score = 2.1, 98th percentile). She had significant learning problems since early childhood and left school early for a placement in a life skills program. She was always in a special class in school, and there was no documentation to suggest that her functioning changed after the onset of seizures. She did not have a history of diabetes or preneoplastic lesions; however, she had anal squamous cell carcinoma at 61 years of age that was treated by chemoradiation therapy. She developed epilepsy at the age of 16 years. Her brain MRI and EEG findings were normal. Her seizures were well controlled on valproate. She had an episode of catatonia at 49 years of age. She underwent a C4-C6 laminectomy for disk bulging and radiculopathy. She has a tall stature, hypertelorism, dolichocephaly, large eyes, hypermetropia, thinning of lateral eyebrows, a small jaw with a maxillary overbite, long digits with large hands and feet, large ears, and a bifid uvula. She developed pes cavus. She worked as a cleaner, but now at the age of 64, she is living on a disability pension. She had two sons and one daughter. Her affected son (1.2) died at the age of 33 years. Her father was in full-time work, and according to her, he was well educated. Her mother did not have any learning problems. Our proband was much taller than her parents.
1.2 Affected son (1.2)
He was born at full term after a normal pregnancy. He had an early global developmental delay and was formally diagnosed with mild intellectual disability on WISC-III assessment at 10 years of age. He was born at 40 weeks via a normal delivery. His birth weight was 4400 g (Z-score = 2.05, 98th percentile), length was 56 cm (99th percentile, Z-score = 2.2), and his head circumference was 35 cm (50th percentile). At 10 and half years of age, his height was 152.8 cm (Z-score = 1.88, 97th percentile), weight was 41.8 kg (Z-score = 0.84, 80th percentile), and his head circumference was 56 cm (Z-score = 2.1, 98th percentile). His body mass index (BMI) was 17.9 kg/m2 (Z-score = 0.6, 73rd percentile).
He had severe language disorder, tall stature, dysmorphic features such as hypertelorism, large eyes, hypermetropia, thinning of lateral eyebrows, a small jaw with a maxillary overbite, long digits with large hands and feet, large ears, and dolichocephaly. He developed epilepsy at the age of 19 years. He passed away at 33 years of age due to an uncontrolled episode of seizures. His CT brain was normal. He did not have an MRI brain.
1.3 Affected daughter (1.3)
Our proband's daughter was 10 years old at the time of her first visit. She had mild intellectual disability, behavioral problems, and facial features similar those of her mother and brother. At the age of 7 years, her WISC-III assessment revealed mild intellectual disability.
Her birth measurements were not known. At 12 years of age, her height was 168 cm (Z-score = 2.3, 99th percentile), weight was 48.6 kg (Z-score = 0.67, 75th percentile), and head circumference was 53.4 cm (Z-score = 0.25, 60th percentile). Her body mass index (BMI) was 17.2 kg/m2 (Z-score = −0.4, 34th percentile).
She had hypertelorism, large eyes, hypermetropia, thinning of lateral eyebrows, a small jaw with a maxillary overbite, long digits with large hands and feet, large ears, and dolichocephaly. She was seen again at the age of 38 years. Her height was 185.5 cm (Z-score = 3, >99th percentile), weight was 111 kg (Z-score = 3.2, >99th percentile), head circumference was 56 cm (Z-score = 1.6, 94th percentile), and her body mass index was 32.3 kg/m2 (Z-score = 1.7, 96th percentile). She has not developed epilepsy; however, she has developed an early pes cavus. Her muscle tone, strength, and deep tendon reflexes were normal. She had no abnormal sensory findings.
1.4 Unaffected son (1.4)
The proband's other son did not have developmental delay or growth abnormalities. He was born at 40 weeks via a normal delivery. His birth weight was 3520 g (56th percentile), length was 51 cm (60th percentile), and his head circumference was 34 cm (40th percentile). His adult height is 180 cm (68th percentile), weight is 69 kg (50th percentile), and his head circumference is 57 cm (91st percentile). His body mass index was 21.3 kg/m2 (Z-score = −0.6, 27th percentile).
