A Japanese patient with Teebi hypertelorism syndrome and a novel CDH11 EC1 domain variant
Abstract
The gene CDH11 encodes cadherin-11, a Type II cadherin superfamily member that contains five extracellular cadherin (EC) domains. Cadherin-11 undergoes trans-dimerization via the EC1 domain to generate cadherin complexes. Compound heterozygous and homozygous loss-of-function CDH11 variants are observed in Elsahy–Waters syndrome (EWS), which shows characteristic craniofacial features, vertebral abnormalities, cutaneous syndactyly in 2–3 digits, genitourinary anomalies, and intellectual disability. Heterozygous CDH11 variants can cause Teebi hypertelorism syndrome (THS), which features widely spaced eyes and hypospadias. We report a THS patient with a novel CDH11 variant involving the EC1 domain. The patient was a 10-month-old male with normal developmental milestones, but had widely spaced eyes, strabismus, hypospadias, shawl scrotum, broad thumbs (right bifid thumb in x-ray), polysyndactyly of the left fourth finger, and cutaneous syndactyly of left third/fourth fingers. Exome sequencing identified a de novo heterozygous CDH11 variant (NM_001797.4:c.229C > T [p.Leu77Phe] NC_000016.9:g.64998856G > A). Clinical features were consistent with previously reported THS patients, but polysyndactyly, broad thumb, and cutaneous syndactyly overlapped phenotypic features of EWS. THS and EWS may represent a spectrum of CDH11-related disorders. Residue Leu77 in this novel CDH11 variant lines a large hydrophobic pocket where side chains of the partner cadherin-11 insert to trans-dimerize, suggesting that the cadherin-11 structure might be altered in this variant.
1 INTRODUCTION
CDH11 encodes cadherin-11, a member of Type II superfamily of cadherins and contains five extracellular cadherin (EC) domains. Cadherin-11 undergoes trans-dimerization via the EC1 domain to form a cytoplasmic interaction with the cadherin complex of opposing cells (Patel et al., 2006). Through these interactions, cadherin-11 is involved in the regulation of cell migration, cellular differentiation, and cellular adhesions (Becker et al., 2013). Deficiencies in cellular adhesion molecules have been associated with the occurrence of syndactyly and other congenital anomalies (Brancati et al., 2010; Suzuki et al., 2000). Compound heterozygous and homozygous loss-of-function CDH11 variants have been found in Elsahy–Waters syndrome (EWS; OMIM#211380; Taskiran et al., 2017). Furthermore, heterozygous CDH11-dominant negative variants have been associated with Teebi hypertelorism syndrome (THS; OMIM#619736). Nine THS families have been reported with widely spaced eyes and hypospadias (Li et al., 2021). In this study, we present a THS patient with a novel heterozygous CDH11 variant.
2 CLINICAL REPORT
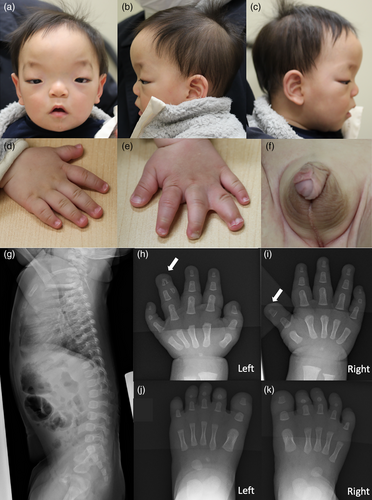
The patient was a 10-month-old male of dizygotic twins born to healthy nonconsanguineous parents. He was delivered at 35 weeks and 5 days gestational age after an uneventful pregnancy, with a birth weight of 2431 g (0.2 SD), birth length of 49.2 cm (1.3 SD), and an occipital–frontal head circumference (OFC) of 32.0 cm (0.2 SD). He was referred to our department at 4 months of age for the assessment of multiple congenital anomalies. At referral, his height, weight, and OFC were 64.0 cm (1.2 SD), 6920 g (0.7 SD), and 41.8 cm (0.8 SD), respectively. His dysmorphic features included a wide forehead, large anterior fontanelle, arched eyebrows, widely spaced eyes, depressed nasal root, short nose, prominent philtrum, down-slanted corners of the mouth, narrow palate, tented upper lip, broad thumb, cutaneous syndactyly of the left third/fourth fingers, and polysyndactyly of the left fourth finger (Figure 1a–e). In addition, he had strabismus, an abnormal gluteal cleft, hypospadias, shawl scrotum (Figure 1f), and a small hemangioma on the left arm (approximately 10 mm in diameter). Neonatal hearing screening test results and cardiac echogram were normal. Skeletal x-ray revealed polysyndactyly of the left fourth finger and that the right broad thumb was the result of a bifid distal phalanx (Figure 1g–k). At 10 months of age, head MRI was normal, but spinal MRI revealed the caudal spinal cord ending at the L3 and L4 vertebral levels. Karyotype was normal. Development and growth were normal. He controlled his head at 3–4 months, rolled over at 5–6 months, sat up at 8 months, and walked with support at 10 months of age. His height was 75.5 cm (1.1 SD), weight was 9935 g (0.9 SD), and OFC was 47.0 cm (0.8 SD) at 10 months of age.
