Microcystic lymphatic malformations in Turner syndrome are due to somatic mosaicism of PIK3CA
Abstract
Turner syndrome (45,X) is caused by a complete or partial absence of a single X chromosome. Vascular malformations occur due to abnormal development of blood and/or lymphatic vessels. They arise from either somatic or germline pathogenic variants in the genes regulating growth and apoptosis of vascular channels. Aortic abnormalities are a common, known vascular anomaly of Turner syndrome. However, previous studies have described other vascular malformations as a rare feature of Turner syndrome and suggested that vascular abnormalities in individuals with Turner syndrome may be more generalized. In this study, we describe two individuals with co-occurrence of Turner syndrome and vascular malformations with a lymphatic component. In these individuals, genetic testing of the lesional tissue revealed a somatic pathogenic variant in PIK3CA—a known and common cause of lymphatic malformations. Based on this finding, we conclude that the vascular malformations presented here and likely those previously in the literature are not a rare part of the clinical spectrum of Turner syndrome, but rather a separate clinical entity that may or may not co-occur in individuals with Turner syndrome.
1 INTRODUCTION
Turner syndrome is a sex chromosome disorder, occurring in about 1 in 2000 to 1 in 2500 live female births, caused by the complete or partial absence of an X chromosome (Bondy and Group, 2007; Gravholt et al., 2017). This syndrome is commonly associated with short stature, webbed neck, widely spaced nipples, and congenital heart disease such as coarctation of the aorta.
Vascular malformations such as “pedal hemangiomas” and ectatic vessels in the gastrointestinal tract have been described as part of the clinical spectrum of Turner syndrome (Kucharska et al., 2018; Paller et al., 1983; Weiss, 1988). Vascular malformations form due to abnormal growth and development of blood and lymphatic vessels (Queisser et al., 2021). Vascular malformations are commonly caused by somatic pathogenic variants in the genes that regulate growth and apoptosis of vascular channels (Queisser et al., 2021). The International Society for the Study of Vascular Anomalies provides a nomenclature for the diagnosis of vascular malformations, which is typically done by a combination of history, physical exam, pathology, genetics, and imaging (Wassef et al., 2015). A previous study described the co-occurrence of other conditions in Turner syndrome and suggested evaluation for dual diagnosis in individuals with Turner syndrome and rare features (Jones et al., 2018).
Two individuals with Turner syndrome and vascular malformations presented to our clinic. Given the previous case series and common somatic causes of vascular malformations, we hypothesized that the vascular malformations were due to a secondary mosaic cause. In this study, we describe two individuals with co-occurrence of Turner syndrome and PIK3CA-related vascular malformations to confirm this association is due to dual diagnosis and is not a rare feature of Turner syndrome.
2 METHODS
2.1 Editorial policies and ethical considerations
Individual 1 and 2 provided informed written consent for photo publication and individual 2 enrolled in a research study with the Center for Applied Genomics (CAG) that was approved by the Children's Hospital of Philadelphia (CHOP) Institutional Review Board.
The Somatic Overgrowth and Vascular Malformations panel was used for both individuals (NCBI, 2020). Briefly, targeted DNA capture of 34 genes, including PIK3CA, was performed via a custom-designed Agilent XT HS Target Enrichment System panel. The targeted regions include all coding exons and 10 bp of flanking intronic regions. Libraries were sequenced on an Illumina NextSeq with 150 bp paired end sequencing. Variant filtering and annotation was performed using Agilent SureCall software using chromosome build GRCh37. The limit of detection for the Somatic Overgrowth and Vascular Malformation panel for clinical reporting is 1% variant allele fraction (VAF) (at 1000× coverage with at least 10 unbiased reads) that is confirmed in a second run of the sample.
