The IFITM5 Ser40Leu variant can manifest as prenatal Caffey disease
Abstract
We report on a female neonate with a clinico-radiological presentation in keeping with a lethal form of prenatal Caffey disease (PCH). She had antenatal and postnatal features of severely bowed long bones, small chest, diaphyseal hyperostosis and polyhydramnios and died shortly after birth. Initial testing excluded COL1A1-related PCH, as an OI gene panel, consisting of COL1A1, COL1A2, CRTAP, and P3H1 genes, was negative. Targeted sequencing using a gene panel was performed and a de novo heterozygous, likely pathogenic variant in IFITM5: c.119C > T(p.Ser40Leu) was identified, which was previously described to cause a severe form of progressively deforming osteogenesis imperfect (OI). To our knowledge, variants in IFITM5 have not been reported in infantile Caffey disease (ICH) or PCH. Given that the pathogenesis of PCH is largely unknown, we postulate that a subset of PCH may be associated with variants in IFITM5.
1 INTRODUCTION
Infantile cortical hyperostosis (ICH), also known as Caffey disease (MIM #114000), is a heterogeneous group of disorders characterized by massive subperiosteal new bone formation in early infancy, usually presenting before 5 months of age and typically involving the long bones, clavicles and mandibles (Caffey, 1957). ICH may present with fever, irritability, bony tenderness and can have a course which flares and remits spontaneously (Couper et al., 2001). Another form of ICH, namely prenatal Caffey disease or prenatal cortical hyperostosis (PCH) presents earlier and is further divided into two groups. The severe prenatal form, which is also known as lethal PCH, presents before 35 weeks of gestation with polyhydramnios, hydrops, anasarca, pulmonary hypoplasia, and involvement of nearly all the long bones. These features are usually absent in the mild prenatal form (Kamoun-Goldrat et al., 2008; Wright Jr. et al., 2005).
A heterozygous pathogenic variant in COL1A1: c.3040C > T (p.Arg1014Cys) has been identified in a subset of individuals with autosomal dominant ICH or PCH (Gensure et al., 2005; Kamoun-Goldrat et al., 2008). However, ICH and PCH have significant etiological and/or genetic heterogeneity, as COL1A1 genetic testing is negative for most affected individuals (Gensure et al., 2005; Nemec et al., 2012). Notably, the etiology of PCH is still poorly understood and remains under debate, and includes cases with an autosomal recessive inheritance pattern involving genes other than COL1A1 (Nemec et al., 2012) and an in-utero acquired condition leading to a PCH-like clinical picture.
Here, we present a family that was referred to Genetics as their infant was suspected to have lethal prenatal Caffey disease. There was no known family history of skeletal dysplasias. The antenatal and postnatal features were suggestive of PCH. Targeted sequencing using a gene panel (TruSight One sequencing panel, Illumina) identified a de novo likely pathogenic variant in IFITM5, previously described in patients with severe deforming osteogenesis imperfecta (OI), distinct from the typical IFITM5 type V OI. This report thus expands the clinical spectrum of IFITM5 p.Ser40Leu to include PCH.
2 CASE REPORT
The proband was the first child born to a 27-year-old mother. First trimester screening at 12 + 5 weeks of gestation showed an absent nasal bone, abnormal nuchal thickness of 2.9 mm (more than 97th centile), a short right humerus and bilateral short and curved femurs. She was found to be at high risk for trisomy 21 (1 in 33) and intermediate risk for trisomy 18 (1 in 590) based on first trimester serum screening.
Her subsequent follow-up antenatal scans at 16 + 1, 20 + 1, and 21 + 5 weeks showed a narrowed chest, short femurs (<3rd centile), bilateral curved and bent humerii, radii, ulnae, femurs, tibiae, and fibulae (Figure 1). There was no evidence of any fractures. There was suspicion for a skeletal dysplasia, with a possible guarded prognosis in view of the risks of severe lung hypoplasia with the narrow chest wall. The couple was extensively counseled and offered testing, but declined both non-invasive and invasive prenatal testing options.
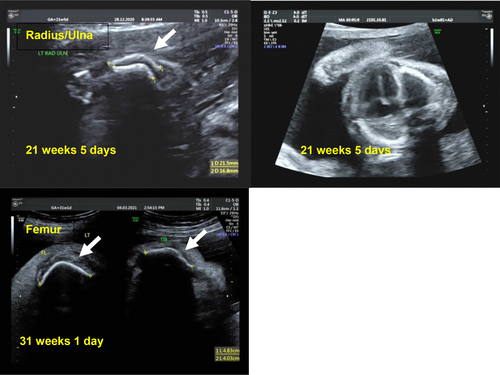
An episode of threatened preterm labor occurred at 25 + 4 weeks gestation and the mother was subsequently discharged. Polyhydramnios and skin edema were noted on her last antenatal scan at 26 + 1 weeks and four amnioreductions were required at 27 + 2, 28 + 6, 31, and 32 + 1 weeks, respectively. The mother agreed to the collection and storage of fetal DNA from amniotic fluid for future testing. She was counseled by the maternal-fetal medicine specialist, neonatologist, and perinatal palliative consultant and decided on comfort care for the baby. She subsequently developed preterm premature rupture of the membranes at 32 + 1 weeks and delivered at 32 + 2 weeks.