3 MATERIALS AND METHODS
The patient and family members were enrolled in an institutional review board-approved study with informed consent at the Kanagawa Children's Medical Center. DNA libraries were enriched for sequencing using SureSelect XT (Agilent Technologies, Inc., Santa Clara, CA). Samples were sequenced by NovaSeq (Illumina Inc., San Diego, CA) using 151-bp pair-end reads. The data were analyzed by the Burrows–Wheeler alignment tool (version 0.7.17) and the Genome Analysis Toolkit pipeline (version 4.2.0.0; Broad Institute, Cambridge, MA). Of the called variants within exons or ± 8 bp from exon–intron boundaries, those registered as having more than 1% of allele frequency in the genome Aggregation Database (gnomAD), the NHLBI GO Exome Sequencing Project 6500, the 1000 Genomes Project, the Human Genetic Variation Database, the Japanese Multi Omics Reference Panel (jMorp) genome variant database, and our in-house Japanese exome data (145 individuals) were removed (Kuroda et al., 2019). For de novo variants, variants absent in these control databases and in-house data were called.
4 RESULTS
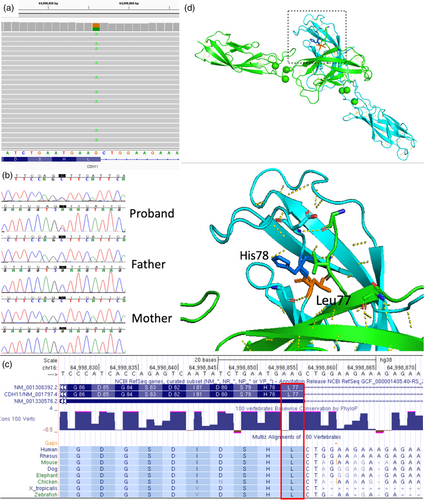
The mean depth of coverage in the patient's exome sequencing was above 112.28 per base, and 96.6% of the coding region was covered by at least 20 reads. We identified candidate variants of 91 ultrarare heterozygous variants and evaluated candidate genes to explain the phenotype of the patient. Finally, exome sequencing identified a novel heterozygous variant in CDH11 (NM_001797.4:c.229C > T [p.Leu77Phe] NC_000016.9:g.64998856G > A) (Figure 2a). The de novo variant was confirmed by trio Sanger sequencing (Figure 2b) and was absent in the population databases. This variant was considered as “likely pathogenic” according to the 2015 ACMG variant interpretation guidelines (PS2 + PM1 + PM2_supporting + PP3 + PP4). The variant is reported in ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar; code SCV003919729, likely pathogenic). The variant is located in the EC1 domain, which is conserved among species (phastCons 1, Figure 2c), and considered an intolerant region as predicted by MetaDome. In fact, the mouse cadherin-11 protein demonstrated high similarity to human cadherin-11 (Patel et al., 2006). From protein structures (2a4e, mouse cadherin-11 EC1-2), residue Leu77 is shown to form a hydrogen bond with Gly108, and the neighboring amino acid His76 forms hydrogen bonds with the partner molecule to form a trans-dimer between EC1 domains (Figure 2d).