2 GENETIC INVESTIGATIONS
The proband and subject 1.3 had a normal SNP microarray, fragile X, and urine metabolic screen. Their endocrine workup involving growth hormone, thyroid function test, and sex hormone analysis was normal. The proband underwent a singleton exome sequencing with a focus on genes related to intellectual disability, epilepsy, and overgrowth. A c.710A>G missense variant in EED (NM_001308007.1) was identified. This was also present in subjects 1.2 and 1.3 but was absent in subject 1.4. This variant was confirmed by Sanger sequencing. This variant is predicted to change the asparagine residue at 237 to glycine. This variant has been reported once in a patient with intellectual disability with overgrowth (Griffiths et al., 2019). This variant was absent in the gnomAD database. In silico prediction supported the pathogenicity of the variant (10 pathogenic vs. 3 benign). This amino acid, aspartic acid, at position 237 is highly conserved. The variant has been submitted to the Decipher database (511790). The variant in our study and previously published variants in EED are shown in Figure S1.
3 DISCUSSION
The current report is the first familial report of three affected members of two generations with significant homogeneity of phenotype. EED is a highly conserved protein required for the methyltransferase activity of EZH2. EED is regionally constrained for missense and loss of function variants (Gudmundsson et al., 2022; Wiel et al., 2019). EED contains a WD40 domain with seven WD40 repeats that make a closed ring propeller-structure acting as a scaffold for the assembly of multiprotein complexes (Rakotobe et al., 2008).
As mentioned before, EZH2, EED, and SUZ12 genes encode proteins that are core components of the polycomb repressive complex 2 (PRC2), with HMTase activity. Pathogenic variants in one of the WD40 repeats in EED are likely to impair its interaction with EZH2.
Our patient's variant (c.710A>G encoding p.Asn237Gly) falls within a conserved protein region of the WD4 repeat region of the WD40 domain. Loss-of-function variants in genes encoding the EZH2 lead to overgrowth, macrocephaly, advanced bone age, variable intellectual disability, and distinctive facial features. COGIS has a significant overlap with the EZH2-associated overgrowth syndrome (Cyrus, Burkardt, et al., 2019). Recently, pathogenic variants in SUZ12 have also been described that present with clinical characteristics like the above-mentioned two syndromes (Cyrus, Cohen, et al., 2019).
All nine variants reported so far are missense variants, that preferentially target WD domains 3 and 4. The predominance of missense rather than protein truncating variants in published patients suggests that COGIS is unlikely to be the result of simple haploinsufficiency of EED. As previously mentioned EED is strongly constrained for the loss of function variants. The total loss of function of EED may lead to a lethal phenotype, therefore, it is absent from the medical literature. Another WD40 domain containing gene, WDFY3 was recently associated with an autosomal dominant primary microcephaly. In the original article, the mechanism was postulated to be dominant negative; however, subsequently, truncating/loss of function variants in WDFY3 were also found to cause the same phenotype (Kadir et al., 2016). Episignature profile has been described for EED related disorder. Unfortunately, this could not be performed in our patients (Levy et al., 2022). All fourteen individuals have an intellectual disability (most frequently in the mild range), a distinctive facial appearance, and tall stature. Other frequently associated clinical features include macrocephaly, umbilical hernia, cryptorchidism, abnormalities of muscle tone, cardiac abnormalities, and cervical spine abnormalities. Individuals with germline EED and EZH2 variants can generally be distinguished in their adulthood. Similar findings in both conditions are frontal bossing, a prominent chin, and horizontal skin crease; however, individuals with COGIS also have prominent eyes, eversion of the lateral aspects of the lower eyelid, telecanthus, prominent nose, and pes cavus (Griffiths et al., 2019) (Table 1).