The exome libraries for individual 2 were made using Twist Human Core Exome Capture Kit (TWIST Bioscience). Sequencing was performed on NovaSeq 6000 sequencers (Illumina, Inc., San Diego, CA, USA) at CAG/CHOP. Data were analyzed using BWA-mem v0.7.12 for alignment and Picard v1.97 for PCR duplication removal. The resulted BAM file was fed to GATK/Queue v2.6.5 for germline variant calling and GATK/Mutect2 v4.1.4.0 for somatic variant calling. ANNOVAR and SnpEff were then used to functionally annotate the variants and collect minor allele frequency (MAF) data from 1000 Genomes Projects, ESP6500SI, ExAC, gnomAD, and Kaviar. Subsequent variant filtration and prioritization were based on MAF in either population dataset and function annotation, such as, nonsynonymous, exonic, splicing-altering, and frameshift. Subsequent gene prioritization was performed on the basis of deleterious prediction and biological relevance by referring to the Online Mendelian Inheritance in Man (OMIM) database and Human Gene Mutation Database (HGMD). In a large cohort of participants with vascular anomalies published by our group, the average coverage for deep exomes was 470× (128–1030×) with the lowest VAF detected 2.3% (Li et al., 2023).
3 CASE REPORTS
Individual 1 is an 11-year-old female with a combined lymphatic-venous malformation of the right foot, lymphedema, and Turner syndrome. The pregnancy was complicated by increased nuchal translucency. She was noted to have lymphedema of the hands and feet, and extra nuchal skin at birth. Karyotype and FISH for X and Y chromosomes confirmed 45,X.
She was born with flesh-colored nodules on the plantar surface of her right foot (Figure 1a). Around 5 years old, the posterior nodule on her became reddish and then blackish and was excised. Pathology from the excision revealed hyperkeratosis, acanthosis, and multiple red blood cell-containing thin-walled dilated channels predominantly in the superficial dermis (Figure 1b). The endothelial lining stained strongly positive for CD31 with focal positivity for D2-40 and was negative for GLUT-1, suggestive of lymphatic origin.
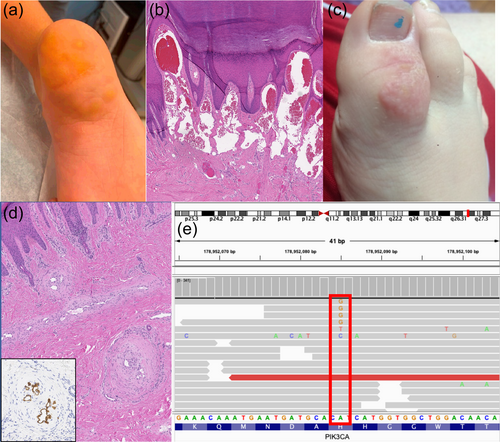
Three years later, at age eight, she was seen again for a vascular lesion of her right great toe (Figure 1c). Debulking procedure was done for the lesion on her right great toe in addition to a split thickness skin graft from her buttock to her right great toe. Pathology of tissue from this lesion revealed irregular and tortuous thick and thin-walled channels within the dermis with irregular luminal “fenestrations” or plexiform-type lesions. These types of changes are most similar to that seen in vessel re-canalization post thrombosis or are akin to the plexiform lesions seen in pulmonary arterial hypertension. The endothelium of these vessels stained positive for both CD31 and D2-40, supporting lymphatic origin. The thick smooth muscle lining of the vessels was highlighted by SMA and h-caldesmon. Based on morphology and immunophenotype, the pathology for both the heel and the great toe sample were consistent with a combined vascular malformation containing both lymphatic and venous components.
Somatic Overgrowth and Vascular Malformation panel performed on genomic DNA isolated from the original heel tissue sample revealed a pathogenic PIK3CA variant (c.3140A>G, p.His1047Arg) with a variant allele frequency of 0.7%–1.0% (9 out of 1320 reads; 12 out of 1116 reads) (Figure 1e). The p.His1047Arg variant is a recurrent pathogenic variant found in lymphatic malformations and has an allele frequency of 0.000004028 in gnomAD (Brouillard et al., 2021; Chen et al., 2022).