5 DISCUSSION
We report a novel de novo heterozygous CDH11 variant in a patient with THS. The patient had widely spaced eyes, hypospadias, digital anomalies, and dysmorphic features that were consistent among 19 patients previously reported (Li et al., 2021). However, he also exhibited shawl scrotum, cutaneous syndactyly, and polysyndactyly, which are uncommon in THS (Table 1). In contrast, the allelic disorder EWS is characterized by distinctive craniofacial morphology, cervical fusion, cutaneous syndactyly in 2–3 digits, genitourinary anomalies, and intellectual disability. In this patient, cutaneous syndactyly was an overlapping phenotype with that of EWS. Although polydactyly and bifid thumb have not been reported in either THS or EWS, skeletal abnormalities in EWS tend to be more severe than those in THS (Table 1). In this patient, overall clinical features were compatible with THS, including normal development and no vertebral abnormality, although atypical and overlapping features with EWS were noted. To date, seven patients have been identified with bi-allelic CDH11 variants in EWS (Castori et al., 2018; Harms et al., 2018; Minatogawa et al., 2021; Taskiran et al., 2017). Likely, CDH11 haploinsufficiency does not contribute to any phenotypes since heterozygous parents of EWS patients have not reported THS phenotypes. Dominant-negative effects may lead to THS, thus, a significantly reduced function of cadherin-11 may be a shared mechanism. Both dominant-negative and bi-allelic loss-of-function variants have been reported to cause similar and overlapping phenotypes with other genes, such as DNM1-related epileptic encephalopathy (Biesecker et al., 2021; Yigit et al., 2022). Thus, it appears that THS and EWS are different syndromes, but may constitute a broad spectrum of CDH11-related disorders.
| This patient | THS (n = 19)a | EWS (n = 7)b | |
|---|---|---|---|
| Variant | Heterozygous | Heterozygous | Homozygous or compound heterozygous |
| Inheritance | de novo | de novo or inherited | |
| Brachycephaly | + | Na | 7/7 |
| Large anterior fontanelle | + | 10/16 | Na |
| Widely spaced eyes | + | 19/19 | 7/7 |
| Proptosis | + | 5/19 | 7/7 |
| Ptosis | + | 5/19 | 6/7 |
| Broad forehead | + | 18/19 | 7/7 |
| Broad, thick, or arched eyebrows | + | 17/19 | 7/7 |
| Eyelid coloboma | − | 2/19 | 1/7 |
| Short nose | + | 15/19 | 7/7 |
| Broad or high nasal root | + | 1/19 | 7/7 |
| Depressed nasal root | + | 13/19 | 7/7 |
| Thin upper lip | + | 16/19 | 6/7 |
| Small chin with central transverse groove | − | 16/19 | 7/7 |
| Umbilical defect | − | 3/13 | Not reported |
| ID/DD | − | 7/19 | 7/7 |
| Strabismus | + | Not reported | 6/7 |
| Cleft palate | − | 1/19 | 2/7 |
| High palate | + | 12/19 | 5/5 |
| Dental cysts | − | Not reported | 5/5 |
| Heart defect | − | 5/19 | Not reported |
| Truncal skeletal abnormality/vertebral fusion | Abnormal gluteal cleft | 3/13 | 4/5 |
| Skin syndactyly | + | 0/18 | 3/7 |
| Polydactyly | + | 0/18 | 0/7 |
| Hallux valgus | − | Na | 4/5 |
| Broad or short phalanx | + | 6/18 | 3/7 |
| Hypospadias | + | 4/8 | 1/3 |
| Shawl scrotum | + | 0/8 | Na |
Intercellular adhesion is affected by CDH11 variants associated with THS (Li et al., 2021). Leu77 is part of the adhesive interface residues (from Val72 to Asp80) and lines a large hydrophobic pocket where the conserved Trp55 and Trp57 side chains of Type II cadherins insert (Patel et al., 2006). Although 8 of 9 reported variants are located in the EC2-3 domain or linker regions, the (p.Trp55Ser) variant in the EC1 domain affects hydrogen bonds between Trp55 and Glu140/Pro141 (Li et al., 2021). Thus, it is assumed that the novel (p.Leu77Phe) variant would also affect cadherin structure.
In conclusion, we report a THS patient with a novel CDH11 EC1 domain variant and skeletal features that overlap those of EWS patients. Our findings suggest that dysfunction of the EC1 domain in cadherin-11 leads to THS.
AUTHOR CONTRIBUTIONS
Kenji Kurosawa designed the study. Yumi Enomoto and Takuya Naruto performed the bioinformatics experiments and contributed to the genetics experiments. Yoko Saito, Yukiko Kuroda, and Kenji Kurosawa recruited and evaluated the study subjects. Kenji Kurosawa supervised Yukiko Kuroda. Yukiko Kuroda prepared the manuscript.
ACKNOWLEDGMENTS
This study was supported by The Initiative on Rare and Undiagnosed Diseases (Grant number 23ek0109549) from the Japan Agency for Medical Research and Development and JSPS KAKENHI Grant Number 23 K14966 (Yukiko Kuroda). Dr Gen Nishimura participated in the evaluation of the radiograph of the patient. We thank the patient and his family for their cooperation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