| Study | Cohen et al. (2015) | Cohen and Gibson (2016) | Imagawa et al. (2017) | Cooney et al. (2017) | Smigiel et al. (2018) | Griffiths et al. (2019) | Griffiths et al. 2019 | Griffiths et al. 2019 | Spellicy et al. (2019) | Spellicy et al. (2019) | Spellicy et al. (2019) | Current study (1.1) | Current study 1.2 | Current study 1.3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant | c.906 A>C | c.772 C>T | c.707G>C | c.904 A>G | c.907G>C, c.919A>G | c.581A>G | c.706A>G | c.710A>G | c.773A>T | c.581A>G | c.772C>T | c.710A>G | c.710A>G | c.710A>G |
| p. Arg302Ser | p.His258Thr | p.Arg236Thr | p.Arg302Gly | p.Arg302Gly, p.Asn307Asp | p.Asn194Ser | p.Arg236Gly | p.Asp237Gly | p.His258Leu | p.Asn194Ser | p.His258Tyr | p.Asp237Gly | p.Asp237Gly | p.Asp237Gly | |
| Inheritance | De novo | De novo | De novo | De novo | De novo | De novo | De novo | De novo | De novo/paternal | De novo | De novo/paternal | U | Maternally | Maternally |
| Age at publication | 27 years | 30 years | 5 years | 16 years | 8 years | 26 years | 11 years | 24 years | 10 years | 15 years | ~10 years | 64 years | Died at 33 years | 38 years |
| Sex | Male | Male | Male | Female | Male | Male | Male | Female | Female | Female | Male | Female | Male | Female |
| Birth weight/SD | 1.1 | 1.6 | 3.1 | 4.2 | 75th percentile | 2.3 | 2.8 | 1.1 | 3 | 0.6 | 4 | U | 2.05 | U |
| Birth length/SD | 0.5 | 1.8 | 2.2 | 2.6 | 2 | U | 3 | 3 | U | 0.9 | 3.4 | U | 2.2 | U |
| Head circumfernce/SD | U | 1.6 | 1.4 | 2.2 | −0.1 | U | U | U | U | U | 1.2 | U | 0 | U |
| Current weight/SD | U | 1.9 | 3.8 | 1.6 | 3.6 | 3.7 | 2.4 | 3.6 | 2.64 | U | U | 3.7 | 0.84 | 3.2 |
| Current height/SD | 1.7 | 1.9 | 4.5 | 2 | 4.8 | 3.1 | 3.7 | 3 | 2.89 | U | U | 1.8 | 1.88 | 3 |
| Current head circumference/SD | 1 | 2.2 | 2.6 | 1.8 | 2 | 3.9 | 2.3 | 2.9 | 4.77 | U | U | 1.08 | 2.1 | 1.6 |
| Degree of ID | Mild | Moderate | Mild | Moderate | Moderate | Mild | Severe | Mild | Mild | Moderate | Mild | Mild | Moderate | Moderate |
| Language delay | Moderate | Mild | Not specified | Moderate, hypernasal and hoarse | Moderate, hoarse voice | Limited speech | Mild | Mild | Moderate | Mild | Mild | Moderate | Mild | |
| Personality | Friendly | Hyperactivity | Friendly | Friendly | Friendly | Friendly | ||||||||
| Cardiopulmonary | Mitral regurgitation | PDA | VSD | Atrial septal defect | Unspecified spontaneously resolved defect | Normal | Normal | Normal | ||||||
| CNS | ||||||||||||||
| Hypertonia | U | No | No | Yes | Yes | |||||||||
| Hypotonia | U | Yes | No | Yes | Yes | Yes | Yes | Yes | ||||||
| MRI/CT brain | ND | Normal | Normal | White matter loss, thinning of corpus callosum | Mild enlargement of lateral ventricles | Normal, cervical spinal canal stenosis | Septum pellucidum cyst, small corpus callosum | Normal | Normal | Normal | ||||
| MRI/CT spine | Fusion of cervical vertebral bodies, lumbar spinal canal stenosis | C4–C6 disk bulging | Lumbar Spine Stenosis | |||||||||||
| Seizures | Yes | Yes | Yes | No | ||||||||||
| EEG findings | Generalized irregular wave pattern | Normal | Normal | Normal | Normal | Not applicable | ||||||||
| Antiepileptics/response | Phenytoin/good | Valproate | Valproate/good | Keprra and valproate/poor response | ||||||||||
| Other CNS features | Dyspraxia | Abnormal gait | Reduced muscle bulk | Catatonia | ||||||||||
| Advanced bone age | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||
| Skeletal | Abnormal metaphysis, kyphoscoliosis | Asymmetric skull, scoliosis, cervical instability, pronation of the feet and bent knees | Scoliosis | Camptodactyly, kyphoscoliosis, cervical spine stenosis, small iliac wings, coxa valga, wide metaphyses, and osteopenia, leg length descrepancy, contracture | Scoliosis, club and flat feet | Cervical spine canal stenosis | Scoliosis | Hip dysplasia | L2–L3 fusion, spondylolyses, spondylolisthesis | Pes cavus | No | Pes cavus | ||
| GU | ||||||||||||||
| Cryptorchidism | Yes | Yes | Yes | Yes | Incompletely descended testes | |||||||||
| Renal anomaly | Nephromegaly, duplicated collecting system | |||||||||||||
| Gastro | Constipation | Poor feeding, functional bowel obstruction | Hirschsprung disease | |||||||||||