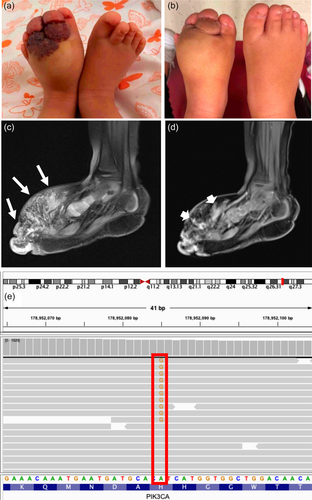
Individual 2 is a 3-year-old female with Turner syndrome diagnosed by chorionic villus sampling due to cystic hygroma. At birth, she was noted to have a lesion on the foot which was thought to be a bruise due to the emergency C-section. When this did not resolve, it was then thought to be a congenital hemangioma. She was referred to our Comprehensive Vascular Anomalies Clinic at Children's Hospital of Philadelphia for diagnosis and treatment of the foot lesion at 15 months of age. Physical exam was notable for long eyelashes, a flared medial eyebrow, and dorsal area of the left foot with overlying patchy port wine stains, angiokeratomas, and soft tissue hypertrophy and plantar surface with similar crusting and ulceration (Figure 2a,b). Intralesional bleomycin sclerotherapy and concurrent tissue biopsy for genetic testing was recommended. MRI with contrast and ultrasound revealed fatty hypertrophy of the dorsal aspect of the left foot intermixed with a microcystic lymphatic malformation (Figure 2c,d). Somatic Overgrowth and Vascular Malformation panel was performed on genomic DNA isolated from lesional tissue biopsy identified a pathogenic PIK3CA variant (c.3140 A>G, p.His1047Arg) with a 3.1% VAF (46 out of 1473 reads) (Figure 2e). Research deep exome sequencing revealed the same somatic variant at a VAF of 4.1% (16 out of 389 reads). Although the lymphatic malformation initially improved following sclerotherapy, she experienced worsening pain, swelling, and bleeding at the foot especially during weight bearing 6 months post-sclerotherapy. Around 30 months of age, she started sirolimus, an mTOR inhibitor, at 0.39 mg by mouth twice per day. After 6 months of therapy, she had decreased pain, ulceration, and size of the lesion.
4 DISCUSSION
A co-occurring genetic disorder has been reported in 152 individuals with Turner syndrome (Jones et al., 2018). The majority (31%) of individuals had Down syndrome as the most common co-occurring genetic condition (Jones et al., 2018). Other genetic conditions reported in individuals with Turner syndrome include Fragile X syndrome, hemophilia, and muscular dystrophy (Jones et al., 2018). However, there were no individuals with a mosaic vascular malformation previously reported. The co-occurrence of lymphatic-venous and lymphatic malformations in these two individuals with Turner syndrome led us to hypothesize that the vascular malformations were due to a secondary mosaic cause. Indeed, we discovered that these vascular malformations are due to mosaic pathogenic variants in PIK3CA, a well-known cause of lymphatic malformations (Brouillard et al., 2021; Luks et al., 2015; Zenner et al., 2019).
Vascular malformations have also been reported to co-occur in Turner syndrome (Paller et al., 1983; Weiss, 1988), though a complicating factor is the many of these reports were prior to or around the time that classification of hemangiomas and vascular malformations was changed to be based on endothelial characteristics (Mulliken & Glowacki, 1982) and prior to the International Society for the Study of Vascular Anomalies classification (Wassef et al., 2015). Four individuals with Turner syndrome and “pedal hemangiomas” were described with varying features, though Bushkell and colleagues questioned whether these were true hemangiomas and possibly could be related to vascular ectasia of the skin (Bushkell & Broughton, 1984). Weiss (1988) described two individuals with Turner syndrome and “pedal hemangiomas” though histologically the malformations could be considered “venous hemangiomas”. Dr. Weiss proposed that these were in fact malformations (rather than a vascular tumor which is what a hemangioma is considered) and that more generalized vascular anomalies could be part of the spectrum of Turner syndrome (Weiss, 1988). We would agree with Dr. Weiss as the descriptions were not consistent with hemangiomas nor were the biopsies. Indeed, the features of the toe lesion of individual 1 closely resemble those described by Weiss; however, immunohistochemical data was not provided in her description (Weiss, 1988). There was another individual with Turner syndrome and angiokeratoma of the foot, which was D2-40 positive on pathology (typically a marker of lymphatic endothelium) (Berk et al., 2010). Finally, one additional individual was reported with a left subclavian cystic lymphangioma (now known as a lymphatic malformation), but no genetic testing was performed on that sample (Gaertner et al., 2016).