A female infant was born at 32 + 2 weeks via normal vaginal delivery, with a birth weight of 2.43 kg (90th to 97th percentile). Her APGAR scores were 2 at 1 min and 2 at 5 min. She did not cry spontaneously and appeared cyanotic, with no improvement in her respiratory status despite the use of continuous positive airway pressure (CPAP). The decision was made to pursue palliative comfort care at 7 min of life. She subsequently passed away at 40 min of life.
On physical examination, there was hydrops, frontal bossing, a bell-shaped chest, short limbs (rhizomelic segments), and bowing of her limbs. Postmortem radiographs showed multiple Wormian bones in the skull, mildly thickened ribs with a beaded appearance, severely bowed long bones with diaphyseal widening and mild cortical thickening and mild hyperostosis of the mandible. The clavicles and scapulae were not affected. The constellation of these findings led to the suspicion of the lethal form of PCH (Figure 2). The wavy appearance of the ribs was presumed to be the result of hyperostosis rather than multiple healing fractures.
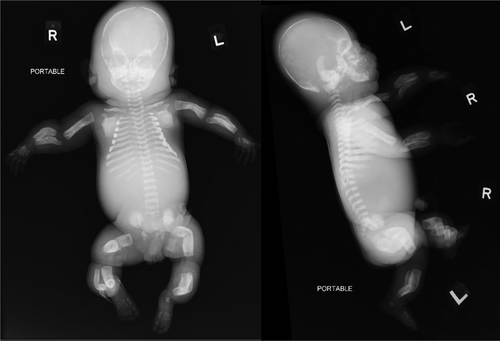
Her parents declined a post-mortem examination but agreed to placental histology and further genetic testing on amniocytes, which were previously obtained during one of the amnioreduction sessions. This was done in view of considerations and recurrence risks for future pregnancies.
An OI panel consisting of COL1A1, COL1A2, CRTAP, and P3H1 genes was done initially to test for COL1A1-related PCH and was negative. The proband was recruited for a research project under institutional review board-approved study (CIRB 2019/2243), and informed consent was obtained from the parents. A heterozygous pathogenic variant c.119C > T (p.Ser40Leu) was identified in IFITM5 (Figure S1), leading to the diagnosis of IFITM5-associated OI. Sanger validation of the IFITM5 confirmed its presence in the proband, and the absence in her parents indicates that the variant is de novo.
3 DISCUSSION
Here, we report on a patient with a previously described likely pathogenic variant in IFITM5: c.119C > T (p.Ser40Leu), with initial clinico-radiological diagnostic suspicion of PCH. IFITM5 is known to underlie type V OI, due to a recurrent heterozygous variant c.-14C > T at the 5′-untranslated region (UTR) of the gene. Type V OI is characterized by calcification of the forearm interosseous membrane with radial head dislocation and hyperplastic callus formation as well as dense metaphyseal bands with metaphyseal flaring in the neonatal period (Glorieux et al., 2000).
The c.119C > T (p.Ser40Leu) found in our patient has been reported in four other patients (Farber et al., 2014; Guillén-Navarro et al., 2014; Hoyer-Kuhn et al., 2014; Liu et al., 2017; Rodriguez Celin et al., 2018). These patients had clinical and radiological features distinct from those of type V OI, with a very severe presentation of prenatal onset. In these patients, the prenatal and perinatal manifestations are that of severely angulated long bones, which later evolves into type III OI with multiple fractures and severe deformities (Rodriguez Celin et al., 2018). The majority of them had bone shortening and bowing seen antenatally, but did not present with other features. At birth, they had short and bowed limbs with or without multiple fractures including in their limbs and ribs. Postnatally, they had short and bowed limbs, hypoplastic thoraces with narrow chests and extreme short stature. All of them had motor development delays and motor disability. (Farber et al., 2014; Guillén-Navarro et al., 2014; Hoyer-Kuhn et al., 2014; Rodriguez Celin et al., 2018).