| Endocrine | Hyperinsulinemic hypoglycaemia subclinical hypothyroidism | Hyperinsulinemic hypoglycaemia | Gynaecomastia, adrenal gland calcification, partial cortisol deficiency | |||||||||||
| Vision | ||||||||||||||
| Myopia | Yes | Yes | Yes | Myopia, choreoretinitis | Yes | Yes | ||||||||
| Hyperopia | ||||||||||||||
| Strabismus | Exotropia | Yes | Yes | |||||||||||
| Astigmatism | Yes | |||||||||||||
| Cataract | Yes | |||||||||||||
| Hernia | Umbilical hernia | Umbilical hernia | Umbilical hernia | Umbilical, inguinal hernia | Umbilical hernia | Umbilical hernia | Umbilical hernia | |||||||
| Dental | Overbite | Wide spaced teeth | Dysplastic enamel, large teeth | |||||||||||
| Dysmorphic features | ||||||||||||||
| Skull | ||||||||||||||
| Abnormal skull shape | Dolichocephaly | Asymmetry | Flat occiput | Fine hair, epicanthus | Telecanthus | Occipital flattening | High frontal hairline | Flat occiput | ||||||
| Increased bifrontal diameter | Yes | Yes | Yes | |||||||||||
| Forehead | ||||||||||||||
| Broad forehead | Yes | Yes | Yes | Yes | Yes | Yes, high anterior hairline | Yes | Yes | Yes | |||||
| Eyebrows | ||||||||||||||
| High arched eyebrows | Yes | Yes | Yes | Thick | Eversion of the lateral lower eyelid | Synophrys | Thick | Thick | Yes | Yes | Yes | |||
| Thick eyebrows | Yes | Yes | Yes | Synophrys | Yes | Thick | Thick | Thick | ||||||
| Eyes | ||||||||||||||
| Almond-shaped PF | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Hypertelorism | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||||
| Ptosis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
| Everted lateral eyelids | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | ||||||
| Upslanting PF | No | No | No | No | Yes | |||||||||
| Downslanting PF | Yes | No | Yes | Yes | Yes | Yes | ||||||||
| Epicanthus | Yes | Yes | Telecanthus | Telecanthus | ||||||||||
| Deep set eyes | Yes | Yes | ||||||||||||
| Ears | ||||||||||||||
| Long ears | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Thick and fleshy | Dysplastic helices, protruding ears | Yes | Yes | Yes | ||
| Other anomaly | Ear pit | |||||||||||||
| Hearing loss | Conductive hearing loss | Bilateral hypoacusis, hearing loss | ||||||||||||
| Nose | ||||||||||||||
| High nasal bridge | Yes | Yes | No | No | Yes | Yes | Yes | Yes | ||||||
| Low nasal bridge | No | Yes | Yes | Flat midface | ||||||||||
| Bulbous nasal tip | Yes | Yes | Flat tip | Yes | Yes | Yes | Yes | |||||||
| Philtrum | ||||||||||||||
| Prominent and/or long philtrum | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Lips | ||||||||||||||
| Full lips | Yes | Yes | Yes | Yes | Yes | |||||||||
| Palate | ||||||||||||||
| Bifid uvula | Yes | Yes | High arched | Yes | No | No | ||||||||
| Chin | ||||||||||||||
| Micrognathia/retrognathia | Yes | Yes | Yes | Yes | Yes | Prognathism | Prognathism | |||||||
| Pointed, “stuck-on” chin with horizontal skin crease | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Hands/feet | ||||||||||||||
| Large hands and feet | Yes | Yes | Yes | Yes | Club foot | Yes | Fleshy | yes | Yes | Yes | Yes | |||
| Digits | ||||||||||||||
| Camptodactyly | Yes | Yes | Yes | Yes | Thick | |||||||||
| Joint hypermobility | Yes | Yes | Yes | |||||||||||
| Joint stiffness | Yes | Yes | ||||||||||||
| Abnormal nails | Yes | Yes | ||||||||||||
| Thumbs/great toes | Broad | Broad | Broad | |||||||||||
| Nails | Fragile and thin | Small | Dysplastic | |||||||||||
| Long digits | Yes | Tapered | Yes | Yes | Yes | |||||||||
| Other features | Widely spaced nipples and several pigmented nevi | Osteopenia, pigmented nevi | Poor weight gain | Fingertip pads | Fingertip pads |
- Abbreviation: U = unknown.
In conclusion, the current report is the first familial report of three affected members of two generations with significant homogeneity of phenotype. These are the oldest reported patients in the literature with EED related disorder. We propose that EED related overgrowth syndrome is phenotypically recognizable from other disorders associated with PRC2 complex disorders.
ACKNOWLEDGMENTS
The authors appreciate the family's involvement in this case report.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