We hypothesize that if genetic testing was performed on the biopsies from the previous case studies, somatic pathogenic variants may have been found as the cause of the vascular malformations. Vascular malformations of the gastrointestinal tract resulting in bleeding are also described as a rare feature of Turner syndrome (Bang & Peter, 2013; Kucharska et al., 2018; Witkowska-Krawczak et al., 2021). In these individuals, further genetic characterization is needed to evaluate for secondary causes, such as Hereditary Hemorrhagic Telangiectasia, as previously recommended (Jones et al., 2018).
We evaluated the prevalence of vascular malformations in the Turner syndrome population seen at CHOP. From January 2020 to July 2022, there were 345 individuals with Turner syndrome seen at CHOP which leads to prevalence of 2 in 345. This is similar to what would be expected, based on the prevalence of vascular malformations in 1.2% of the population (Tasnadi, 2009). Future work studying the natural history of lymphatic anomalies may allow us to investigate this further.
Determination of additional genetic causes have important clinical implications, especially in the current era of theragnostics (Queisser et al., 2021). Recently, there have been many advances in the use of targeted inhibitors for treatment of vascular malformations. Sirolimus led to a partial response in 82% of participants with complicated vascular anomalies (Adams et al., 2016). In individual 2, the discovery of PIK3CA mosaicism as a secondary cause for the lymphatic malformation led to the addition of sirolimus. Notably, she had clinical improvement after the initiation of sirolimus. She will be eligible for alpelisib, a selective PI3Kα inhibitor, if there is worsening of disease (Delestre et al., 2021; Venot et al., 2018).
In summary, we report the co-occurrence of PIK3CA-related vascular malformations with Turner syndrome to conclude that generalized vascular anomalies are not part of the spectrum of Turner syndrome.
AUTHOR CONTRIBUTIONS
Bede N. Nriagu: writing − original draft, and writing − review and editing; Lydia S. Williams: writing − original draft, and writing − review and editing; Niambi Brewer: formal analysis, writing − original draft, and writing − review and editing; Lea F. Surrey: writing − original draft, and writing − review and editing; Abhay S. Srinivasan: writing − original draft, and writing − review and editing; Dong Li: formal analysis, writing − original draft, and writing − review and editing; Allison Britt: formal analysis and writing − review and editing; James Treat: formal analysis and writing − review and editing; T. Blaine Crowley: formal analysis and writing − review and editing; Nora O'Connor: project administration and writing − review and editing; Arupa Ganguly: formal analysis and writing − review and editing; David Low: formal analysis and writing − review and editing; Maria Queenan: formal analysis and writing − review and editing; Theodore G. Drivas: formal analysis and writing − review and editing; Elaine H. Zackai: formal analysis and writing − review and editing; Denise M. Adams: funding acquisition and writing − review and editing; Hakon Hakonarson: funding acquisition and writing − review and editing; Kristen M. Snyder: formal analysis and writing − review and editing; Sarah E. Sheppard: conceptualization, writing − original draft, and writing − review and editing.
ACKNOWLEDGMENTS
The authors thank the individuals and their families for participating in this manuscript. The authors thank the Comprehensive Vascular Anomalies Program for clinical care. The work was supported by a Children's Hospital of Philadelphia Frontier Program Grant Research (Denise M. Adams, Hakon Hakonarson). Sarah E. Sheppard is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number HD009003-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
Dr. Hakon Hakonarson and CHOP are equity holders in Nobias Therapeutics Inc., developing MEK inhibitor therapy for complex lymphatic anomalies. Dr. Denise M. Adams is a consultant for Novartis and Nobias and Dr. Kristen M. Snyder are consultants for Novartis Pharmaceuticals, which makes Vijoice (alpelisib), a selective PI3Kα inhibitor.
Open Research
DATA AVAILABILITY STATEMENT
The data that support this study are presented in the figures. Data from the clinical testing is not publicly available due to privacy or ethical restrictions. Exome data for individual 2 is available from the authors upon request.