On the other hand, in prenatal lethal Caffey disease, typical radiographic features are that of a rather symmetrical hyperostosis of the long bones, scapulae and mandible. The humeri and femora are short and appear angulated without fractures. The ribs can be involved, with less severe signs of periosteal thickening. Bony sclerosis and ballooning of the diaphysis of the long bones with periosteal sclerosis can give rise to a “bone within bone” appearance, with relative sparing of the metaphyses. There is also sparing of the spine, clavicles and small bones of the hands and feet (Burton & Glanc, 2016; Dahlstrom et al., 2001; Hoen et al., 2015; Kamoun-Goldrat et al., 2008; Kim et al., 2019; Schweiger et al., 2003; Wright Jr. et al., 2005). The patient presented here was thus suspected of having lethal PCH. In retrospect, however, the prenatal bowing, diaphyseal widening and a wavy appearance of the ribs may have been the result of numerous occult fractures.
It can be challenging to differentiate between subperiosteal cortical hyperostosis, which classically occurs in Caffey disease and hyperplastic callus formation, which is a distinctive feature in type V OI. This is because the variable course of hyperplastic callus formation can resemble that of Caffey disease (Glorieux, 2005). Subperiosteal cortical hyperostosis involves periosteal/cortical thickening and dense laminated subperiosteal new bone formation. This results in an increase in cortical width and density, which can appear to be fairly well-defined with a smooth osseous surface (Lachman, 2007). On the other hand, hyperplastic callus formation, which can either be precipitated by fractures or arise spontaneously, can have a variable evolution (Cheung et al., 2007). It starts with the proliferation of soft tissue at the level of the periosteum, forming at times and sites of periosteal apposition. There are distinctive zones with the outer regions of the callus containing edematous tissue with a loose collagenous network to the innermost region showing hypercellular trabeculae of woven bone and small cartilaginous islands (Brenner et al., 1989). After the hyperplastic callus mineralises, it usually stabilizes for some years. Smaller lesions can resolve while larger lesions can result in the destruction of normal bone anatomy (Cheung et al., 2007).
The clinical and causal heterogeneity of PCH remains elusive. In a review of 44 cases of PCH, 26 patients (59%) had the severe prenatal form as was seen in our patient (Schweiger et al., 2003). The outcomes of the severe prenatal form were poor with 11 (42%) being stillborn. All were born premature, which was likely due to polyhydramnios and fetal hydrops, like in the case of our patient. Most presented first with polyhydramnios and had fetal hydrops, skin edema and hepatosplenomegaly. The earliest manifestation of bowing or shortening of limbs was reported at 14 weeks of gestation. The majority also had all long bones involved antenatally. The major cause of mortality was that of respiratory complications caused by prematurity or pulmonary hypoplasia. Of note, in the cases who survived, significant recovery of the bones was observed weeks after birth. In all of these cases, a molecular etiology was not identified.
Skeletal dysplasias are a complex group of bone and cartilage disorders with significant clinical and genetic heterogeneity. The lack of family history and limited clinical symptoms can lead to difficulties in arriving at an accurate diagnosis. The advancements in next-generation sequencing and molecular technology have led to the ability to achieve clearer genetic diagnoses (LaDuca et al., 2017; Retterer et al., 2016). There is thus a role for genetic testing, as an accurate diagnosis has important implications in prognostication, family counseling and subsequent targeted management plans. In this report, we brought attention to an example of a subset of IFITM5-associated OI, which presents as a phenotype of severe prenatal PCH.
4 CONCLUSION
In conclusion, we report finding a known variant in the IFITM5 in a patient with an unusual clinico-radiological presentation, namely that of PCH. Our experience suggests that IFITM5-associated OI can manifest as not only type V OI and a phenotype of severely angulated long bones, but also, as PCH. Given that the pathogenesis of PCH is largely unknown, IFITM5-associated OI may constitute a subset of PCH. This report illustrates the importance of using precise radiographic evaluation together with genetic investigation to enable an accurate diagnosis of the heterogeneous group of lethal cortical hyperostosis.
AUTHOR CONTRIBUTIONS
Jia Ying Celeste Yap: conduct of literature review, writing—original draft, writing—review and editing. Jiin Ying Lim: conceptualization and design of study, writing—original draft, writing—review and editing. Gen Nishimura and Shahida Moosa: conceptualization and design of study, writing—review and editing, supervision and mentorship. Anju Bhatia, Vic Khi June Tan, Stephanie Koo, Ai Ling Koh, Ene Choo Tan, and Nikki Fong: writing—review and editing. Saumya Shekhar Jamuar: conceptualization and design of study, writing—review and editing, supervision and mentorship.
FUNDING INFORMATION
The study was supported by National Medical Research Council Centre Grant (NMRC/CG1/006/2021). Saumya Shekhar Jamuar is supported by National Medical Research Council Clinician Scientist Award (NMRC/CSAINVJun21-0003).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




